Chemical Profile and Antioxidant, Anti-Inflammatory, Antimutagenic and Antimicrobial Activities of Geopropolis from the Stingless Bee Melipona orbignyi
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis
2.1.1. Determination of Phenolic Compounds and Flavonoids
2.1.2. Chemical Composition of HEGP by High Performance Liquid Chromatography Coupled to Diode Array Detector and Mass Spectrometry (HPLC-DAD-MS)
2.2. Antioxidant Activity
2.2.1. Capture of Free Radicals DPPH• and ABTS•+
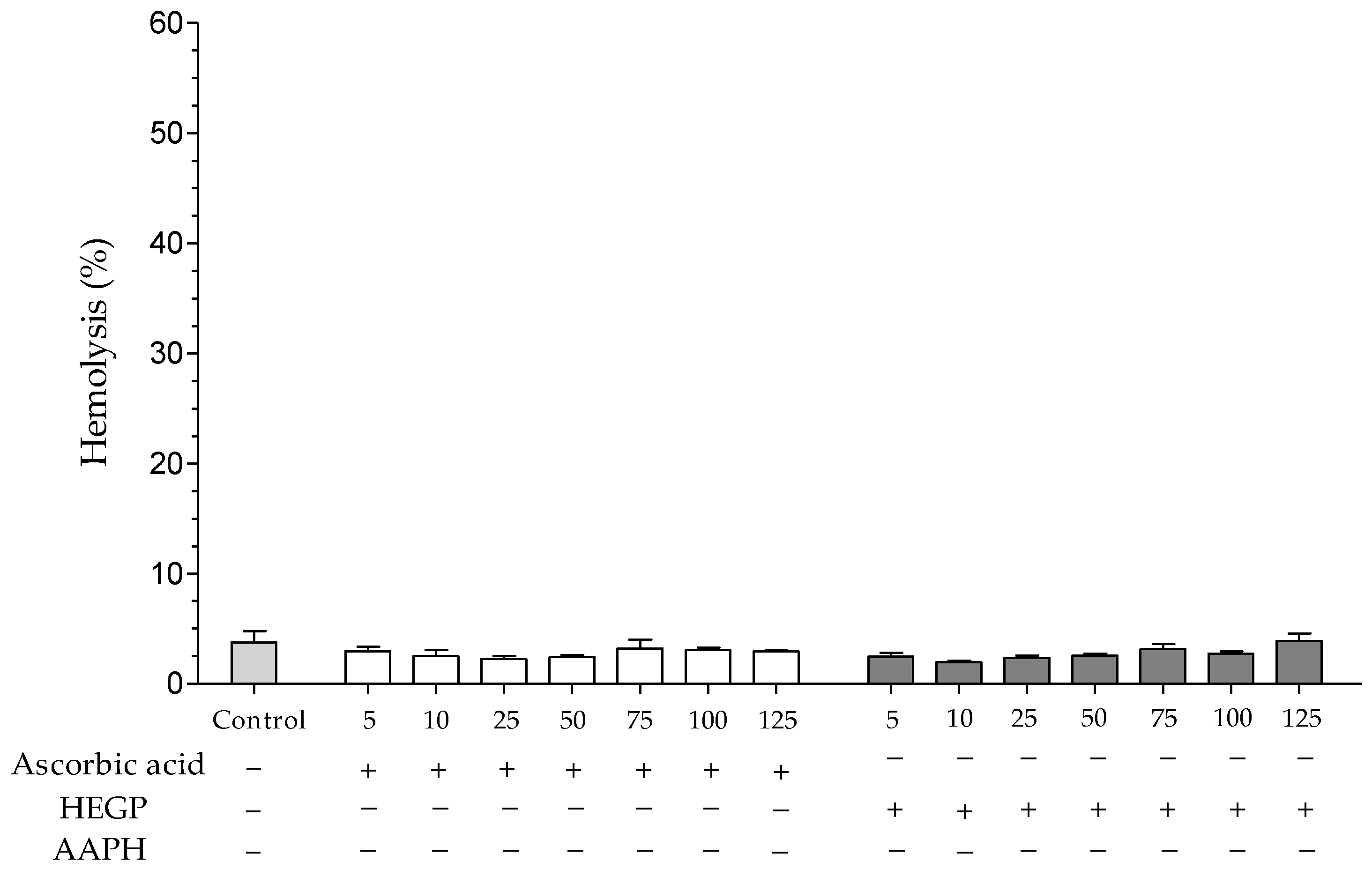
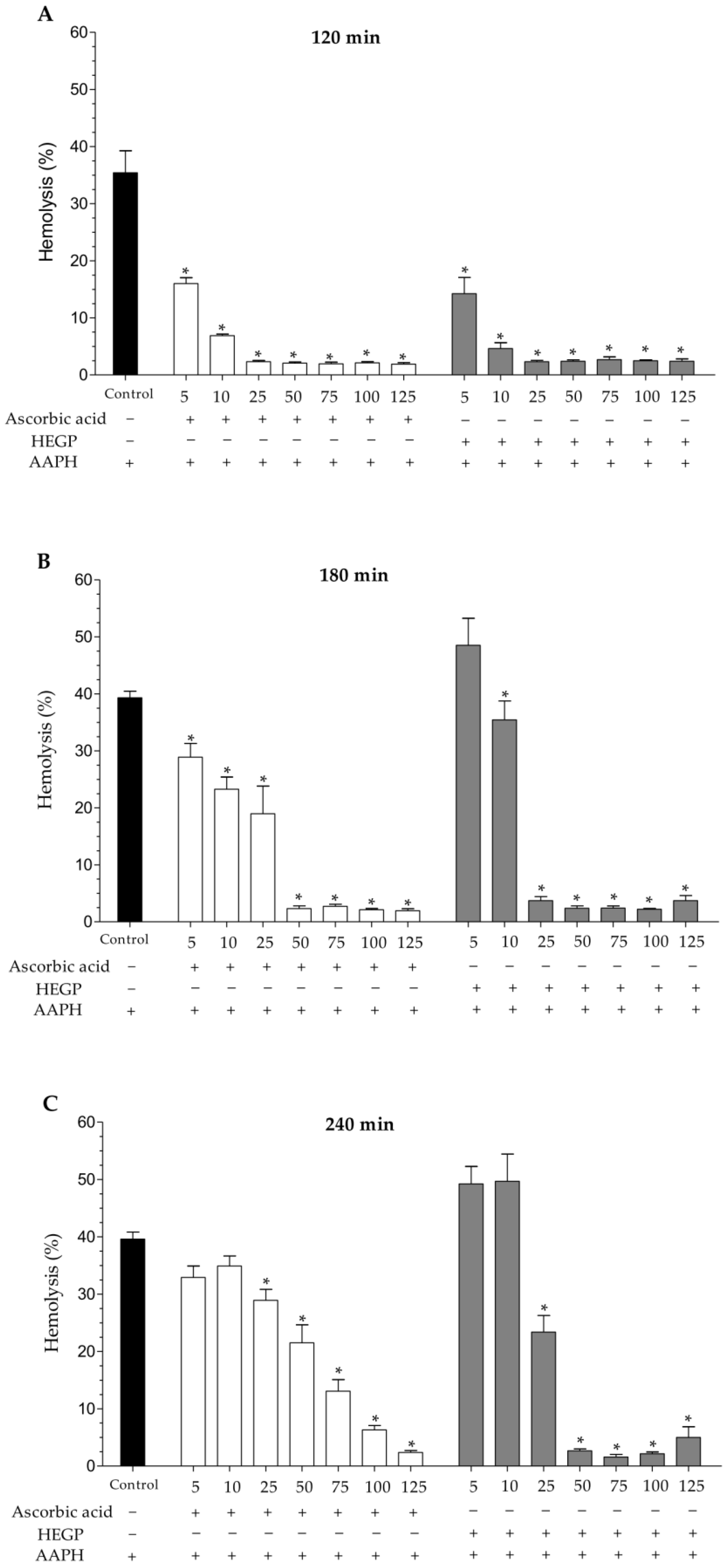
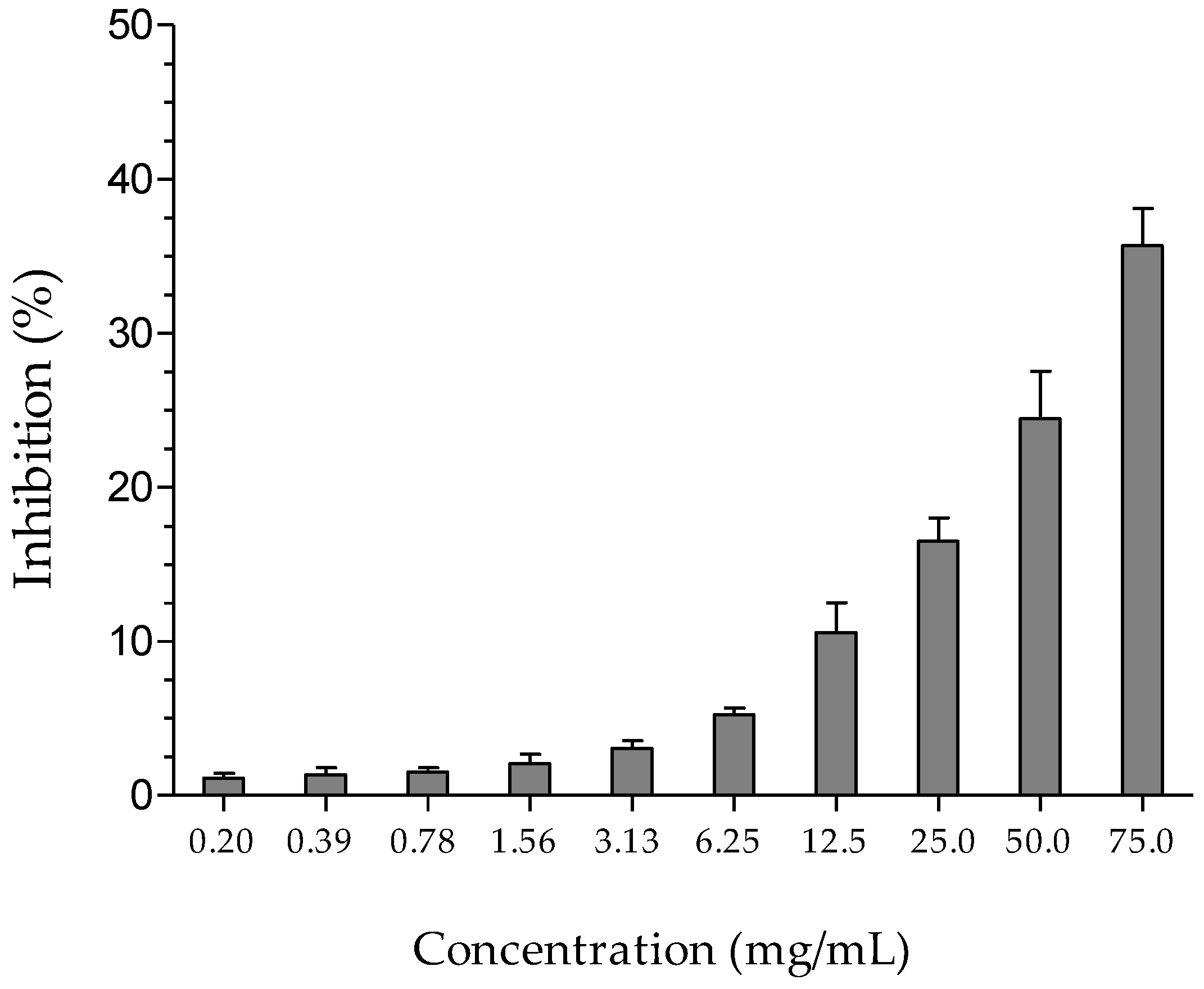
2.2.2. Hemolytic Activity and Inhibition of Oxidative Hemolysis
2.2.3. Dosage of Malondialdehyde
2.3. Anti-Inflammatory Activity
2.4. Antimutagenic Activity
2.5. Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Preparation of the Hydroethanolic Extract of Geopropolis
4.2. Determination of Phenolic Compounds and Flavonoids
4.2.1. Phenolic Compounds
4.2.2. Total Flavonoids
4.3. HPLC-DAD-MS Analyses
4.4. Antioxidant Activities
4.4.1. DPPH• Free Radical Capture Activity
4.4.2. ABTS•+ Free Radical Capture Activity
4.4.3. Antioxidant Assays in Human Erythrocytes
Preparation of the Erythrocyte Suspensions
Inhibition of Oxidative Hemolysis
Dosage of Malondialdehyde (MDA)
4.5. Anti-Inflammatory Activity—Hyaluronidase Assay
4.6. Antimutagenic Activity
4.7. Antimicrobial Activity
