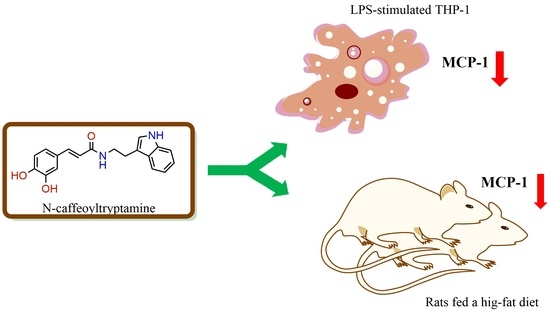
N-Caffeoyltryptamine, a Potent Anti-Inflammatory Phenolic Amide, Suppressed MCP-1 Expression in LPS-stimulated THP-1 Cells and Rats Fed a High-Fat Diet
Abstract
:1. Introduction
2. Results
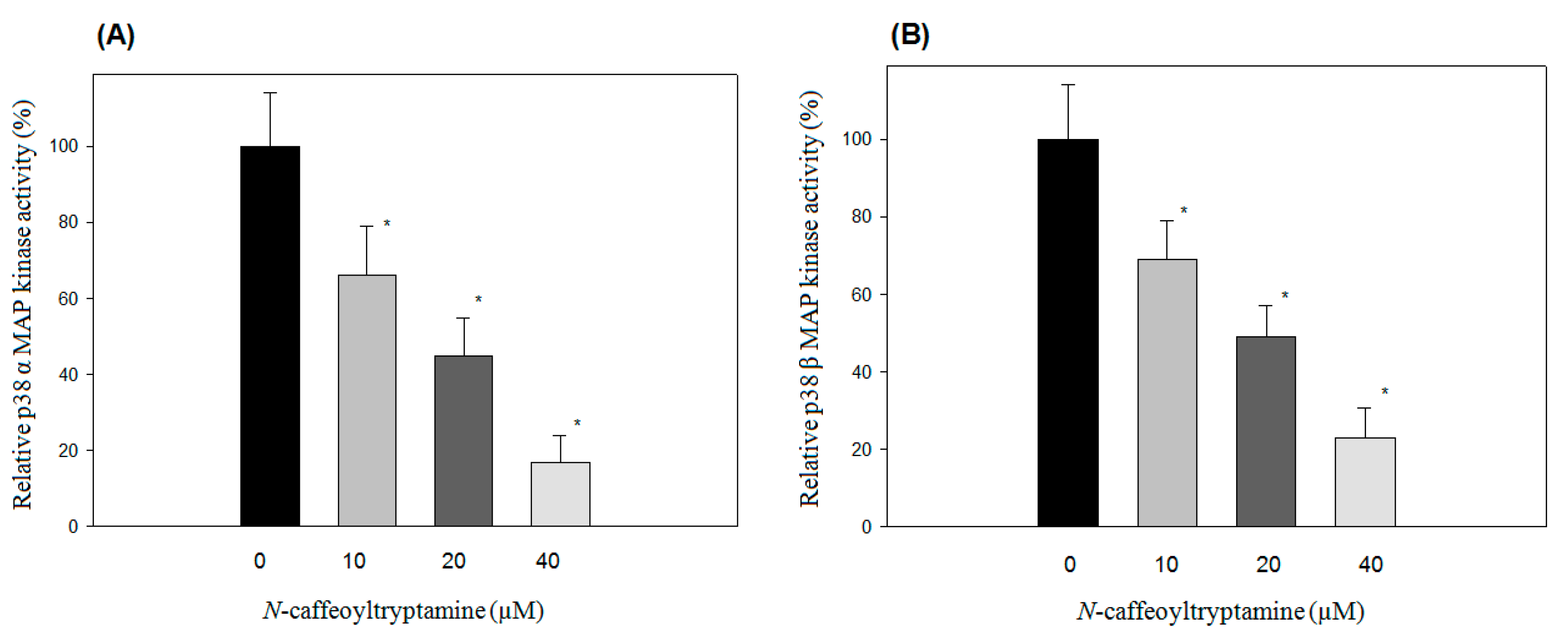
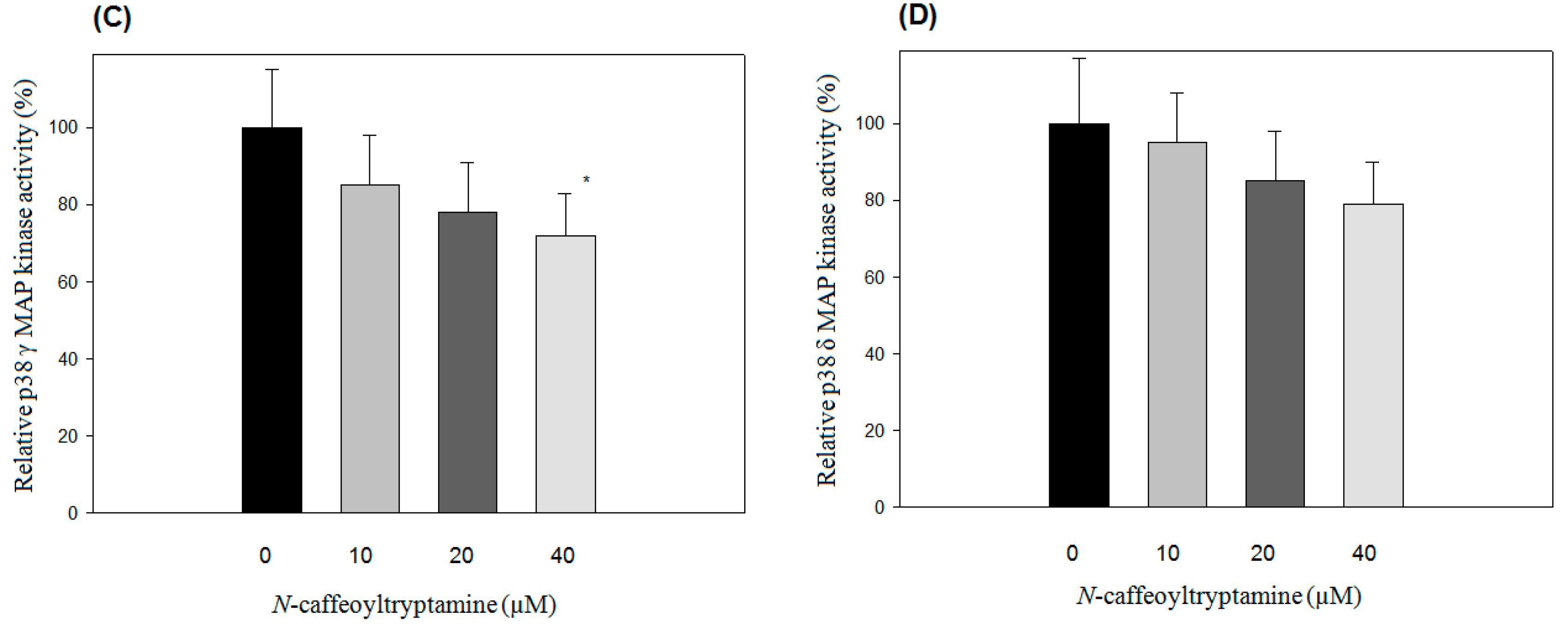
2.1. Effects of N-Caffeoyltryptamine on p38 MAP Kinase Activity
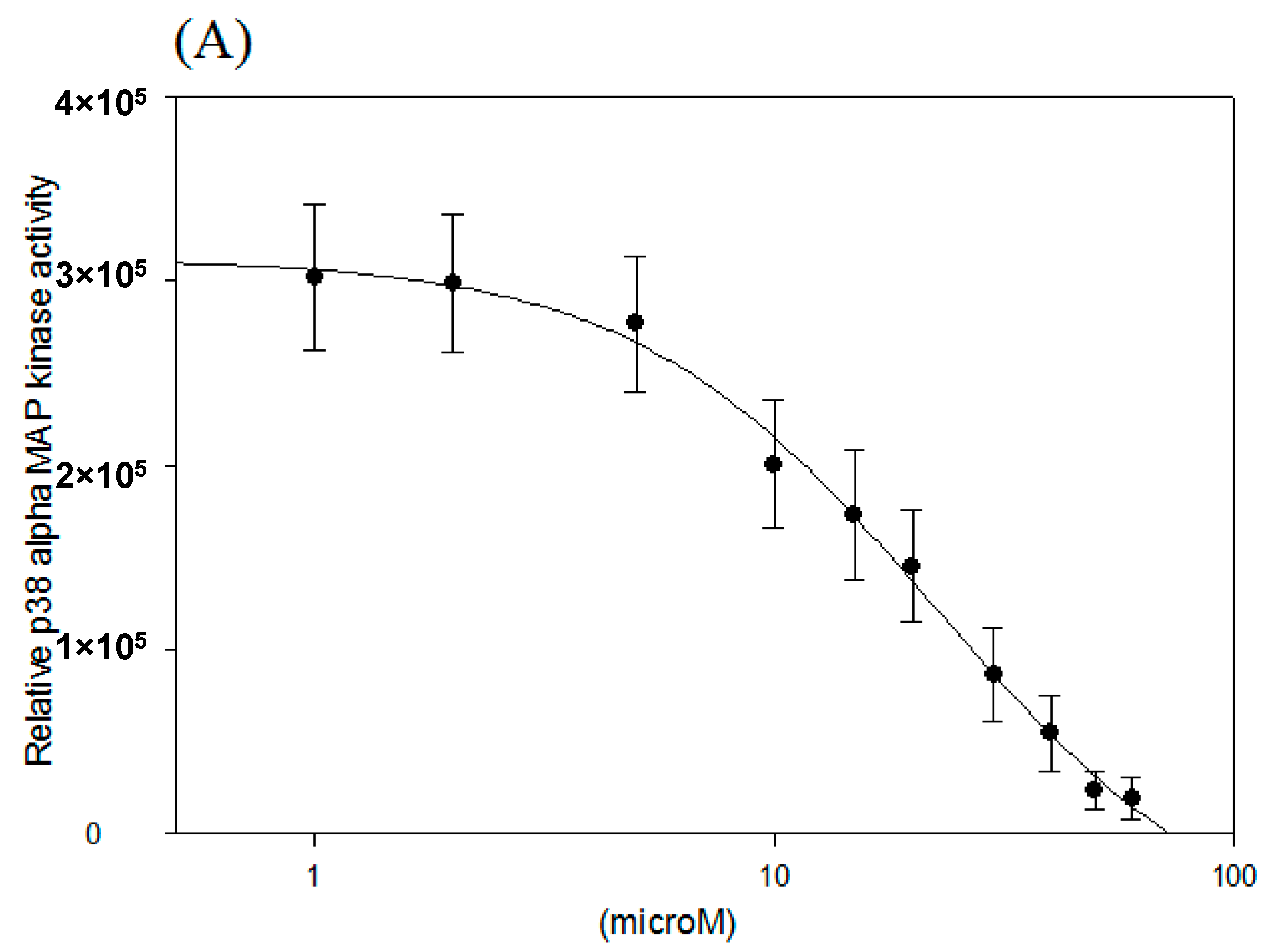
2.2. IC50 of p38 MAP Kinase α and β Inhibition
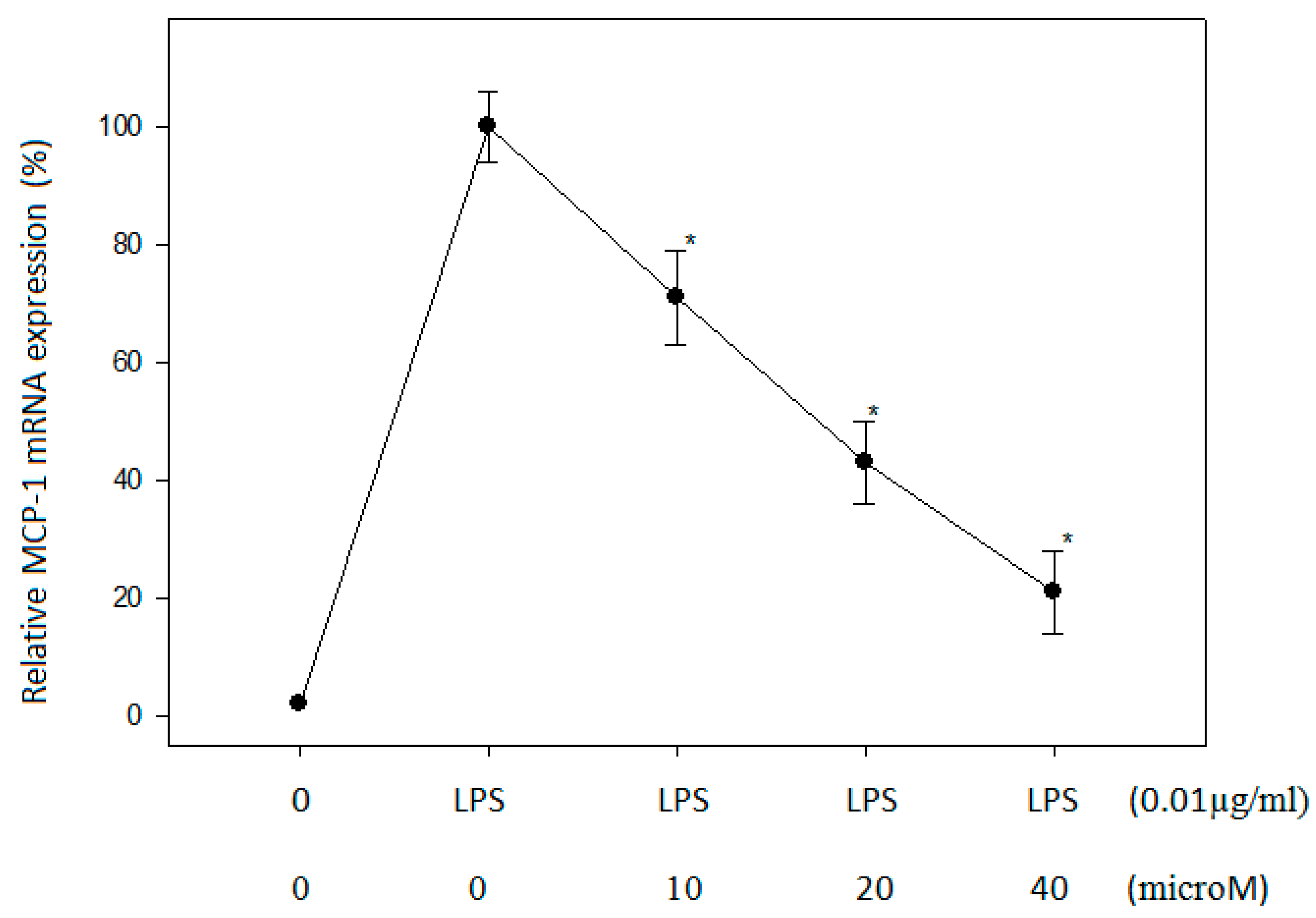
2.3. Effect of N-Caffeoyltryptamine on MCP-1 mRNA in THP-1 Cells
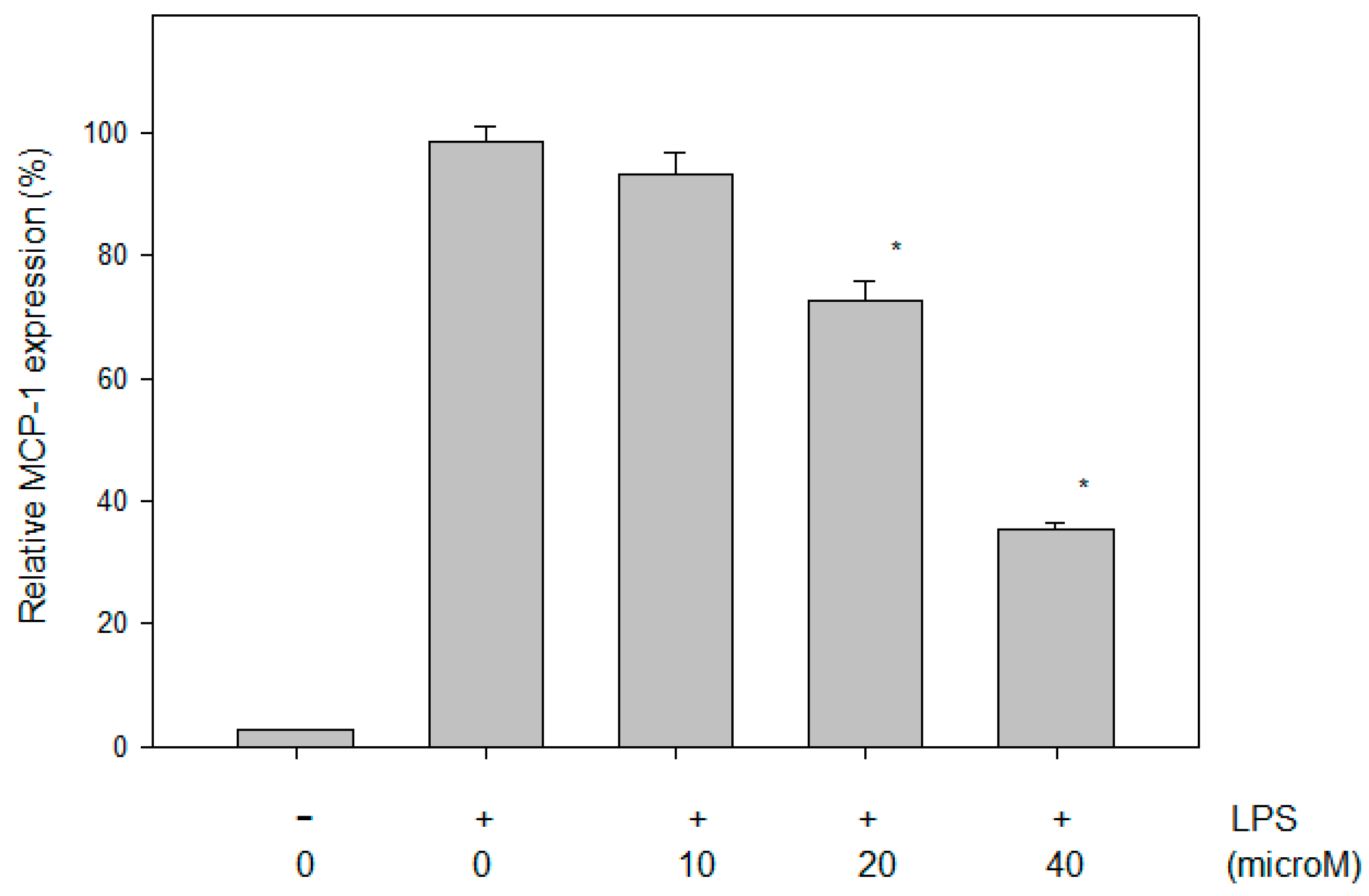
2.4. Effect of N-Caffeoyltryptamine on the MCP-1 Protein in THP-1 Cells
2.5. In Vivo Effects of N-Caffeoyltryptamine on MCP-1 Expression
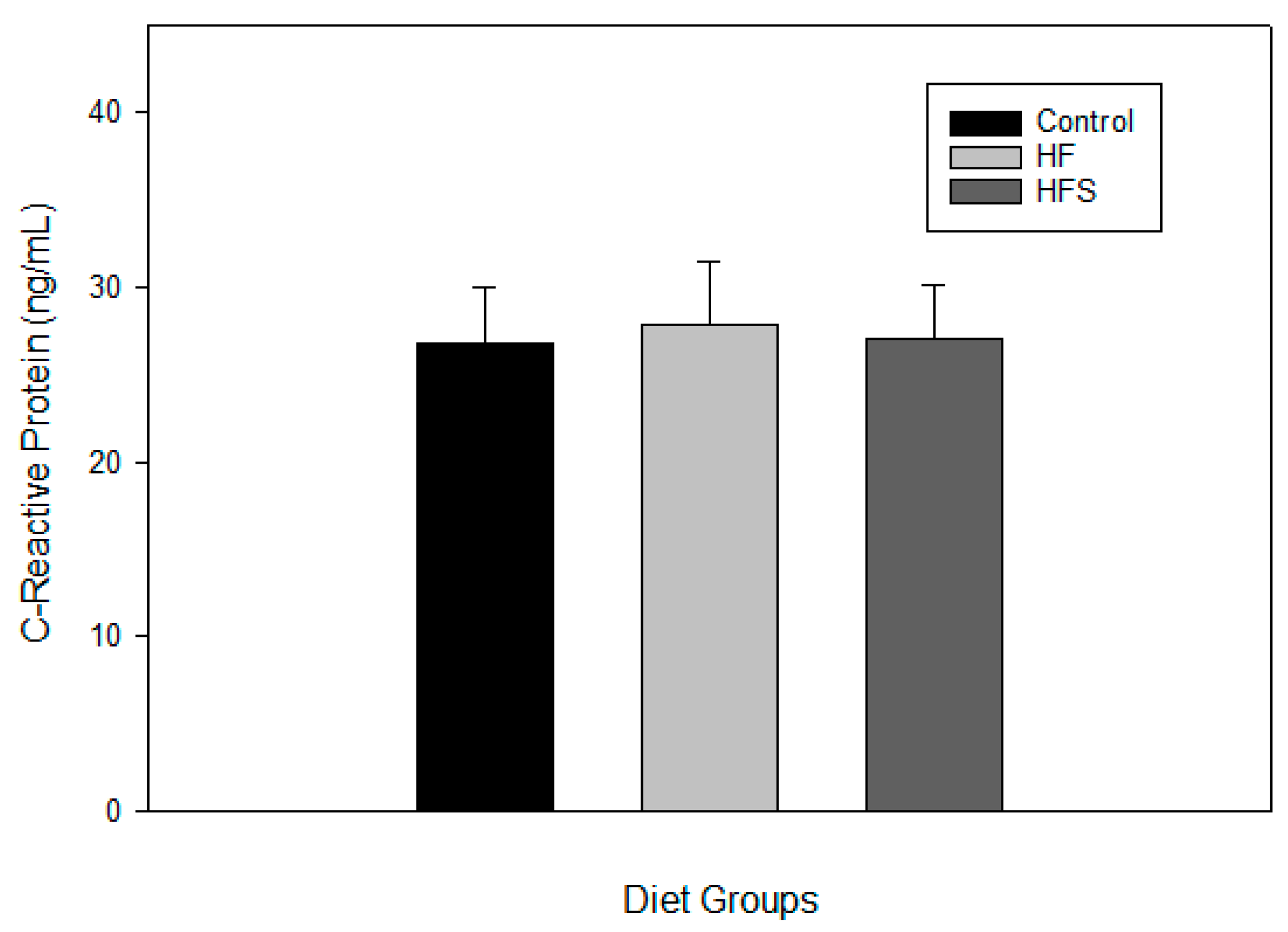
2.6. Effects of N-Caffeoyltryptamine on Plasma C-Reactive Protein
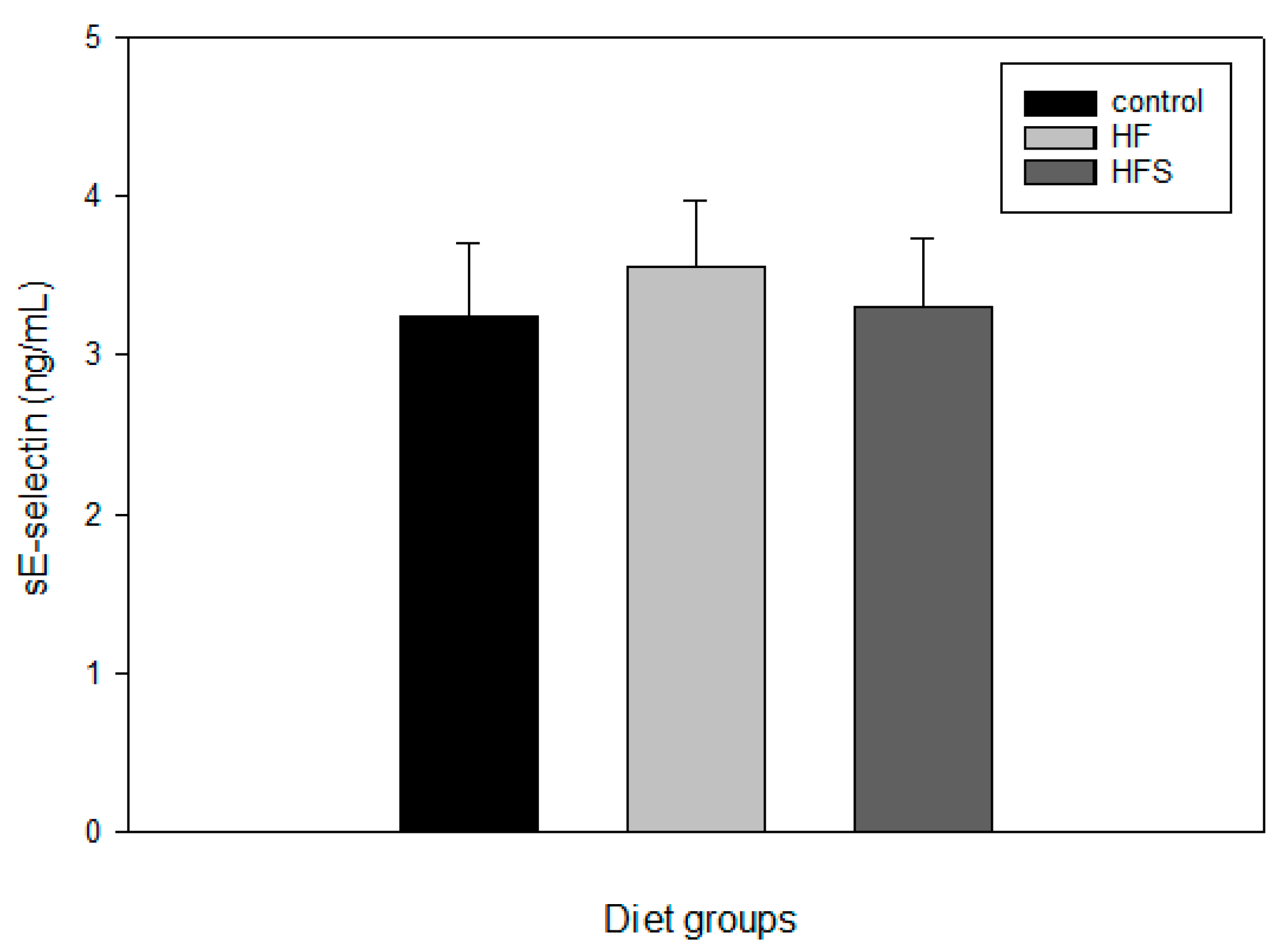
2.7. Effects of N-Caffeoyltryptamine on Plasma sE-Selectin
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. p38 Kinase Activity Assays
4.3. Cell Culture
4.4. Real Time RT-PCR Quantization of MCP-1 mRNA
4.5. Determination of MCP-1 Protein in Cell Culture Medium
4.6. Animal Study
4.7. Assays for Plasma Cholesterol, high-density lipoprotein cholesterol and Triglyceride
4.8. Assays for Plasma MCP-1, C-Reactive Protein and sE-Selectin
