Mammalian Metallothionein-3: New Functional and Structural Insights
Abstract
:1. Introduction
2. Structural and Chemical Properties of Metallothionein-3
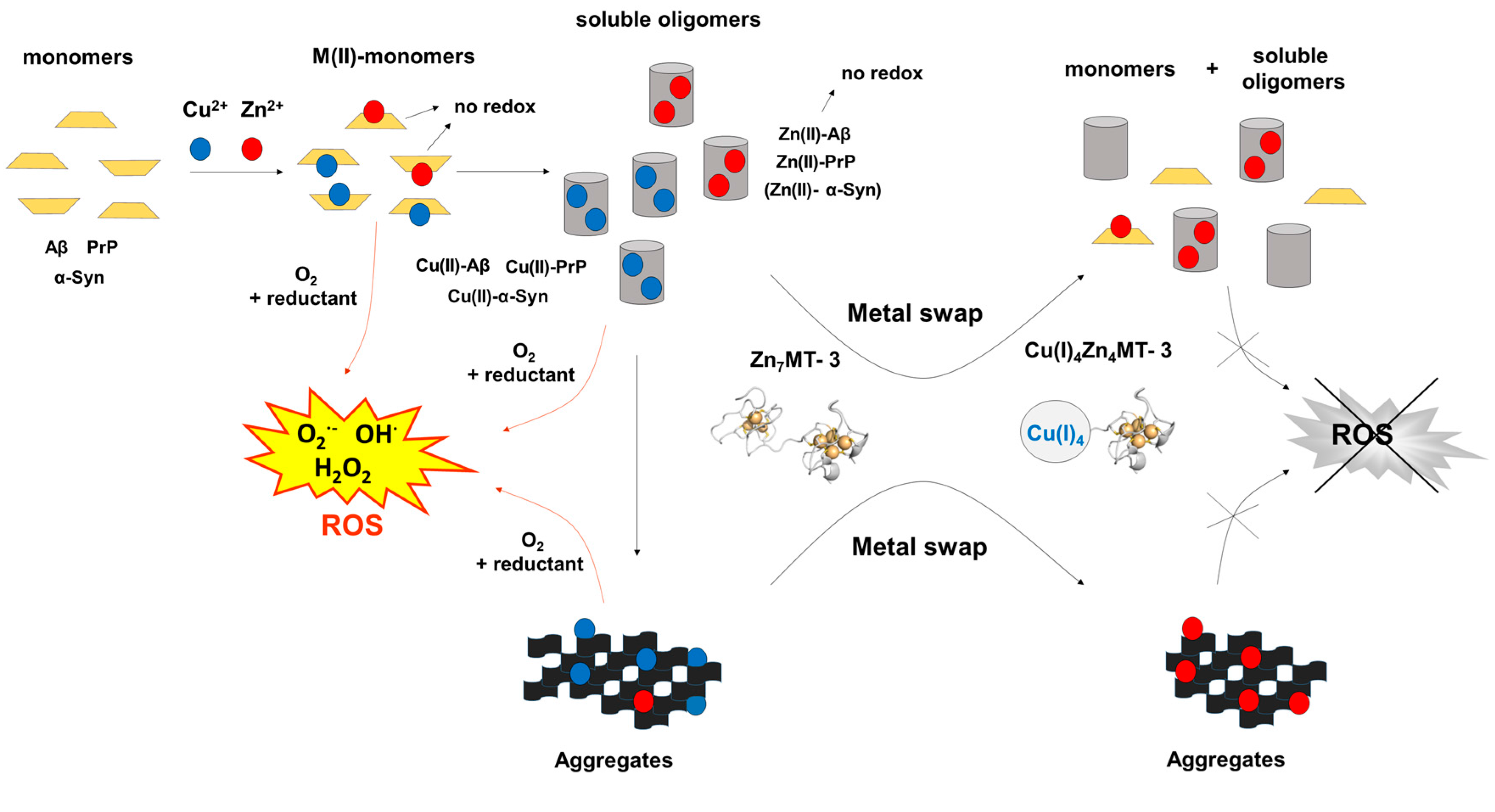
3. Metallothionein-3 in Metal-Linked Neurodegenerative Disorders
4. Toxicological and Neuropathological Aspects of Lead (Pb) Toxicity in Relation to Mammalian Metallothioneins
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta |
| AD | Alzheimer’s Disease |
| ALAD | δ-aminolevulinic acid dehydratase |
| ALS | Amyotrophic lateral sclerosis |
| APP | Amyloid precursor protein |
| AREs | Antioxidant response elements |
| BACE | Beta-secretase |
| CJD | Creutzfeldt–Jakob disease |
| CNS | Central nervous system |
| EXAFS | Extended X-ray absorption fine structure |
| FAD | Familiar Alzheimer’s Disease |
| GREs | Glucocorticoid response elements |
| ITC | Isothermal Titration Calorimetry |
| MRE | Metal response elements |
| MT | Metallothionein |
| MTF-1 | Metal-regulatory transcription factor 1 |
| PD | Parkinson’s Disease |
| PrP | Prion Protein |
| PSEN1 | Presenilin 1 |
| PSEN2 | Presenilin 1 |
| SAD | Sporadic Alzheimer’s Disease |
| SOD | Superoxide Dismutase |
| α-Syn | Alpha-Synuclein |
References
- Mocchegiani, E.; Malavolta, M.; Costarelli, L.; Giacconi, R.; Cipriano, C.; Piacenza, F.; Tesei, S.; Basso, A.; Pierpaoli, S.; Lattanzio, F. Zinc, metallothioneins and immunosenescence. Proc. Nutr. Soc. 2010, 69, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem. Rev. 2006, 106, 1995–2044. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug. Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Takio, K.; Titani, K.; Ihara, Y.; Tomonaga, M. The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron 1991, 7, 337–347. [Google Scholar] [CrossRef]
- Masters, B.A.; Quaife, C.J.; Erickson, J.C.; Kelly, E.J.; Froelick, G.J.; Zambrowicz, B.P.; Brinster, R.L.; Palmiter, R.D. Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J. Neurosci. 1994, 14, 5844–5857. [Google Scholar] [PubMed]
- Miles, A.T.; Hawksworth, G.M.; Beattie, J.H.; Rodilla, V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 35–70. [Google Scholar] [CrossRef] [PubMed]
- Tanji, K.; Irie, Y.; Uchida, Y.; Mori, F.; Satoh, K.; Mizushima, Y.; Wakabayashi, K. Expression of metallothionein-III induced by hypoxia attenuates hypoxia-induced cell death in vitro. Brain Res. 2003, 976, 125–129. [Google Scholar] [CrossRef]
- Wang, B.; Wood, I.S.; Trayhurn, P. PCR arrays identify metallothionein-3 as a highly hypoxia-inducible gene in human adipocytes. Biochem. Biophys. Res. Commun. 2008, 368, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D. Constitutive expression of metallothionein-III (MT-III), but not MT-I, inhibits growth when cells become zinc deficient. Toxicol. Appl. Pharmacol. 1995, 135, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Keung, W.M. Anti-amyloid beta activity of metallothionein-III is different from its neuronal growth inhibitory activity: Structure-activity studies. Brain Res. 2003, 960, 228–234. [Google Scholar] [CrossRef]
- Sewell, A.K.; Jensen, L.T.; Erickson, J.C.; Palmiter, R.D.; Winge, D.R. Bioactivity of metallothionein-3 correlates with its novel beta domain sequence rather than metal binding properties. Biochemistry 1995, 34, 4740–4747. [Google Scholar] [CrossRef] [PubMed]
