The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism
Abstract
:1. A 60-Year Old Conundrum about a Protein’s Function
2. Relationships among Human Metallothioneins
3. Galvanization of Human Metallothioneins’ Gene Expression
4. Function in Human Cellular Zinc Metabolism
5. Metal Composition: Native Mammalian Metallothioneins Contain Copper
6. Cu+ Affinity for Metallothionein
7. Zn2+ Affinity for Human Metallothionein
8. Metallothionein: A Metamorphic Protein with a Structure Depending on Metal Load and Redox State
Acknowledgments
Conflicts of Interest
Abbreviations
| NTA | Nitrilotriacetic acid |
| BAPTA | 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid |
| H2KTSM | 3-Ethoxy-2-oxobutyraldehyde-bis(N4-methylthiosemicarbazone) |
References
- Margoshes, M.; Vallee, B.L. A cadmium protein from equine kidney cortex. J. Am. Chem. Soc. 1957, 79, 4813–4814. [Google Scholar] [CrossRef]
- Kägi, J.H.; Vallee, B.L. Metallothionein: A cadmium- and zinc-containing protein from equine renal cortex. J. Biol. Chem. 1960, 235, 3460–3465. [Google Scholar] [PubMed]
- Hunziker, P.E.; Kägi, J.H. Isolation and characterization of six human hepatic isometallothioneins. Biochem. J. 1985, 231, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maret, W. Human metallothionein metallomics. J. Anal. At. Spectrom. 2008, 23, 1055–1062. [Google Scholar] [CrossRef]
- Uchida, Y.; Takio, K.; Titani, K.; Ihara, Y.; Tomonaga, M. The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron 1991, 7, 337–347. [Google Scholar] [CrossRef]
- Quaife, C.J.; Findley, S.D.; Erickson, J.C.; Froelick, G.J.; Kelly, E.J.; Zambrowicz, B.P.; Palmiter, R.D. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry 1994, 33, 7250–7259. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Berger, C.; Vallee, B.L.; Kägi, J.H. Amino-acid sequence of equine renal metallothionein-1B. Proc. Natl. Acad. Sci. USA 1976, 73, 3413–3417. [Google Scholar] [CrossRef] [PubMed]
- Messerle, B.A.; Schäffer, A.; Vašák, M.; Kägi, J.H.; Wüthrich, K. Three-dimensional structure of human [113Cd7]metallothionein-2 in solution determined by nuclear magnetic resonance spectroscopy. J. Mol. Biol. 1990, 214, 765–779. [Google Scholar] [CrossRef]
- Robbins, A.H.; McRee, D.E.; Williamson, M.; Collett, S.A.; Xuong, N.H.; Furey, W.F.; Wang, B.C.; Stout, C.D. Refined crystal structure of Cd, Zn metallothionein at 2.0 Å resolution. J. Mol. Biol. 1991, 221, 1269–1293. [Google Scholar] [PubMed]
- Meloni, G.; Polanski, T.; Braun, O.; Vašák, M. Effects of Zn2+, Ca2+, and Mg2+ on the structure of Zn7metallothionein-3: Evidence for an additional zinc binding site. Biochemistry 2009, 48, 5700–5707. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.E.K.; Willans, M.J.; Stillman, M.J. Single domain metallothioneins: Supermetalation of human MT 1a. J. Am. Chem. Soc. 2012, 134, 3290–3299. [Google Scholar] [CrossRef] [PubMed]
- Blindauer, C. Metallothioneins. In Transport and Storage of Metal Ions in Biological Cells; Maret, W., Wedd, A.G., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 606–665. [Google Scholar]
- Binz, P.-A.; Kägi, J.H.R. Metallothionein: Molecular evolution and classification. In Metallothionein IV; Klaassen, C., Ed.; Birkhäuser Verlag: Basel, Switzerland, 1999; pp. 7–13. [Google Scholar]
- Freisinger, E. Structural features specific to plant metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tamaru, H.; Khan, S.I.; Horton, J.R.; Keefe, L.J.; Selker, E.U.; Cheng, X. Structure of the Neurospora SET domain protein DIM-5, a histone H3 lysine methyltransferase. Cell 2002, 111, 117–127. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, J.; Feng, Y.; Wang, J.; Ye, K. Solution structure of MSL2 CXC domain reveals an unusual Zn3Cys9 cluster and similarity to Pre-SET domains of histone lysine methyltransferases. PLoS ONE 2012, 7, e45437. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.E.; Choo, K.H. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc. Natl. Acad. Sci. USA 1993, 90, 8088–8092. [Google Scholar] [CrossRef] [PubMed]