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Younus, A.; Aneni, E.C.; Spatz, E.S.; Osondu, C.U.; Roberson, L.; Ogunmoroti, O.; Malik, R.; Ali, S.S.; Aziz, M.; Feldman, T.; et al. Systematic review of the prevalence and outcomes of ideal cardiovascular health in US and non-US populations. Mayo Clin. Proc. 2016, 91, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y.; Ali, F. Atherosclerotic cardiovascular disease: A review of initiators and protective factors. Inflammopharmacology 2016, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Horio, E.; Kadomatsu, T.; Miyata, K.; Arai, Y.; Hosokawa, K.; Doi, Y.; Ninomiya, T.; Horiguchi, H.; Endo, M.; Tabata, M.; et al. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Teague, H.; Mehta, N.N. The link between inflammatory disorders and coronary heart disease: A look at recent studies and novel drugs in development. Curr. Atheroscler. Rep. 2016, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Aichele, S.; Herkommer, M.; Bigalke, B.; Stellos, K.; Htun, P.; Fateh-Moghadam, S.; May, A.E.; Flather, M.; Gawaz, M.; et al. Impact of inflammatory markers on platelet inhibition and cardiovascular outcome including stent thrombosis in patients with symptomatic coronary artery disease. Atherosclerosis 2010, 213, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Cipollone, F.; Marini, M.; Fazia, M.; Pini, B.; lezzi, A.; Reale, M.; Paloscia, L.; Materazzo, G.; D’Annunzio, E.; Conti, P.; et al. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Boring, L.; Gosling, J.; Cleary, M.; Charo, I.F. Decreased lesion formation in CCR2–/– mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998, 394, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kakkar, V.; Lu, X. Impact of MCP-1 in atherosclerosis. Curr. Pharm. Des. 2014, 20, 4580–4588. [Google Scholar] [CrossRef] [PubMed]
- Reape, T.J.; Groot, P.H. Chemokines and atherosclerosis. Atherosclerosis 1999, 147, 213–225. [Google Scholar] [CrossRef]
- Boyle, J.J. Macrophage activation in atherosclerosis: Pathogenesis and pharmacology of plaque rupture. Curr. Vasc. Pharmacol. 2005, 3, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.N.; Nelken, N.A.; Coughlin, S.R.; Gordon, D.; Schall, T.J. Local expression of inflammatory cytokines in human atherosclerotic plaques. J. Atheroscler. Thromb. 1994, 1, S10–S13. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, U.; Matsui, K.; Murakami, Y.; Shimada, K. Monocyte chemoattractant protein-1 and coronary artery disease. Clin. Cardiol. 2002, 25, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Andrianaivoravelona, J.O.; Terreaux, C.; Sahpaz, S.; Rasolondramanitra, J.K.; Hostettmann, A. A phenolic glycoside and N-(p-coumaroyl)-tryptamine from Ravensara anisata. Phytochemistry 1999, 52, 1145–1148. [Google Scholar] [CrossRef]