4.8. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAPH | 2,2′-Azobis (2-methylpropionamidine) dihydrochloride |
| ABTS•+ | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| ATCC | American Type Culture Collection |
| BHT | Butylated hydroxytoluene |
| DPPH• | 2,2-Diphenyl-1-picrylhydrazyl |
| EMS | Ethyl methanesulfonate |
| ESA | Escola Superior Agrária |
| GAE | Gallic acid equivalents |
| HEGP | Hydroalcoholic extract of geopropolis |
| MDA | Malondialdehyde |
| MBC | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| MFC | Minimum fungicidal concentration |
| M. orbignyi | Melipona orbignyi |
| NaCl | Sodium chloride |
| QE | Quercetin equivalents |
| SEM | Standard error of the mean |
| TBA | Thiobarbituric acid |
| TTC | 2,3,5-Triphenyl-2H-Tetrazoliumchloride |
References
- Freitas, M.O.; Ponte, F.A.; Lima, M.A.S.; Silveira, E.R. Flavonoids and triterpenes from the nest of the stingless bee Trigona spinipes. J. Braz. Chem. Soc. 2008, 19, 532–535. [Google Scholar] [CrossRef]
- Bartolomeu, A.R.; Frión-Herrera, Y.; da Silva, L.M.; Romagnoli, G.G.; de Oliveira, D.E.; Sforcin, J.M. Combinatorial effects of geopropolis produced by Melipona fasciculata Smith with anticancer drugs against human laryngeal epidermoid carcinoma (HEp-2) cells. Biomed. Pharmacother. 2016, 81, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.; Paiva, L.K.O.Y.J.; Aguiar, L.; Plácido, F.; Oliveira, M. Valor nutricional do mel e pólen de abelhas sem ferrão da região amazônica. Acta Amazon. 2004, 34, 1–4. [Google Scholar] [CrossRef]