- Atrian, S.; Capdevila, M. Metallothionein-protein interactions. Biomol. Concepts 2013, 4, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.E.; Ngu, T.T.; Chan, J.; Salgado, M.T.; Merrifield, M.E.; Stillman, M.J. Peptide folding, metal-binding mechanisms, and binding site structures in metallothioneins. Exp. Biol. Med. 2006, 231, 1488–1499. [Google Scholar] [CrossRef]
- Hidalgo, J.; Aschner, M.; Zatta, P.; Vašák, M. Roles of the metallothionein family of proteins in the central nervous system. Brain Res. Bull. 2001, 55, 133–145. [Google Scholar] [CrossRef]
- West, A.K.; Hidalgo, J.; Eddins, D.; Levin, E.D.; Aschner, M. Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. Neurotoxicology 2008, 29, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Hao, Q.; Maret, W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007, 463, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Henkel, G.; Krebs, B. Metallothioneins: Zinc, cadmium, mercury, and copper thiolates and selenolates mimicking protein active site features—Structural aspects and biological implications. Chem. Rev. 2004, 104, 801–824. [Google Scholar] [CrossRef] [PubMed]
- Vašák, M.; Hasler, D.W. Metallothioneins: New functional and structural insights. Curr. Opin. Chem. Biol. 2000, 4, 177–183. [Google Scholar] [CrossRef]
- Vašák, M.; Romero-Isart, N. Metallothioneins. In Encyclopedia of Inorganic Chemistry, 2nd ed.; King, R.B., Ed.; J. Wiley & Sons Ltd.: New York, NY, USA, 2005; pp. 3208–3221. [Google Scholar]
- Faller, P. Neuronal growth-inhibitory factor (metallothionein-3): Reactivity and structure of metal-thiolate clusters. FEBS J. 2010, 277, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Vašák, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Bruinink, A.; Faller, P.; Sidler, C.; Bogumil, R.; Vašák, M. Growth inhibitory factor and zinc affect neural cell cultures in a tissue specific manner. Chem. Biol. Interact. 1998, 115, 167–174. [Google Scholar] [CrossRef]
- Faller, P.; Vašák, M. Distinct metal-thiolate clusters in the N-terminal domain of neuronal growth inhibitory factor. Biochemistry 1997, 36, 13341–13348. [Google Scholar] [CrossRef] [PubMed]
- Hasler, D.W.; Faller, P.; Vašák, M. Metal-thiolate clusters in the C-terminal domain of human neuronal growth inhibitory factor (GIF). Biochemistry 1998, 37, 14966–14973. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Hasler, D.W.; Zerbe, O.; Klauser, S.; Winge, D.R.; Vašák, M. Evidence for a dynamic structure of human neuronal growth inhibitory factor and for major rearrangements of its metal-thiolate clusters. Biochemistry 1999, 38, 10158–10167. [Google Scholar] [CrossRef] [PubMed]
- Oz, G.; Zangger, K.; Armitage, I.M. Three-dimensional structure and dynamics of a brain specific growth inhibitory factor: Metallothionein-3. Biochemistry 2001, 40, 11433–11441. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Q.; Cai, B.; Li, H.; Sze, K.H.; Huang, Z.X.; Wu, H.M.; Sun, H. Solution structure and dynamics of human metallothionein-3 (MT-3). FEBS Lett. 2006, 580, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Roschitzki, B.; Vašák, M. A distinct Cu4-thiolate cluster of human metallothionein-3 is located in the N-terminal domain. J. Biol. Inorg. Chem. 2002, 7, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.; Faller, P.; Vasak, M. Redox silencing of copper in metal-linked neurodegenerative disorders: Reaction of Zn7metallothionein-3 with Cu2+ ions. J. Biol. Chem. 2007, 282, 16068–16078. [Google Scholar] [CrossRef] [PubMed]