- Sarker, S.D.; Laird, A.; Nahar, L.; Kumarasamy, Y.; Jaspars, M. Indole alkaloids from the seeds of Centaurea cyanus (Asteraceae). Phytochemistry 2001, 57, 1273–1276. [Google Scholar] [CrossRef]
- Kang, S.; Kang, K.; Chung, G.C.; Choi, D.; Ishihara, A.; Lee, D.S.; Back, K. Functional analysis of the amine substrate specificity domain of pepper tyramine and serotonin N-hydroxycinnamoyltransferases. Plant Physiol. 2006, 140, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, A. N-(p-coumaroyl)-tryptamine and N-ferulyltryptamine in kernels of Zea mays. Phytochemistry 1974, 13, 1979–1983. [Google Scholar] [CrossRef]
- Park, J.B. Serotomide and safflomide modulate forskolin-stimulated cAMP formation via 5-HT1 receptor. Phytomedicine 2008, 15, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Wang, T.T. Safflomide increases the expression of adiponectin in vitro and in vivo: Potential implication for hypoadiponectemia, visceral obesity, and insulin resistance. J. Agric. Food Chem. 2012, 60, 4048–4052. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Siew, L.; Christensen, J.; Plumb, J.; Clarke, G.W.; Greenaway, S.; Perros-Huguet, C.; Clarke, N.; Kilty, I.; Tan, L. Oral and inhaled p38 MAPK inhibitors: Effects on inhaled LPS challenge in healthy subjects. Eur. J. Clin. Pharmacol. 