Nobiletin Inhibits Angiogenesis by Regulating Src/FAK/STAT3-Mediated Signaling through PXN in ER+ Breast Cancer Cells
Abstract
:1. Introduction
2. Results
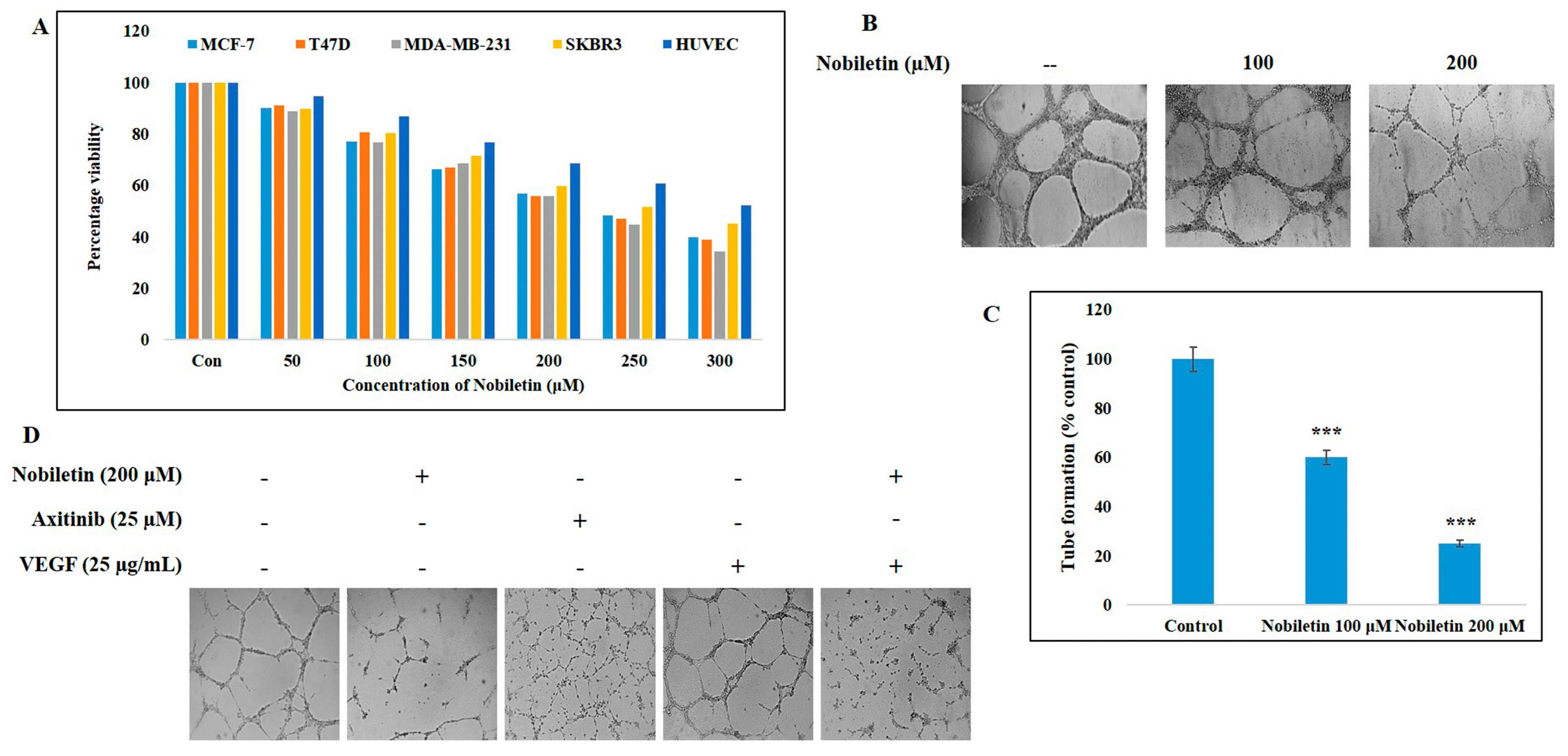
2.1. Nobiletin Inhibits Cell Proliferation of Various Breast Cancer Cell Lines and an Endothelial Cell Line
2.2. Nobiletin Inhibits VEGF-Dependent In Vitro Angiogenesis
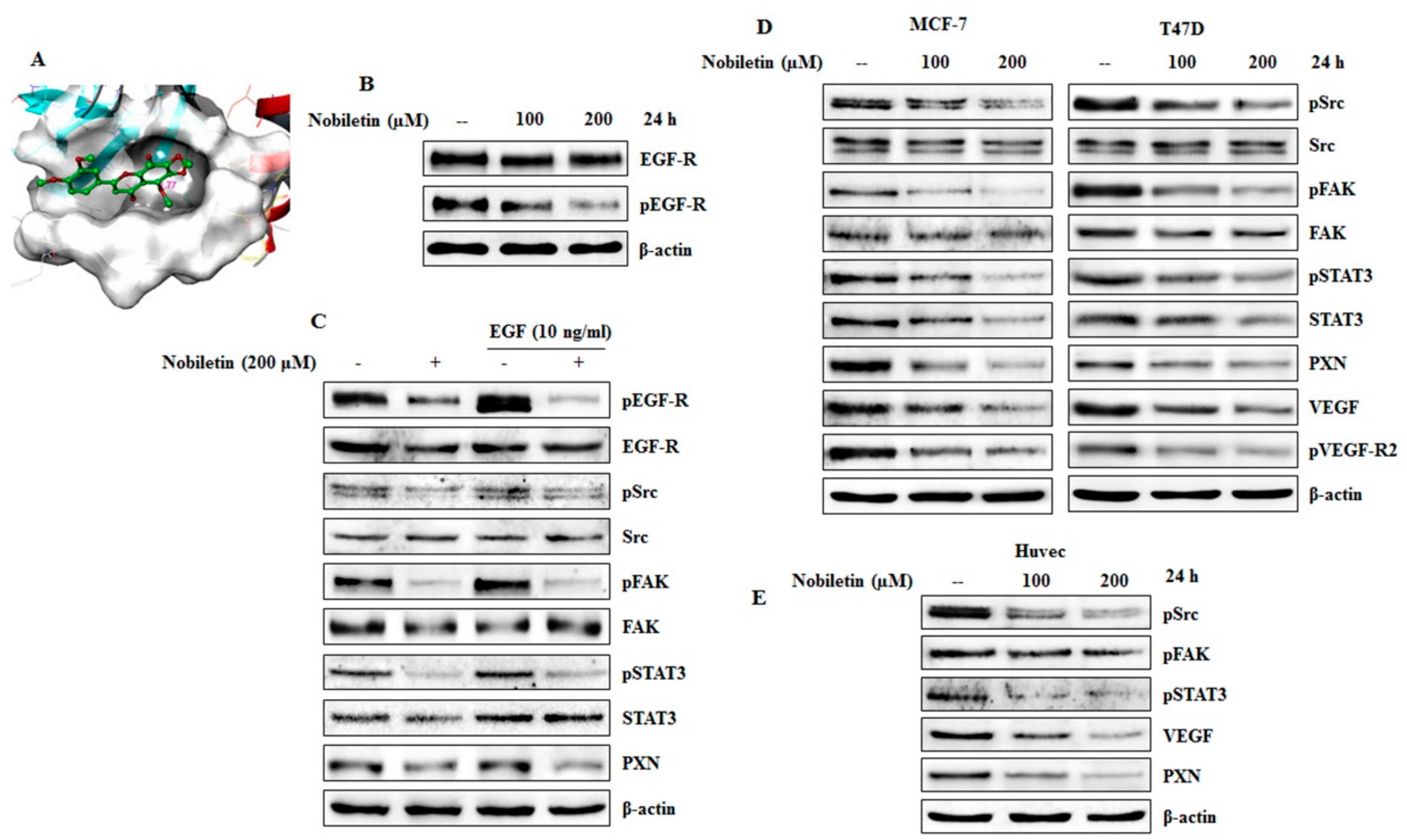
2.3. Nobiletin Inhibits EGFR Activity and Downregulates Src/FAK/STAT3 Signaling
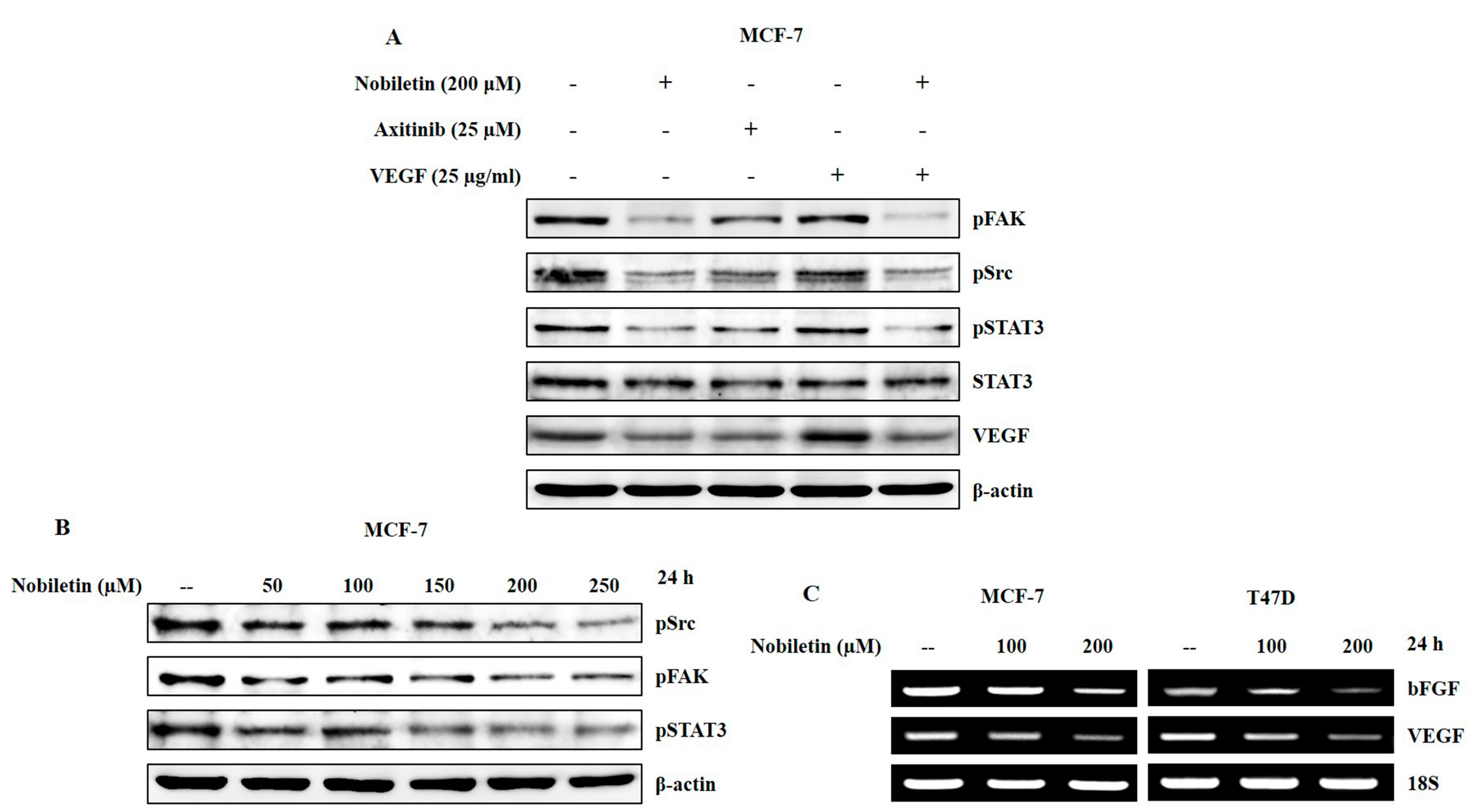
2.4. VEGF and Concentration-Dependent Inhibition of Src/FAK/STAT3 Signaling and Downregulation of Angiogenic Factors by Nobiletin
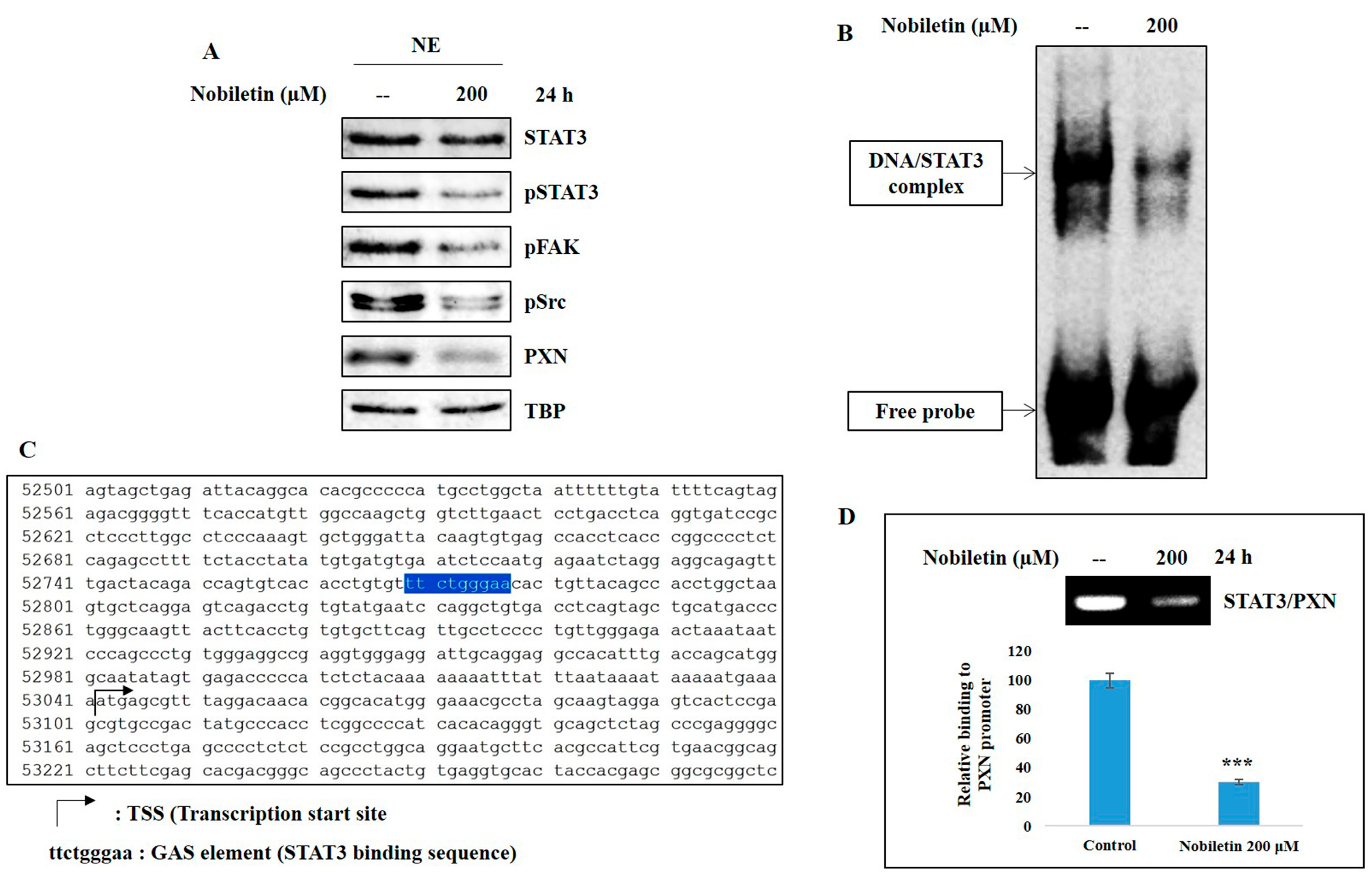
2.5. Nobiletin Inhibits Nuclear Translocation and DNA Binding Activity of STAT3
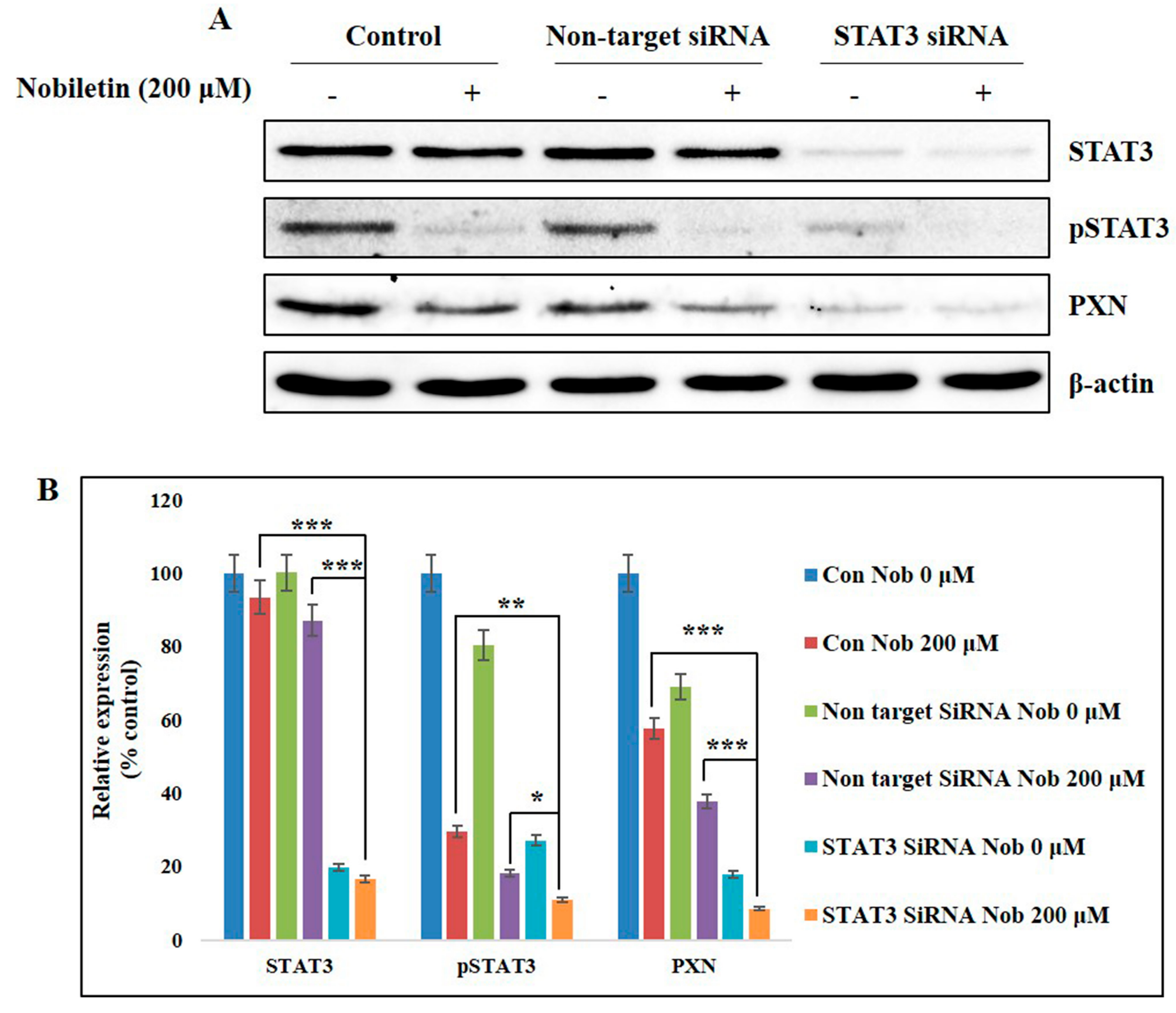
2.6. Nobiletin Inhibits PXN Expression in a STAT3-Dependent Manner
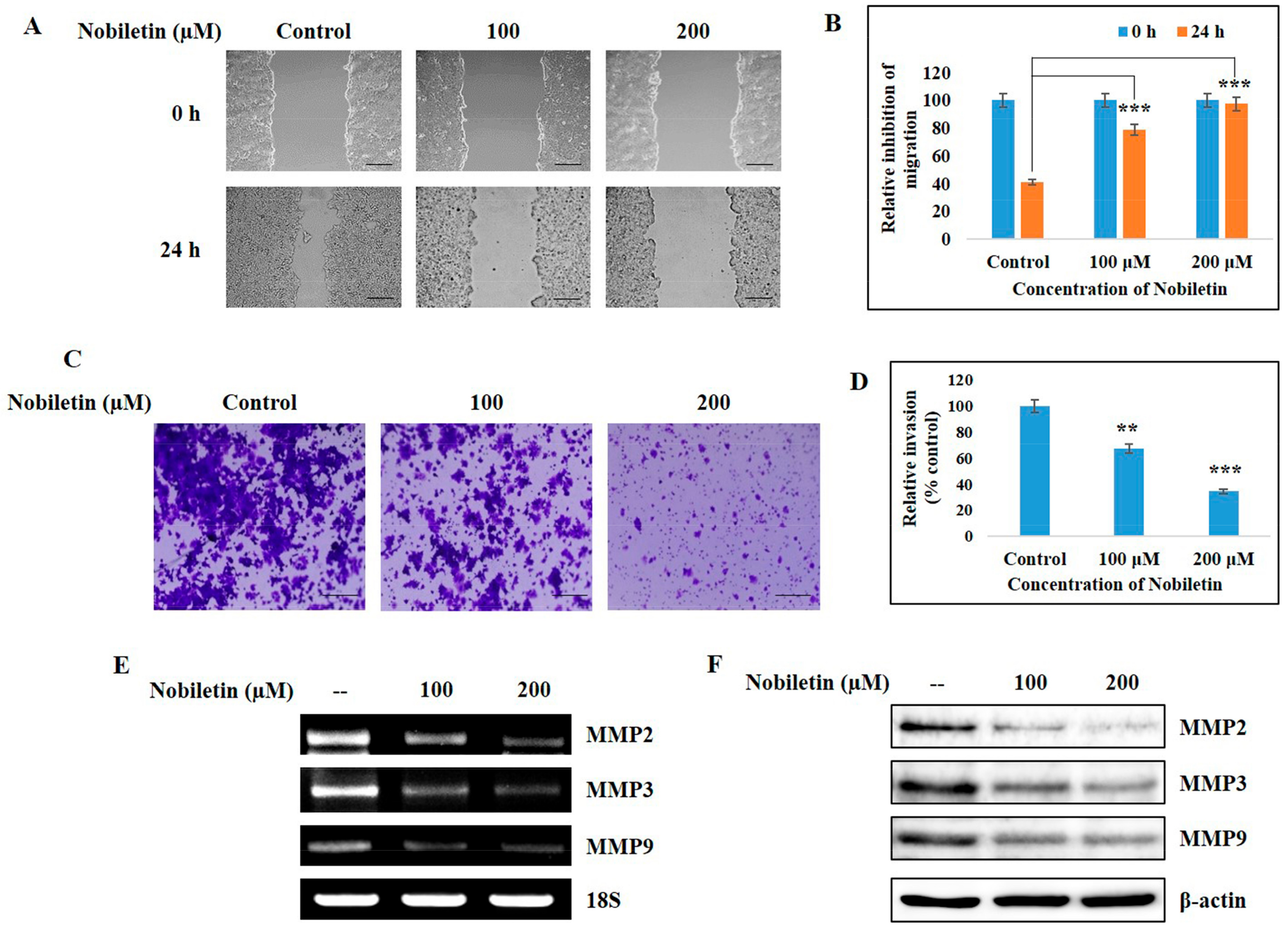
2.7. Nobiletin Inhibits Migration and Cellular Invasive Potential
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Culture and Treatment
4.3. Cell Proliferation Inhibition
4.4. In Vitro Angiogenesis Assay
4.5. Western Blotting
4.6. Reverse Transcription-PCR
4.7. EMSA
4.8. ChIP Assay
4.9. Wound Healing Assay