- Araújo, M.J.A.M.; Búfalo, M.C.; Conti, B.; Junior, A.F.; Trusheva, B.; Bankova, V.; Sforcin, J.M. The chemical composition and pharmacological activities of geopropolis produced by Melipona fasciculata Smith in Northeast Brazil. J. Mol. Pathophysiol. 2015, 4, 12–20. [Google Scholar] [CrossRef]
- Cunha, M.G.; Franchin, M.; Galvão, L.C.D.C.; Bueno-Silva, B.; Ikegaki, M.; Alencar, S.M.; Rosalen, P.L. Apolar bioactive fraction of Melipona scutellaris geopropolis on Streptococcus mutans biofilm. Evid.-Based Complement. Altern. Med. 2013, 2013, 256287. [Google Scholar] [CrossRef] [PubMed]
- Franchin, M.; Cunha, M.G.; Denny, C.; Napimoga, M.H.; Cunha, T.M.; Bueno-Silva, B.; Alencar, S.M.; Ikegaki, M.; Rosalen, P.L. Bioactive fraction of geopropolis from Melipona scutellaris decreases neutrophils migration in the inflammatory process: Involvement of nitric oxide pathway. Evid.-Based Complement. Altern. Med. 2013, 2013, 907041. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.P.; Abreu, B.V.B.; Cunha, M.S.; Batista, M.C.A.; Torres, L.M.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Phenolic acids, hydrolyzable tannins, and antioxidant activity of geopropolis from the stingless bee Melipona fasciculata Smith. J. Agric. Food Chem. 2014, 62, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Moure’s bee catalogue. Available online: http://moure.cria.org.br (accessed on 11 November 2016).