2015, 71, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Warner, G.M.; Yin, P.; Knudsen, B.E.; Cheng, J.; Butters, K.A.; Lien, K.R.; Gray, C.E.; Garovic, V.D.; Lerman, L.O.; et al. Inhibition of p38 MAPK attenuates renal atrophy and fibrosis in a murine renal artery stenosis model. Am. J. Physiol. Ren. Physiol. 2013, 304, F938–F947. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; McClelland, R.L.; Bank, A.; Bluemke, D.A.; Cushman, M.; Szalai, A.J.; Jain, N.; Gomes, A.S.; Heckbert, S.R.; Hundley, W.G.; et al. Biomarkers of inflammation and hemostasis associated with left ventricular mass: The Multiethnic Study of Atherosclerosis (MESA). Int. J. Mol. Epidemiol. Genet. 2011, 2, 391–400. [Google Scholar] [PubMed]
- Sakurai, S.; Kitamura, A.; Cui, R.; Yamagishi, K.; Tanigawa, T.; Iso, H. Relationships of soluble E-selectin and high-sensitivity C-reactive protein with carotid atherosclerosis in Japanese men. J. Atheroscler. Thromb. 2009, 16, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Y.; Jensen, L.E.; Huang, Y.; Kealey, C.; Blair, I.A.; Whitehead, A.S. The up-regulation of monocyte chemoattractant protein-1 (MCP-1) in Ea.hy 926 endothelial cells under long-term low folate stress is mediated by the p38 MAPK pathway. Atherosclerosis 2009, 205, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Gögebakan, Ö.; Osterhoff, M.A.; Schüler, R.; Pivovarova, O.; Kruse, M.; Seltmann, A.C.; Mosig, A.S.; Rudovich, N.; Nauck, M.; Pfeiffer, A.F. GIP