- Bogumil, R.; Faller, P.; Pountney, D.L.; Vašák, M. Evidence for Cu(I) clusters and Zn(II) clusters in neuronal growth-inhibitory factor isolated from bovine brain. Eur. J. Biochem. 1996, 238, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Bogumil, R.; Faller, P.; Binz, P.A.; Vašák, M.; Charnock, J.M.; Garner, C.D. Structural characterization of Cu(I) and Zn(II) sites in neuronal-growth-inhibitory factor by extended X-ray absorption fine structure (EXAFS). Eur. J. Biochem. 1998, 255, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Roschitzki, B.; Vašák, M. Redox labile site in a Zn4 cluster of Cu4,Zn4-metallothionein-3. Biochemistry 2003, 42, 9822–9828. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Atrian, S.; Capdevila, M. Zn- and Cu-thioneins: A functional classification for metallothioneins? J. Biol. Inorg. Chem. 2011, 16, 991–1009. [Google Scholar] [CrossRef] [PubMed]
- Artells, E.; Palacios, O.; Capdevila, M.; Atrian, S. In vivo-folded metal-metallothionein 3 complexes reveal the Cu-thionein rather than Zn-thionein character of this brain-specific mammalian metallothionein. FEBS J. 2014, 281, 1659–1678. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.W.; Osman, D.; Robinson, N.J. Metal preferences and metallation. J. Biol. Chem. 2014, 289, 28095–28103. [Google Scholar] [CrossRef] [PubMed]
- Dudev, T.; Lim, C. Metal binding affinity and selectivity in metalloproteins: Insights from computational studies. Annu. Rev. Biophys. 2008, 37, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.; Jung, H.; Meloni, G. Copper metallothioneins. IUBMB Life 2017, 69, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, C.A.; Asanuma, M.; Sogawa, N.; Miyazaki, I.; Nakanishi, T.; Furuta, H.; Ogawa, N. Localization, regulation, and function of metallothionein-III/growth inhibitory factor in the brain. Acta Med. Okayama 2001, 55, 1–9. [Google Scholar] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. The pathogenesis of senile plaques. J. Neuropathol. Exp. Neurol. 1997, 56, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–990. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C.; La Penna, G. Metal ions and intrinsically disordered proteins and peptides: From Cu/Zn amyloid-β to general principles. Acc. Chem. Res. 2014, 47, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Keung, W.M. Metallothionein-III antagonizes the neurotoxic and neurotrophic effects of amyloid beta peptides. Biochem. Biophys. Res. Commun. 2001, 282, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.; Sonois, V.; Delaine, T.; Guilloreau, L.; Gillet, A.; Teissie, J.; Faller, P.; Vašák, M. Metal swap between Zn7-metallothionein-3 and amyloid-β-Cu protects against amyloid-β toxicity. Nat. Chem. Biol. 2008, 4, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.T.; Hureau, C.; Hemmingsen, L.; Heegaard, N.H.; Ostergaard, J.; Vašák, M.; Faller, P. Rapid exchange of metal between Zn7-metallothionein-3 and amyloid-β peptide promotes amyloid-related structural changes. Biochemistry 2012, 51, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.; Meloni, G.; Talmard, C.; Vašák, M.; Faller, P. Zinc release of Zn7-metallothionein-3 induces fibrillar type amyloid-beta aggregates. Metallomics 2010, 2, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Manso, Y.; Carrasco, J.; Comes, G.; Meloni, G.; Adlard, P.A.; Bush, A.I.; Vašák, M.; Hidalgo, J. Characterization of the role of metallothionein-3 in an animal model of Alzheimer’s disease. Cell. Mol. Life Sci. 2012, 69, 3683–3700. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.S.; Howells, C.; Eaton, E.D.; Shabala, L.; Zovo, K.; Palumaa, P.; Sillard, R.; Woodhouse, A.; Bennett, W.R.; Ray, S.; et al. The native copper- and zinc-binding protein metallothionein blocks copper-mediated Abeta aggregation and toxicity in rat cortical neurons. PLoS ONE 2010, 5, e12030. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Powell, D.; Howlett, D.R.; Tew, D.G.; Meek, T.D.; Chapman, C.; Gloger, I.S.; Murphy, K.E.; Southan, C.D.; Ryan, D.M.; et al. Identification of a novel aspartic protease (Asp 2) as β-secretase. Mol. Cell. Neurosci. 1999, 14, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Anderson, J.P.; Barbour, R.; Basi, G.S.; Caccavello, R.; Davis, D.; Doan, M.; Dovey, H.F.; Frigon, N.; Hong, J.; et al. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature 1999, 402, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Bienkowski, M.J.; Shuck, M.E.; Miao, H.; Tory, M.C.; Pauley, A.M.; Brashier, J.R.; Stratman, N.C.; Mathews, W.R.; Buhl, A.E.; et al. Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature 1999, 402, 533–537. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active γ-Secretase complex. Neuron 2003, 38, 9–12. [Google Scholar] [CrossRef]
- Andreasson, U.; Portelius, E.; Andersson, M.E.; Blennow, K.; Zetterberg, H. Aspects of β-amyloid as a biomarker for Alzheimer’s disease. Biomark. Med. 2007, 1, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Takio, K.; Ogawara, M.; Selkoe, D.J. Mass spectrometry of purified amyloid β protein in Alzheimer’s disease. J. Biol. Chem. 1992, 267, 17082–17086. [Google Scholar] [PubMed]
- Moore, B.D.; Chakrabarty, P.; Levites, Y.; Kukar, T.L.; Baine, A.M.; Moroni, T.; Ladd, T.B.; Das, P.; Dickson, D.W.; Golde, T.E. Overlapping profiles of Aβ peptides in the Alzheimer’s disease and pathological aging brains. Alzheimers Res. Ther. 2012, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Portelius, E.; Bogdanovic, N.; Gustavsson, M.K.; Volkmann, I.; Brinkmalm, G.; Zetterberg, H.; Winblad, B.; Blennow, K. Mass spectrometric characterization of brain amyloid β isoform signatures in familial and sporadic Alzheimer’s disease. Acta Neuropathol. 2010, 120, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Drew, S.C.; Noble, C.J.; Masters, C.L.; Hanson, G.R.; Barnham, K.J. Pleomorphic copper coordination by Alzheimer’s disease amyloid-β peptide. J. Am. Chem. Soc. 2009, 131, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Rozga, M.; Sokolowska, M.; Protas, A.M.; Bal, W. Human serum albumin coordinates Cu(II) at its N-terminal binding site with 1 pM affinity. J. Biol. Inorg. Chem. 2007, 12, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Mital, M.; Wezynfeld, N.E.; Fraczyk, T.; Wiloch, M.Z.; Wawrzyniak, U.E.; Bonna, A.; Tumpach, C.; Barnham, K.J.; Haigh, C.L.; Bal, W.; et al. A Functional Role for Abeta in Metal Homeostasis? N-Truncation and High-Affinity Copper Binding. Angew. Chem. Int. Ed. Engl. 2015, 54, 10460–10464. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.N.; Nicklin, H.G. A possible role for the species peroxonitrite in nitrification. Biochim. Biophys. Acta 1970, 222, 660–661. [Google Scholar] [CrossRef]
- Wezynfeld, N.E.; Stefaniak, E.; Stachucy, K.; Drozd, A.; Plonka, D.; Drew, S.C.; Krezel, A.; Bal, W. Resistance of Cu(Aβ4–16) to Copper Capture by Metallothionein-3 Supports a Function for the Aβ4–42 Peptide as a Synaptic Cu(II) Scavenger. Angew. Chem. Int. Ed. Engl. 2016, 55, 8235–8238. [Google Scholar] [CrossRef] [PubMed]