- Nogueira-Neto, P. Arquitetura dos ninhos. In Vida e criação de abelhas indígenas sem ferrão; Urna Edição Nogueirapis: Nogueirapis São Paulo, Brazil, 1997; pp. 47–60. [Google Scholar]
- Souza, S.A.; Camara, C.A.; Silva, E.M.S.; Silva, T.M.S. Composition and antioxidant activity of geopropolis collected by Melipona subnitida (Jandaíra) bees. Evid.-Based Complement. Altern. Med. 2013, 2013, 801383. [Google Scholar]
- Franchin, M.; Cunha, M.G.; Denny, C.; Napimoga, M.H.; Cunha, T.M.; Koo, H.; Alencar, S.M.; Ikegaki, M.; Rosalen, P.L. Geopropolis from Melipona scutellaris decreases the mechanical inflammatory hypernociception by inhibiting the production of IL-1β and TNF-α. J. Ethnopharmacol. 2012, 143, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Junior, J.A.; Franchin, M.; Cavallini, M.E.; Denny, C.; Alencar, S.M.; Ikegaki, M.; Rosalen, P.L. Gastroprotective effect of geopropolis from Melipona scutellaris is dependent on production of nitric oxide and prostaglandin. Evid.-Based Complement. Altern. Med. 2015, 2015, 459846. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.R.; Mendonça, R.Z.; Vilar, K.D.S.; Figueiredo, C.A.; Badari, J.C.; Taniwaki, N.; Namiyama, G.; Oliveira, M.I.; Curti, S.P.; Silva, P.E.; et al. Antiviral action of hydromethanolic extract of geopropolis from Scaptotrigona postica against antiherpes simplex virus (HSV-1). Evid.-Based Complement. Altern. Med. 2015, 2015, 296086. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.P.; Nogueira, A.M.C.; Marques, R.R.D.O.; Costa, M.C.P.; Ribeiro, M.N.S. Pharmacognostic evaluation of geopropolis of Melipona fasciculata Smith from Baixada Maranhense, Brazil. Rev. Bras. Farmacogn. 2008, 18, 557–562. [Google Scholar] [CrossRef]
- Chemical Abstract Service database. Available online: https://scifinder.cas.org (accessed on 15 January 2017).
- Campos, J.F.; Santos, U.P.; Macorini, L.F.B.; Melo, A.M.M.F.; Balestieri, J.B.P.; Gamero, E.J.P.; Cardoso, C.A.L.; Souza, K.P.; Santos, E.L. Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae). Food Chem. Toxicol. 2014, 65, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R. Ultraviolet-visible absorption spectroscopy. In Techniques of Flavonoid Identification; Academic Press: London, UK, 1982; pp. 36–51. [Google Scholar]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Justino, G.C.; Borges, C.M.; Florencio, M.H. Electrospray ionization tandem mass spectrometry fragmentation of protonated flavone and flavonol aglycones: A re-examination. Rapid. Commun. Mass Spectrom. 2009, 23, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Christov, R.; Marcucci, C.; Popov, S. Constituents of Brazilian geopropolis. Z. Naturforsch. C 1998, 53, 402–406. [Google Scholar]
- Costa da Silva, E.C.; Muniz, M.P.; Nunomura, R.C.S.; Nunomura, S.M.; Zilse, G.A.C. Phenolic constituents and antioxidant activity of geopropolis from two species of Amazonian stingless bees. Quim. Nov. 2013, 36, 628–633. [Google Scholar]
- Wang, J.B.; Qin, Y.; Kong, W.J.; Wang, Z.W.; Zeng, L.N.; Fang, F.; Jin, C.; Zhao, Y.L.; Xiao, X.H. Identification of the antidiarrhoeal components in official rhubarb using liquid chromatography–tandem mass spectrometry. Food Chem. 2011, 129, 1737–1743. [Google Scholar] [CrossRef]