4.10. Matrigel Invasion Assay
4.11. Small Interference RNA (siRNA) Analysis
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anders, C.K.; Carey, L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Fernández, L.M.G. Use of statistics to assess the global burden of breast cancer. Breast J. 2006, 12, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.M.; Clark, G.M.; Osborne, C.K.; Allred, D.C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999, 17, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.R.; Anderson, A.R.A.; Chaplain, M.A.J. Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: Clinical implications and therapeutic targeting strategies. J. Theor. Biol. 2006, 241, 564–589. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, T.; Rofstad, E.K. VEGF, bFGF and EGF in the angiogenesis of human melanoma xenografts. Int. J. Cancer 1998, 76, 836–841. [Google Scholar] [CrossRef]
- Sieg, D.J.; Hauck, C.R.; Ilic, D.; Klingbeil, C.K.; Schaefer, E.; Damsky, C.H.; Schlaepfer, D.D. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000, 2, 249–256. [Google Scholar] [PubMed]
- Sieg, D.J.; Hauck, C.R.; Schlaepfer, D.D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 1999, 112, 2677–2691. [Google Scholar] [PubMed]
- Roskoski, R. Src protein-tyrosine kinase structure and regulation. Biochem. Biophys. Res. Commun. 2004, 324, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M. Src signaling in cancer invasion. J. Cell. Physiol. 2010, 223, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, S.; Zhong, J.; Zhang, W.; Hua, X.; Li, B.; Sun, H. CD151 mediates netrin-1-induced angiogenesis through the Src-FAK-Paxillin pathway. J. Cell. Mol. Med. 2017, 21, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive STAT3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C.H. STAT3: A critical transcription activator in angiogenesis. Med. Res. Rev. 2008, 28, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Deramaudt, T.B.; Dujardin, D.; Noulet, F.; Martin, S.; Vauchelles, R.; Takeda, K.; Ronde, P. Altering FAK-paxillin interactions reduces adhesion, migration and invasion processes. PLoS ONE 2014, 9, e92059. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.L.; Naora, H.; Liu, J.; Cheng, W.; Montell, D.J. Activated signal transducer and activator of transcription STAT3: Localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004, 64, 3550–3558. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Sato, T.; Takayama, Y.