- Huse, J.T.; Liu, K.; Pijak, D.S.; Carlin, D.; Lee, V.M.; Doms, R.W. β-secretase processing in the trans-Golgi network preferentially generates truncated amyloid species that accumulate in Alzheimer’s disease brain. J. Biol. Chem. 2002, 277, 16278–16284. [Google Scholar] [CrossRef] [PubMed]
- Barritt, J.D.; Viles, J.H. Truncated Amyloid-β(11–40/42) from Alzheimer Disease Binds Cu2+ with a Femtomolar Affinity and Influences Fiber Assembly. J. Biol. Chem. 2015, 290, 27791–27802. [Google Scholar] [PubMed]
- Gunn, A.P.; Masters, C.L.; Cherny, R.A. Pyroglutamate-Abeta: Role in the natural history of Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2010, 42, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Naslund, J.; Schierhorn, A.; Hellman, U.; Lannfelt, L.; Roses, A.D.; Tjernberg, L.O.; Silberring, J.; Gandy, S.E.; Winblad, B.; Greengard, P.; et al. Relative abundance of Alzheimer Aβ amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. USA 1994, 91, 8378–8382. [Google Scholar] [CrossRef] [PubMed]
- Binolfi, A.; Lamberto, G.R.; Duran, R.; Quintanar, L.; Bertoncini, C.W.; Souza, J.M.; Cervenansky, C.; Zweckstetter, M.; Griesinger, C.; Fernandez, C.O. Site-specific interactions of Cu(II) with α and β-synuclein: Bridging the molecular gap between metal binding and aggregation. J. Am. Chem. Soc. 2008, 130, 11801–11812. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.R.; Shin, H.J.; Lee, J.H. Metal-catalyzed oxidation of α-synuclein in the presence of Copper(II) and hydrogen peroxide. Arch. Biochem. Biophys. 2000, 378, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Drew, S.C.; Leong, S.L.; Pham, C.L.; Tew, D.J.; Masters, C.L.; Miles, L.A.; Cappai, R.; Barnham, K.J. Cu2+ binding modes of recombinant α-synuclein-insights from EPR spectroscopy. J. Am. Chem. Soc. 2008, 130, 7766–7773. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Dawson, V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Heikenwalder, M. Pathogenesis of prion diseases: Current status and future outlook. Nat. Rev. Microbiol. 2006, 4, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Molecular biology of prion diseases. Science 1991, 252, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.; Vašák, M. Redox activity of α-synuclein-Cu is silenced by Zn7-metallothionein-3. Free Radic. Biol. Med. 2011, 50, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.; Crameri, A.; Fritz, G.; Davies, P.; Brown, D.R.; Kroneck, P.M.; Vašák, M. The catalytic redox activity of prion protein-Cu(II) is controlled by metal exchange with the Zn(II)-thiolate clusters of Zn7metallothionein-3. ChemBioChem 2012, 13, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Braun, W.; Vašák, M.; Robbins, A.H.; Stout, C.D.; Wagner, G.; Kägi, J.H.; Wüthrich, K. Comparison of the NMR solution structure and the X-ray crystal structure of rat metallothionein-2. Proc. Natl. Acad. Sci. USA 1992, 89, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Piscator, M. On Cadmium in Normal Human Kidneys with a Report on the Isolation of Metallothioneine from Cadmium-Exposed Rabbit Livers. Nord. Hyg. Tidskr. 1964, 45, 76–82. [Google Scholar] [PubMed]
- Michalska, A.E.; Choo, K.H. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc. Natl. Acad. Sci. USA 1993, 90, 8088–8092. [Google Scholar] [CrossRef] [PubMed]