- Walker, P.; Crane, E. Constituents of propolis. Apidologie 1987, 18, 327–334. [Google Scholar] [CrossRef]
- Patricio, E.F.L.R.A.; Cruz-Lopez, L.; Maile, R.; Tentschert, J.; Jones, G.R.; Morgan, E.D. The propolis of stingless bees: Terpenes from the tibia of three Frieseomelitta species. J. Insect Physiol. 2002, 48, 249–254. [Google Scholar] [CrossRef]
- Marquez Hernandez, I.; Cuesta-Rubio, O.; Campo Fernandez, M.; Perez, A.R.; Porto, R.M.O.; Piccinelli, A.L.; Rastrelli, L. Studies on the constituents of yellow cuban propolis: GC-MS determination of triterpenoids and flavonoids. J. Agric. Food Chem. 2010, 58, 4725–4730. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.G.; Rosalen, P.L.; Franchin, M.; Alencar, S.M.; Ikegaki, M.; Ransom, T.; Beutler, J.A. Antiproliferative constituents of geopropolis from the bee Melipona scutellaris. Planta Med. 2016, 82, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zeng, S.F.; Yang, C.R.; Zhang, Y.J. A new hydrolyzable tannin from Balanophora harlandii with radical-scavenging activity by scavenging activity. Helv. Chim. Acta 2009, 92, 1817–1822. [Google Scholar] [CrossRef]
- Karamać, M. Chelation of Cu (II), Zn (II), and Fe (II) by tannin constituents of selected edible nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Espinoza, M.C.; Zafimahova, A.; Alvarado, P.G.M.; Dubreucq, E.; Poncet-Legrand, C. Grape seed and apple tannins: Emulsifying and antioxidant properties. Food Chem. 2015, 178, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Prasad, N.R. Effect of ursolic acid, a triterpenoid antioxidant, on ultraviolet-B radiation-induced cytotoxicity, lipid peroxidation and DNA damage in human lymphocytes. Chem. Biol. Interact. 2008, 176, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Prasad, R.; Pugalendi, K.V.; Menon, V.P. Modulation of UVB-induced oxidative stress by ursolic acid in human blood lymphocytes. Asian J. Biochem. 2008, 3, 11–18. [Google Scholar]
- Shoaib, M.; Shah, I.; Ali, N.; Adhikari, A.; Tahir, M.N.; Shah, S.W.; Ishtiaq, S.; Khan, J.; Khan, S.; Umer, M.N. Sesquiterpene lactone! A promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement. Altern. Med. 2017, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Mackay, C.R. Moving targets: Cell migration inhibitors as new anti-inflammatory therapies. Nat. Immunol. 2008, 9, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Rawdin, B.J.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Reiter, R.J.; Jiménez-Aranda, A.; Ibán-Arias, R.; Navarro-Alarcón, M.; Marchal, J.A.; Adem, A.; Fernández-Vázquez, G. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 2013, 54, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Veja, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feás, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Formisano, C.; Sanna, C.; Ballero, M.; Chianese, G.; Sirignano, C.; Rigano, D.; Millán, E.; Muñoz, E.; Taglialatela-Scafati, O. Anti-inflammatory sesquiterpene lactones from Onopordum illyricum L. (Asteraceae), an Italian medicinal plant. Fitoterapia 2017, 116, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Francisco, V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Intracellular signaling pathways modulated by phenolic compounds: Application for new anti-inflammatory drugs discovery. Curr. Med. Chem. 2012, 19, 2876–2900. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Serrilli, A.M.; Rizza, L.; Frasca, G.; Cardile, V.; Bonina, F.P.; Bianco, A. Aromadendrine, a new component of the flavonoid pattern of Olea europaea L. and its anti-inflammatory activity. Nat. Prod. Res. 2012, 27, 1–10. [Google Scholar]