increases adipose tissue expression and blood levels of MCP-1 in humans and links high energy diets to inflammation: A randomised trial. Diabetologia 2015, 58, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Emami, H.; Vucic, E.; Subramanian, S.; Abdelbaky, A.; Fayad, Z.A.; Du, S.; Roth, E.; Ballantyne, C.M.; Mohler, E.R.; Farkouh, M.E.; et al. The effect of BMS-582949, a P38 mitogen-activated protein kinase (P38 MAPK) inhibitor on arterial inflammation: A multicenter FDG-PET trial. Atherosclerosis 2015, 240, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Cohen, S.B.; Wofsy, D.; Weinblatt, M.E.; Firestein, G.S.; Brahn, E.; Strand, V.; Baker, D.G.; Tong, S.E. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. J. Rheumatol. 2011, 38, 846–854. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W.; Allan, R.J.; Jones, I.; De Salvo, M.C.; Tan, L.F. Efficacy and safety of the oral p38 inhibitor PH-797804 in chronic obstructive pulmonary disease: A randomised clinical trial. Thorax 2013, 68, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Lomas, D.A.; Lipson, D.A.; Miller, B.E.; Willits, L.; Keene, O.; Barnacle, H.; Barnes, N.C.; Tal-Singer, R. Losmapimod Study Investigators. An oral inhibitor of p38 MAP kinase reduces plasma fibrinogen in patients with chronic obstructive pulmonary disease. J. Clin. Pharmacol. 