- Masters, B.A.; Kelly, E.J.; Quaife, C.J.; Brinster, R.L.; Palmiter, R.D. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc. Natl. Acad. Sci. USA 1994, 91, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Kojima, Y. Metallothionein and Other Low Molecular Weight Metal-Binding Proteins. In Metallothionein; Kägi, J.H.R., Nordberg, M., Eds.; Birkhäuser: Basel, Switzerland, 1979; pp. 41–124. [Google Scholar]
- Liu, Y.; Liu, J.; Iszard, M.B.; Andrews, G.K.; Palmiter, R.D.; Klaassen, C.D. Transgenic mice that overexpress metallothionein-I are protected from cadmium lethality and hepatotoxicity. Toxicol. Appl. Pharmacol. 1995, 135, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Basha, M.R.; Brock, B.; Cox, D.P.; Cardozo-Pelaez, F.; McPherson, C.A.; Harry, J.; Rice, D.C.; Maloney, B.; Chen, D.; et al. Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): Evidence for a developmental origin and environmental link for AD. J. Neurosci. 2008, 28, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Coon, S.; Stark, A.; Peterson, E.; Gloi, A.; Kortsha, G.; Pounds, J.; Chettle, D.; Gorell, J. Whole-body lifetime occupational lead exposure and risk of Parkinson’s disease. Environ. Health Perspect. 2006, 114, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, M.P.; Liu, J.; Goyer, R.A.; Diwan, B.A. Metallothionein-I/II double knockout mice are hypersensitive to lead-induced kidney carcinogenesis: Role of inclusion body formation. Cancer Res. 2004, 64, 7766–7772. [Google Scholar] [CrossRef] [PubMed]
- Zuo, P.; Qu, W.; Cooper, R.N.; Goyer, R.A.; Diwan, B.A.; Waalkes, M.P. Potential role of alpha-synuclein and metallothionein in lead-induced inclusion body formation. Toxicol. Sci. 2009, 111, 100–108. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, M.; Darabedian, N.; Liang, Y.; Wu, D.; Xiang, J.; Zhou, F. pH-dependent coordination of Pb2+ to metallothionein2: Structures and insight into lead detoxification. Inorg. Chem. 2014, 53, 2822–2830. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.C.; Shami Shah, A.; DeSilva, S.; Gleaton, A.; Su, A.; Goundie, B.; Croteau, M.L.; Stevenson, M.J.; Wilcox, D.E.; Austin, R.N. Thermodynamics of Pb(II) and Zn(II) binding to MT-3, a neurologically important metallothionein. Metallomics 2016, 8, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Gonick, H.C. Lead-binding proteins: A review. J. Toxicol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.A. Roles of lead-binding proteins in mediating lead bioavailability. Environ. Health Perspect. 1998, 106 (Suppl. S6), 1585–1587. [Google Scholar] [CrossRef] [PubMed]
- Church, H.J.; Day, J.P.; Braithwaite, R.A.; Brown, S.S. Binding of lead to a metallothionein-like protein in human erythrocytes. J. Inorg. Biochem. 1993, 49, 55–68. [Google Scholar] [CrossRef]
- Heuchel, R.; Radtke, F.; Georgiev, O.; Stark, G.; Aguet, M.; Schaffner, W. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. Embo J. 1994, 13, 2870–2875. [Google Scholar] [PubMed]
- Dalton, T.; Palmiter, R.D.; Andrews, G.K. Transcriptional induction of the mouse metallothionein-I gene in hydrogen peroxide-treated Hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res. 1994, 22, 5016–5023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Georgiev, O.; Hagmann, M.; Gunes, C.; Cramer, M.; Faller, P.; Vašák, M.; Schaffner, W. Activity of metal-responsive transcription factor 1 by toxic heavy metals and H2O2 in vitro is modulated by metallothionein. Mol. Cell. Biol. 2003, 23, 8471–8485. [Google Scholar] [CrossRef] [PubMed]