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem. Pharmacol. 2003, 65, 2065–2071. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Miyazaki, K.; Sakai, S.; Yawo, H.; Nakata, N.; Moriguchi, S.; Fukunaga, K.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid with neurotrophic action, augments protein kinase A-mediated phosphorylation of the AMPA receptor subunit, GluR1, and the postsynaptic receptor response to glutamate in murine hippocampus. Eur. J. Pharmacol. 2008, 578, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T.; Okuyama, Y.; Miyata, Y.; Kosano, H.; Takahashi, H.; Natsugari, H. Nobiletin metabolites: Synthesis and inhibitory activity against matrix metalloproteinase-9 production. Bioorganic Med. Chem. Lett. 2011, 21, 4540–4544. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ono, M.; Takeshima, M.; Nakano, S. Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Res. 2014, 34, 1785–1792. [Google Scholar] [PubMed]
- Chien, S.-Y.; Hsieh, M.-J.; Chen, C.-J.; Yang, S.-F.; Chen, M.-K. Nobiletin inhibits invasion and migration of human nasopharyngeal carcinoma cell lines by involving ERK1/2 and transcriptional inhibition of MMP-2. Expert Opin. Ther. Targets 2015, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.D.; Liao, Y.C.; Shih, Y.W.; Tsai, L.Y. Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways in HGF-treated liver cancer HepG2 cells. Phytomedicine 2013, 20, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Kunimasa, K.; Ikekita, M.; Sato, M.; Ohta, T.; Yamori, Y.; Ikeda, M.; Kuranuki, S.; Oikawa, T. Nobiletin, a citrus polymethoxyflavonoid, suppresses multiple angiogenesis-related endothelial cell functions and angiogenesis in vivo. Cancer Sci. 2010, 101, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Hong, D.Y.; Park, J.H.; Joung, Y.H.; Darvin, P.; Kim, S.Y.; Na, Y.M.; Hwang, T.S.; Ye, S.K.; Moon, E.S.; et al. Methylsulfonylmethane suppresses breast cancer growth by down-regulating STAT3 and STAT5b pathways. PLoS ONE 2012, 7, e33361. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Yoo, Y.B.; Joung, Y.H.; Kang, D.Y.; Kim, D.N.; Hwang, T.S.; Kim, S.Y.; Kim, W.S.; Lee, H.K.; Cho, B.W.; et al. The combination of methylsulfonylmethane and tamoxifen inhibits the Jak2/STAT5b pathway and synergistically inhibits tumor growth and metastasis in ER-positive breast cancer xenografts. BMC Cancer 2015, 15, 474. [Google Scholar]