2012, 52, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Bison, S.; Razzoli, M.; Arban, R.; Michielin, F.; Bertani, S.; Carboni, L. Effect of the p38 MAPK inhibitor SB-239063 on Lipopolysaccharide-induced psychomotor retardation and peripheral biomarker alterations in rats. Eur. J. Pharmacol. 2011, 661, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Elkhawad, M.; Rudd, J.H.; Sarov-Blat, L.; Cai, G.; Wells, R.; Davies, L.C.; Collier, D.J.; Marber, M.S.; Choudhury, R.P.; Fayad, Z.A.; et al. Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis. JACC Cardiovasc. Imaging 2012, 5, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Zomer, E.; Gurusamy, K.; Leach, R.; Trimmer, C.; Lobstein, T.; Morris, S.; James, W.P.; Finer, N. Interventions that cause weight loss and the impact on cardiovascular risk factors: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]


| Composition/Calories | C | HF | HFS |
|---|---|---|---|
| Casein | 207 | 207 | 207 |
| Cornstarch | 400 | 400 | 400 |
| Dyetrose | 200 | — | — |
| Cellulose | 80 | 80 | 80 |
| Lard | 50 | 250 | 250 |
| dl-Methionine | 3 | 3 | 3 |
| AIN-93 mineral mixture | 50 | 50 | 50 |
| AIN-93 vitamin mixture | 10 | 10 | 10 |
| N-Caffeoyltryptamine | — | — | 2 mg/day |
| Calories (kcal/g) | 3.678 | 4.678 | 4.678 |
| Diet and Calorie Intake/Bodyweight | C | HF | HFS |