- Simões-Ambrosio, L.M.; Gregório, L.E.; Sousa, J.P.; Figueiredo-Rinhel, A.S.; Azzolini, A.E.; Bastos, J.K.; Lucisano-Valim, Y.M. The role of seasonality on the inhibitory effect of Brazilian green propolis on the oxidative metabolism of neutrophils. Fitoterapia 2010, 81, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, S.K.; Chaudhary, M.; Gadbail, A.R.; Sharma, A.; Tekade, S. Oxidative and antioxidative mechanisms in oral cancer and precancer: A review. Oral Oncol. 2014, 50, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Cano-Lamadrid, M.; Marhuenda-Egea, F.C.; Hernández, F.; Rosas-Burgos, E.C.; Burgos-Hernández, A.; Carbonell-Barrachina, A.A. Biological activity of conventional and organic pomegranate juices: Antioxidant and antimutagenic potential. Plant Foods Hum. Nutr. 2016, 71, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.H.; Guterres, Z.R.; Garcez, W.S.; Lopes, S.M.; Corsino, J.; Garcez, F.R. Assessment of the (anti) genotoxicity of brown propolis extracts from Brazilian Cerrado biome in a Drosophila melanogaster model. Food Res. Int. 2014, 62, 20–26. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Mata, A.M.O.F.; Aguiar, R.P.S.; Paz, M.F.C.J.; Alencar, M.V.O.B.; Ferreira, P.M.P.; Melo-Cavalcante, A.A.C. Therapeutic potential of essential oils focusing on diterpenes. Phytother. Res. 2016, 30, 1420–1444. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.F.; Santos, U.P.; Rocha, P.S.; Damião, M.J.; Balestieri, J.B.P.; Cardoso, C.A.L.; Gamero, E.J.P.; Estevinho, L.M.; Picoli Souza, K.; Santos, E.L. Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of propolis from the stingless bee Tetragonisca fiebrigi (Jataí). Evid.-Based Complement. Altern. Med. 2015, 2015, 296186. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Hawkey, P.M.; Jones, A.M. The changing epidemiology of resistance. J. Antimicrob. Chemother. 2009, 64, i3–i10. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.A.; Lin, Y.T.; Labbe, R.G.; Shetty, K. Phenolic antioxidant mobilization in cranberry pomace by solid-state bioprocessing using food grade fungus Lentinus edodes and effect on antimicrobial activity against select food borne pathogens. Innov. Food Sci. Emerg. Technol. 2004, 5, 81–91. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A. Scope of hydrolysable tannins as possible antimicrobial agent. Phytother. Res. 2016, 30, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.F.; Castro, D.T.H.; Damião, M.J.; Torquato, H.F.V.; Paredes-Gamero, E.J.; Carollo, C.A.; Estevinho, L.M.; Picoli Souza, K.; Santos, E.L. The chemical profile of Senna velutina leaves and their antioxidant and cytotoxic effects. Oxid. Med. Cell Longev. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
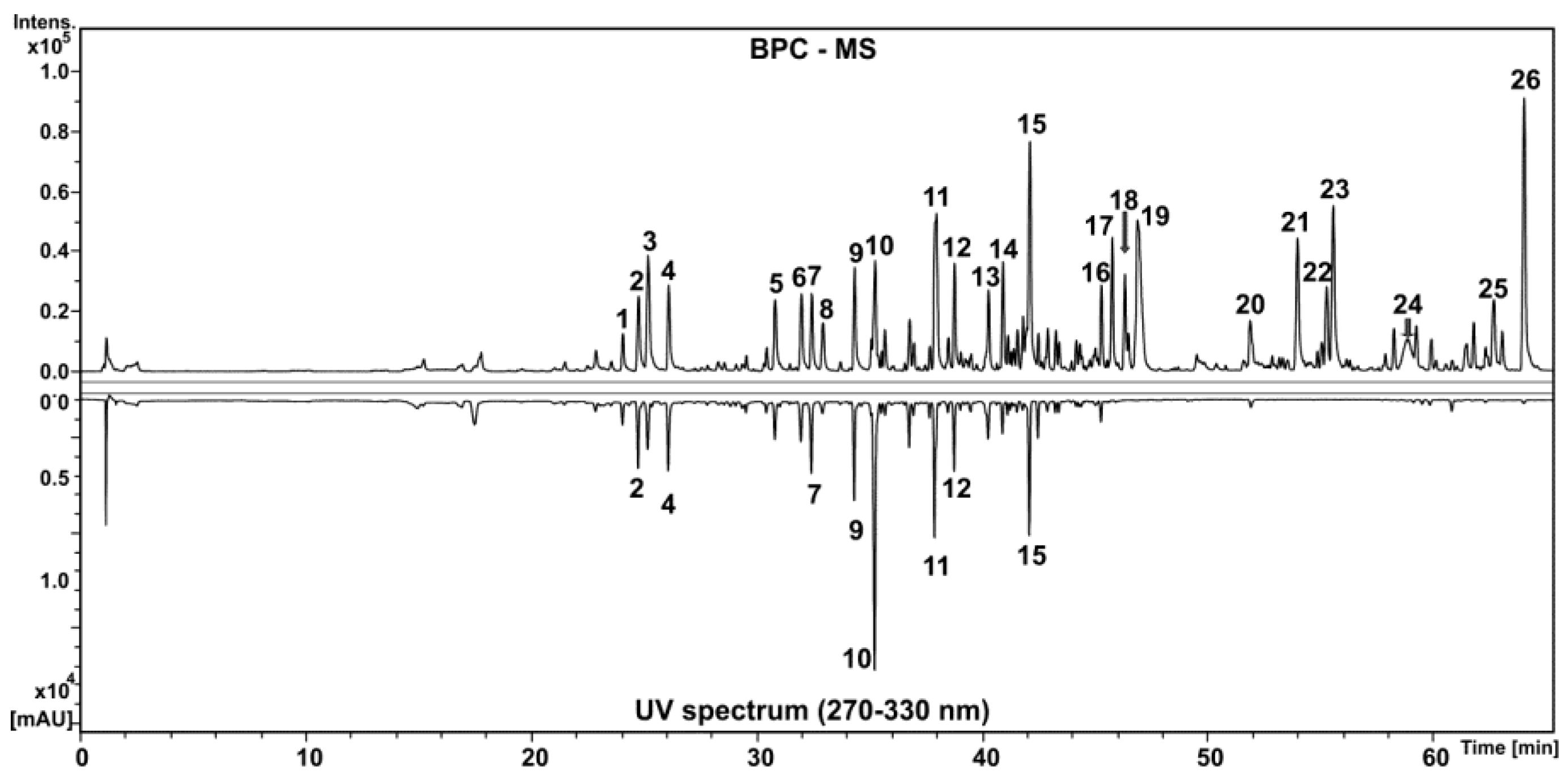

| Peak | Identification | Molecular Formula | RT (min) | UV (nm) | [M-H]− m/z | Error (ppm) | MS/MS m/z |
|---|---|---|---|---|---|---|---|