- Laity, J.H.; Andrews, G.K. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1). Arch. Biochem. Biophys. 2007, 463, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Magyar, J.S.; Weng, T.C.; Stern, C.M.; Dye, D.F.; Rous, B.W.; Payne, J.C.; Bridgewater, B.M.; Mijovilovich, A.; Parkin, G.; Zaleski, J.M.; et al. Reexamination of lead(II) coordination preferences in sulfur-rich sites: Implications for a critical mechanism of lead poisoning. J. Am. Chem. Soc. 2005, 127, 9495–9505. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Hayashi, Y.; Watabe, K.; Inuzuka, T.; Hozumi, I. Metallothionein-III prevents neuronal death and prolongs life span in amyotrophic lateral sclerosis model mice. Neuroscience 2011, 189, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Cho, K.S.; Kim, H.N.; Kim, H.J.; Koh, J.Y. Role of zinc metallothionein-3 (ZnMT3) in epidermal growth factor (EGF)-induced c-Abl protein activation and actin polymerization in cultured astrocytes. J. Biol. Chem. 2011, 286, 40847–40856. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Kolbe, H.V.; Lepage, P.; Faure, T.; Sauerwald, R.; de la Salle, H.; Muller, C.; Bihoreau, N.; Paolantonacci, P.; Roitsch, C.; et al. Thrombin cleavage analysis of a novel antihaemophilic factor variant, factor VIII delta II. Eur. J. Biochem. 1991, 195, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.R.; Kim, D.K.; Koh, J.Y. Obesity and downregulated hypothalamic leptin receptors in male metallothionein-3-null mice. Neurobiol. Dis. 2011, 44, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Clifford, K.S.; MacDonald, M.J. Survey of mRNAs encoding zinc transporters and other metal complexing proteins in pancreatic islets of rats from birth to adulthood: Similar patterns in the Sprague-Dawley and Wistar BB strains. Diabetes Res. Clin. Pract. 2000, 49, 77–85. [Google Scholar] [CrossRef]
- Hozumi, I.; Suzuki, J.S.; Kanazawa, H.; Hara, A.; Saio, M.; Inuzuka, T.; Miyairi, S.; Naganuma, A.; Tohyama, C. Metallothionein-3 is expressed in the brain and various peripheral organs of the rat. Neurosci. Lett. 2008, 438, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.R.; Choi, J.A.; Koh, J.Y. The role of metallothionein-3 in streptozotocin-induced β-islet cell death and diabetes in mice. Metallomics 2014, 6, 1748–1757. [Google Scholar] [CrossRef] [PubMed]

| MT-3 Form | Absorption | Luminescence (77 K) | |||||
|---|---|---|---|---|---|---|---|
| High-Energy Band | Low-Energy Band | ||||||
| 1st LMCT a Band (nm) | λem (nm) | τ b (μs) | λmax Excitation Spectra (nm) | λem (nm) | τ (μs) | λmax Excitation Spectra (nm) | |
| Zn7MT-3 | ~230 | - | - | - | - | - | - |
| Cd7MT-3 | ~250 | - | - | - | - | - | - |
| Cu(I)4Zn4MT-3 | ~265 | 425 | ~40 | envelope < 350 | 565 | ~135 | ~265; ~300 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vašák, M.; Meloni, G. Mammalian Metallothionein-3: New Functional and Structural Insights. Int. J. Mol. Sci. 2017, 18, 1117. https://doi.org/10.3390/ijms18061117
Vašák M, Meloni G. Mammalian Metallothionein-3: New Functional and Structural Insights. International Journal of Molecular Sciences. 2017; 18(6):1117. https://doi.org/10.3390/ijms18061117
Chicago/Turabian StyleVašák, Milan, and Gabriele Meloni. 2017. "Mammalian Metallothionein-3: New Functional and Structural Insights" International Journal of Molecular Sciences 18, no. 6: 1117. https://doi.org/10.3390/ijms18061117