- Masters, B.A.; Kelly, E.J.; Quaife, C.J.; Brinster, R.L.; Palmiter, R.D. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc. Natl. Acad. Sci. USA 1994, 91, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D. The elusive function of metallothioneins. Proc. Natl. Acad. Sci. USA 1998, 95, 8428–8430. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Raudenska, M.; Gumulec, J.; Podlaha, O.; Sztalmachova, M.; Babula, P.; Eckschlager, T.; Adam, V.; Krizek, R.; Masarik, M. Metallothionein polymorphisms in pathological processes. Metallomics 2014, 6, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Benz, F.W.; Cai, J.; Pierce, W.M.; Kang, Y.J. Metallothionein disulfides are present in metallothionein-overexpressing transgenic mouse heart and increase under conditions of oxidative stress. J. Biol. Chem. 2006, 281, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Krężel, A.; Maret, W. Zinc buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006, 11, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Krężel, A.; Maret, W. Different redox states of metallothionein/thionein in biological tissue. Biochem. J. 2007, 402, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Bühler, R.H.; Kägi, J.H. Human hepatic metallothioneins. FEBS Lett. 1974, 39, 229–234. [Google Scholar] [CrossRef]
- Alavarez, L.; Gonzales-Iglesias, H.; Garcia, M.; Ghosh, S.; Sanz-Medel, A.; Coca-Prados, M. The stoichiometric transition from Zn6Cu1-metallothionein to Zn7-metallothionein underlies the up-regulation of metallothionein (MT) expression: Quantitative analysis of MT-metal load in eye cells. J. Biol. Chem. 2012, 287, 28456–28469. [Google Scholar] [CrossRef] [PubMed]
- Jara-Biedma, R.; González-Dominguez, R.; García-Barrera, T.; Lopez-Barea, J.; Pueyo, C.; Gómez-Ariza, J.L. Evolution of metallothionein isoforms complexes in hepatic cells of Mus musculus along cadmium exposure. Biometals 2013, 26, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Vallee, B.L. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc. Natl. Acad. Sci. USA 1998, 95, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. The function of zinc metallothionein: A link between cellular zinc and redox state. J. Nutr. 2000, 130, 1455S–1458S. [Google Scholar] [PubMed]
- Krężel, A.; Maret, W. Dual nanomolar and picomolar Zn(II) binding properties of metallothionein. J. Am. Chem. Soc. 2007, 129, 10911–10921. [Google Scholar] [CrossRef] [PubMed]
- Vašák, M.; Hawkes, G.E.; Nicholson, J.K.; Sadler, P.J. 113Cd NMR studies of reconstituted seven-cadmium metallothionein: Evidence for structural flexibility. Biochemistry 1985, 24, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Munoz, A.; Shaw, C.F.; Petering, D.H. Reaction of 111Cd7-metallothionein with EDTA—A reappraisal. J. Biol. Chem. 1995, 270, 5339–5345. [Google Scholar] [CrossRef] [PubMed]
- Kochańczyk, T.; Drozd, A.; Krężel, A. Relationship between the architecture of zinc coordination and zinc binding affinity in proteins—Insights into zinc regulation. Metallomics 2015, 7, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, M.; Krężel, A.; Otlewski, J. Femtomolar Zn2+ affinity of LIM domain of PDLIM1 protein uncovers crucial contribution of protein-protein interactions to protein stability. J. Inorg. Biochem. 