| 1 | O-coumaroyl O-galloyl-hexoside | C22H22O12 | 24.1 | 289 and 309 | 477.1029 | 2.0 | 313 (C13H13O9)−, 271 (C11H11O8)−, 169 (C7H5O5)− |
| 2 | O-coumaroyl O-galloyl-hexoside | C22H22O12 | 24.8 | 289 and 309 | 477.1022 | 3.4 | 313 (C13H13O9)−, 265 (C13H13O6)−, 235 (C12H11O5)−, 205 (C11H9O4)−, 169 (C7H5O5)− |
| 3 | Aromadendrin | C15H12O6 | 25.2 | 289 | 287.0553 | 2.7 | 259 (C14H11O5)−, 177 (C10H9O3)− |
| 4 | Di-O-galloyl Ocoumaroyl-hexoside | C29H26O16 | 26.1 | 286 and 308 | 629.1122 | 4.2 | 465 (C20H17O13)−, 459 (C22H19O11)−, 313 (C13H13O9)−, 271 (C11H11O8)−, 169 (C7H5O5)− |
| 5 | O-cinnamoyl O-galloyl-hexoside | C22H22O11 | 30.8 | 280 | 461.1085 | 1.0 | 211 (C9H7O6)−, 169 (C7H5O5)−, 161 (C10H9O2)− |
| 6 | Di-O-galloyl O-cinnamoyl-hexoside | C29H26O15 | 32 | 280 | 613.1186 | 2.1 | 465 (C20H17O13)−, 313 (C13H13O9)−, 271 (C11H11O8)−, 211 (C9H7O6)−, 169 (C7H5O5)− |
| 7 | Di-O-coumaroyl-hexoside | C24H24O10 | 32.4 | 299 and 311 | 471.1287 | 2.0 | 325 (C15H17O8)−, 307 (C15H15O7)−, 265 (C13H13O6)−, 163 (C9H7O3)−, 145 (C9H5O2)− |
| 8 | Naringenin | C15H12O5 | 32.9 | 284 | 271.0614 | 0.9 | – |
| 9 | Di-O-coumaroyl O-galloyl-hexoside | C31H28O14 | 34.3 | 290 and 311 | 623.1405 | 0.2 | 459 (C22H19O11)−, 313 (C13H13O9)−, 271 (C11H11O8)−, 211 (C9H7O6)−, 169 (C7H5O5)−,163 (C9H7O3)− |
| 10 | Methyl aromadendrin | C16H14O6 | 35.3 | 290 | 301.0721 | 1.0 | 273 (C15H13O5)−, 240 (C14H8O4)−, 179 (C8H3O5)−, 165 (C8H5O4)− |
| 11 | O-Cinnamoyl-O-coumaroyl-hexoside | C24H24O9 | 38 | 285 and 310 | 455.1359 | 2.5 | 163 (C9H7O3)−, 145 (C9H5O2)− |
| 12 | O-Cinnamoyl O-coumaroyl O-Galloyl-hexoside | C31H28O13 | 38.8 | 285 and 310 | 607.1462 | 0.8 | 461 (C22H21O11)−, 443 (C22H19O10)−, 313 (C13H13O9)−, 271 (C11H11O8)−, 211 (C9H7O6)−, 169 (C7H5O5)− |
| 13 | Methyl naringenin | C16H14O5 | 40.3 | 285 | 285.0777 | 2.9 | 165 (C8H5O4)− |
| 14 | Unknown | C24H22O7 | 40.9 | 293 | 421.1289 | 1.0 | 393 (C23H21O6)− |
| 15 | Unknown | C24H22O7 | 42.1 | 295 | 421.1288 | 0.9 | 393 (C23H21O6)− |
| 16 | Unknown | C24H22O6 | 45.3 | 288 | 405.1337 | 1.6 | – |
| 17 | Diterpene | C20H32O3 | 45.8 | – | 319.2260 | 3.4 | – |
| 18 | Diterpene | C20H32O3 | 46.3 | – | 319.2261 | 0.9 | – |
| 19 | Sesquiterpene | C15H22O4 | 46.9 | – | 265.1454 | 3.3 | – |
| 20 | Diterpene ester | C22H34O4 | 51.9 | 280 | 361.2356 | 4.0 | 301 (C20H29O2)− |
| 21 | Triterpene | C30H48O4 | 54 | – | 471.3459 | 4.4 | – |
| 22 | Triterpene | C30H48O4 | 55.3 | – | 471.3464 | 3.3 | – |
| 23 | Triterpene | C30H46O4 | 55.6 | – | 469.3305 | 3.9 | – |
| 24 | Unknown | C24H38O3 | 58.8 | – | 373.2736 | 3.3 | 329 (C23H37O)− |
| 25 | Triterpene | C31H48O3 | 62.7 | – | 467.3509 | 4.6 | – |
| 26 | Unknown | C24H36O3 | 64 | – | 371.2580 | 3.0 | 327 (C23H35O)− |
| DPPH• | ABTS•+ | |||||
|---|---|---|---|---|---|---|
| Samples | IC50 (µg/mL) | Maximum Activity | IC50 (µg/mL) | Maximum Activity | ||
| % | µg/mL | % | µg/mL | |||
| Ascorbic acid | 3.2 ± 0.9 | 93 ± 2.1 | 10 | 1.8 ± 0.05 | 96 ± 2.4 | 5 |
| BHT | 20.3 ± 5.6 | 79 ± 2.6 | 50 | 8.1 ± 0.7 | 98 ± 0.2 | 50 |
| HEGP | 18.3 ± 2.8 | 95 ± 0.5 | 50 | 10.3 ± 0.5 | 98 ± 0.1 | 50 |
| Treatments | Survival (%) | Gene Conversion Colonies/105 | Mutant Colonies/106 | |
|---|---|---|---|---|
| HEGP (mg/mL) | EMS (mg/mL) | |||
| 0.0 | 0.0 | 96.2 ± 2.35 | 0.77 ± 0.02 | 0.49 ± 0.01 |