|---|---|---|---|
| Diet intake (g/day) | 23.2 ± 3.7 | 24.1 ± 4.1 | 23.9 ± 3.6 |
| Initial body weight (g) | 311 ± 39 | 306 ± 42 | 312 ± 41 |
| Final body weight (g) | 617 ± 51 | 659 ± 45 | 642 ± 41 |
| Calorie intake (kcal/day) | 85.3 ± 9.1 | 112.7 ± 19.1 | 111.8 ± 9.2 |
| Lipid Profiles | C | HF | HFS |
|---|---|---|---|
| TG (mg/dL) | 110 ± 18.3 | 131 ± 17.3 | 127 ± 19.2 |
| Total-C (mg/dL) | 80 ± 6.4 | 82 ± 7.8 | 81 ± 6.7 |
| HDL-C (mg/dL) | 51 ± 8.8 | 50 ± 8.7 | 49 ± 7.8 |
| LDL-C (mg/dL) | 11 ± 1.9 | 12 ± 2.2 | 12 ± 2.1 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.B. N-Caffeoyltryptamine, a Potent Anti-Inflammatory Phenolic Amide, Suppressed MCP-1 Expression in LPS-stimulated THP-1 Cells and Rats Fed a High-Fat Diet. Int. J. Mol. Sci. 2017, 18, 1148. https://doi.org/10.3390/ijms18061148
Park JB. N-Caffeoyltryptamine, a Potent Anti-Inflammatory Phenolic Amide, Suppressed MCP-1 Expression in LPS-stimulated THP-1 Cells and Rats Fed a High-Fat Diet. International Journal of Molecular Sciences. 2017; 18(6):1148. https://doi.org/10.3390/ijms18061148
Chicago/Turabian StylePark, Jae B. 2017. "N-Caffeoyltryptamine, a Potent Anti-Inflammatory Phenolic Amide, Suppressed MCP-1 Expression in LPS-stimulated THP-1 Cells and Rats Fed a High-Fat Diet" International Journal of Molecular Sciences 18, no. 6: 1148. https://doi.org/10.3390/ijms18061148