- Chen, J.; Chen, A.; Huang, H.; Ye, X.; Rollyson, W.; Perry, H.; Brown, K.; Rojanasakul, Y.; Rankin, G.; Dasgupta, P.; et al. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway. Int. J. Oncol. 2015, 46, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.H.; Alex, D.; Lam, I.K.; Tsui, S.K.W.; Yang, Z.F.; Lee, S.M.Y. Nobiletin, a polymethoxylated flavonoid from citrus, shows anti-angiogenic activity in a zebrafish in vivo model and HUVEC in vitro model. J. Cell. Biochem. 2011, 112, 3313–3321. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G. VEGF receptor signaling in tumor angiogenesis. Oncologist 2000, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Shah, A.N.; Zhang, J.; Gallick, G.E. Regulation of angiogenesis and vascular permeability by Src family kinases: Opportunities for therapeutic treatment of solid tumors. Expert Opin. Ther. Targets 2007, 11, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Lechertier, T.; Hodivala-Dilke, K. Focal adhesion kinase and tumour angiogenesis. J. Pathol. 2012, 226, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.J.; Donais, K.; Whitmore, L.A.; Thomas, S.M.; Turner, C.E.; Parsons, J.T.; Horwitz, A.F. FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004, 6, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, O.A.; Chasovskikh, S.; Lonskaya, I.; Tarasova, N.I.; Khavrutskii, L.; Tarasov, S.G.; Zhang, X.; Korostyshevskiy, V.R.; Cheema, A.; Zhang, L.; et al. Mechanisms of unphosphorylated STAT3 transcription factor binding to DNA. J. Biol. Chem. 2012, 287, 14192–14200. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.L.; Milkiewicz, M.; Davis, S.J.; Zhou, A.L.; Egginton, S.; Brown, M.D.; Madri, J.A.; Hudlicka, O. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, 1540–1547. [Google Scholar]
- Palmisano, R.; Itoh, Y. Analysis of MMP-dependent cell migration and invasion. Methods Mol. Biol. 2010, 622, 379–392. [Google Scholar] [PubMed]
| Sl No | Gene | Annealing Temperature (°C) | Product Size (bp) | Sequence (5′–3′) |
|---|---|---|---|---|
| 1 | bFGF | 58 | 498 | Forward: gagaagagcgaccctcaca |
| Reverse: tagctttctgcccaggtcc | ||||
| 2 | VEGF | 58 | 405 | Forward: aggagggcagaatcatcacg |
| Reverse: caaggcccacagggattttc | ||||
| 3 | 18S | 58 | 490 | Forward: agccttcggctgactggctgg |
| Reverse: ctgcccatcatcatgacctgg | ||||
| 4 | MMP2 | 53 | 665 | Forward: gagttggcagtgcaatacct |
| Reverse: gccatccttctcaaagttgt | ||||
| 5 | MMP3 | 60 | 432 | Forward: cctgctttgtcctttgatgc |
| Reverse: tgagtcaatccctggaaagt | ||||