| 0.0 | 5.0 | 85.2 ± 2.40 a | 51.3 ± 0.44 a | 391.3 ± 7.80 a |
| 1.5 | 5.0 | 49.8 ± 1.11 b | 43.8 ± 2.79 b | 73.7 ± 4.35 b |
| 3.0 | 5.0 | 38.1 ± 3.79 c | 37.2 ± 1.32 b | 53.2± 1.71 b |
| Microorganisms | HEGP (mg/mL) | Gentamicin (µg/mL) | |
| Gram-positive bacteria | MIC | MBC | MBC |
| Staphylococcus aureus ATCC 6538™ | 6.13 ± 0.10 | 8.50 ± 0.28 | 2.00 ± 0.28 |
| S. aureus ESA 175 Methicillin-resistant | 6,42 ± 0.46 | 8.75 ± 0.62 | 2.67 ± 0.16 |
| S. aureus ESA 159 Methicillin-resistant | 6.92 ± 0.30 | 9.08 ± 0.58 | 2.50 ± 0.28 |
| Enterococcus faecalis ATCC 43300™ | 7.08 ± 0.58 | 10.5 ± 0.22 | 2.83 ± 0.30 |
| E. faecalis ESA 201 Vancomycin-resistant | 8.17 ± 0.44 | 10.9 ± 0.30 | 3.25 ± 0.14 |
| E. faecalis ESA 361 Vancomycin-resistant | 9.08 ± 0.08 | 11.2 ± 0.38 | 3.33 ± 0.16 |
| Gram-negative bacteria | |||
| Escherichia coli ATCC 29998™ | 10.5 ± 0.82 | 13.2 ± 0.52 | 4.58 ± 0.30 |
| E. coli ESA 37 Cephalosporins-resistant | 11.2 ± 0.32 | 13.8 ± 0.72 | 4.67 ± 0.22 |
| E. coli ESA 54 Cephalosporins-resistant | 11.5 ± 0.76 | 13.7 ± 0.90 | 4.92 ± 0.08 |
| Pseudomonas aeruginosa ATCC 15442™ | 12.9 ± 0.50 | 16.5 ± 0.30 | 5.00 ± 0.28 |
| P.aeruginosa ESA 22 Imipenem-resistant | 13.3 ± 0,60 | 17.0 ± 1.08 | 6.17 ± 0.16 |
| P.aeruginosa ESA 23 Imipenem-resistant | 13.5 ± 1.22 | 17.7 ± 1.02 | 6.50 ± 0.28 |
| Microorganisms | HEGP (mg/mL) | Amphotericin-B (µg/mL) | |
| Fungi | MIC | MFC | MFC |
| Cryptococcus neoformans ATCC 32264 ™ | 19.3 ± 0.60 | 25.0 ± 1.04 | 0.13 ± 0.07 |
| C. neoformans ESA 211 Amphotericin-B resistant | 20.9 ± 0.79 | 26.0 ± 1.04 | 0.25 ± 0.14 |
| C. neoformans ESA 105 Amphotericin-B resistant | 21.4 ± 1.10 | 26.1 ± 1.58 | 0.38 ± 0.22 |
| Candida albicans ATCC 10231™ | 23.0 ± 1.06 | 34.0 ± 1.12 | 0.29 ± 0.16 |
| C.albicans ESA 100 Amphotericin-B resistant | 23.7 ± 1.29 | 35.0 ± 1.23 | 0.14 ± 0.08 |
| C.albicans ESA 97 Amphotericin-B resistant | 24.4 ± 1.83 | 36.1 ± 0.50 | 0.25 ± 0.14 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, H.F.d.; Campos, J.F.; Santos, C.M.d.; Balestieri, J.B.P.; Silva, D.B.; Carollo, C.A.; De Picoli Souza, K.; Estevinho, L.M.; Dos Santos, E.L. Chemical Profile and Antioxidant, Anti-Inflammatory, Antimutagenic and Antimicrobial Activities of Geopropolis from the Stingless Bee Melipona orbignyi. Int. J. Mol. Sci. 2017, 18, 953. https://doi.org/10.3390/ijms18050953
Santos HFd, Campos JF, Santos CMd, Balestieri JBP, Silva DB, Carollo CA, De Picoli Souza K, Estevinho LM, Dos Santos EL. Chemical Profile and Antioxidant, Anti-Inflammatory, Antimutagenic and Antimicrobial Activities of Geopropolis from the Stingless Bee Melipona orbignyi. International Journal of Molecular Sciences. 2017; 18(5):953. https://doi.org/10.3390/ijms18050953
Chicago/Turabian StyleSantos, Helder Freitas dos, Jaqueline Ferreira Campos, Cintia Miranda dos Santos, José Benedito Perrella Balestieri, Denise Brentan Silva, Carlos Alexandre Carollo, Kely De Picoli Souza, Leticia Miranda Estevinho, and Edson Lucas Dos Santos. 2017. "Chemical Profile and Antioxidant, Anti-Inflammatory, Antimutagenic and Antimicrobial Activities of Geopropolis from the Stingless Bee Melipona orbignyi" International Journal of Molecular Sciences 18, no. 5: 953. https://doi.org/10.3390/ijms18050953