| 6 | MMP9 | 58 | 455 | Forward: cctgccagtttccattcatc |
| Reverse: gccattcacgtcgtccttat | ||||
| 7 | PXN (ChIP assay) | 60 | 181 | Forward: gcccctctcagagccttttc |
| Reverse: gcagctactgaggtcacagc |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sp, N.; Kang, D.Y.; Joung, Y.H.; Park, J.H.; Kim, W.S.; Lee, H.K.; Song, K.-D.; Park, Y.-M.; Yang, Y.M. Nobiletin Inhibits Angiogenesis by Regulating Src/FAK/STAT3-Mediated Signaling through PXN in ER+ Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 935. https://doi.org/10.3390/ijms18050935
Sp N, Kang DY, Joung YH, Park JH, Kim WS, Lee HK, Song K-D, Park Y-M, Yang YM. Nobiletin Inhibits Angiogenesis by Regulating Src/FAK/STAT3-Mediated Signaling through PXN in ER+ Breast Cancer Cells. International Journal of Molecular Sciences. 2017; 18(5):935. https://doi.org/10.3390/ijms18050935
Chicago/Turabian StyleSp, Nipin, Dong Young Kang, Youn Hee Joung, Jong Hwan Park, Wan Seop Kim, Hak Kyo Lee, Ki-Duk Song, Yeong-Min Park, and Young Mok Yang. 2017. "Nobiletin Inhibits Angiogenesis by Regulating Src/FAK/STAT3-Mediated Signaling through PXN in ER+ Breast Cancer Cells" International Journal of Molecular Sciences 18, no. 5: 935. https://doi.org/10.3390/ijms18050935