2012, 115, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Miłoch, A.; Krężel, A. Metal binding of zinc finger metallome insights into variations in stability. Metallomics 2014, 6, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Kochańczyk, T.; Nowakowski, M.; Wojewska, D.; Kocyła, A.; Ejchart, A.; Koźmiński, W.; Krężel, A. Metal-coupled folding as the driving force for the extreme stability of Rad50 zinc hook dimer assembly. Sci. Rep. 2016, 6, 36346. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Maret, W. The regulatory and signaling functions of zinc ions in human cellular physiology. In Cellular and Molecular Biology of Metals; Zalups, R.K., Koropatnick, J., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 181–284. [Google Scholar]
- Vallee, B.L.; Maret, W. The functional potential and the potential functions of metallothioneins: A personal perspective. In Metallothionein III. Biological Roles and Medical Implications; Suzuki, K.T., Imura, N., Kimura, M., Eds.; Birkhäuser: Basel, Switzerland, 1993; pp. 1–27. [Google Scholar]
- Fischer, E.H.; Davie, E.W. Recent excitement about metallothionein. Proc. Natl. Acad. Sci. USA 1998, 95, 3333–3334. [Google Scholar] [CrossRef] [PubMed]
- Laukens, D.; Waeytens, A.; de Bleser, P.; Cuvelier, C.; de Vos, M. Human metallothionein expression under normal and pathological conditions: Mechanisms of gene regulation based on in silico promoter analysis. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, P.E.; Kaur, P.; Wan, M.; Känzig, A. Primary structures of seven metallothioneins from rabbit tissue. Biochem. J. 1995, 306, 265–270. [Google Scholar] [CrossRef] [PubMed]
- West, A.K.; Stallings, R.; Hildebrand, C.E.; Chiu, R.; Karin, M.; Richards, R.I. Human metallothionein genes: Structure of the functional locus at 16q13. Genomics 1990, 8, 513–518. [Google Scholar] [CrossRef]
- Stennard, F.A.; Holloway, A.F.; Hamilton, J.; West, A.K. Characterisation of six additional human metallothionein genes. Biochim. Biophys. Acta 1994, 1218, 357–365. [Google Scholar] [CrossRef]
- Tang, C.-M.; Westling, J.; Seto, E. Trans repression of the human metallothioinein IIA gene promoter by PZ120, a novel 120-kilodalton zinc finger protein. Mol. Cell. Biol. 1999, 19, 680–689. [Google Scholar]
- Naruse, S.; Igarashi, S.; Furuya, T.; Kobayashi, H.; Miyatake, T.; Tsuji, S. Structures of the human and mouse growth inhibitory factor-encoding genes. Gene 1994, 144, 283–287. [Google Scholar] [PubMed]
- Günther, V.; Lindert, U.; Schaffner, W. The taste of heavy metals: Gene regulation by MTF-1. Biochim. Biophys. Acta 2012, 1823, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Georgiev, O.; Hagmann, M.; Günes, C.; Cramer, M.; Faller, P.; Vašák, M.; Schaffner, W. Activity of metal-responsive transcription factor 1 by toxic heavy metals and H2O2 in vitro is modulated by metallothionein. Mol. Cell. Biol. 2003, 23, 8471–8485. [Google Scholar] [CrossRef] [PubMed]
- DeMoor, J.M.; Kennette, W.A.; Collins, O.M.; Koropatnick, J. Zinc-metallothionein levels are correlated with enhanced glucocorticoid responsiveness in mouse cells exposed to ZnCl2, HgCl2, and heat shock. Toxicol. Sci. 2001, 64, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.H.; Tam, S.C.; Hesketh, J.E.; Reid, M.; Beattie, J.H. Metal- and tissue-dependent relationship between metallothionein mRNA and protein. Toxicol. Appl. Pharmacol. 2002, 182, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Maret, W.; Vallee, B.L. Differential fluorescence labeling of cysteinyl clusters uncovers high tissue levels of thionein. Proc. Natl. Acad. Sci. USA 2001, 98, 5556–5559. [Google Scholar] [CrossRef] [PubMed]
- Krężel, A.; Maret, W. Thionein/metallothionein control Zn(II) availability and the activity of enzymes. J. Biol. Inorg. Chem. 2008, 13, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, A.; Shaw, C.F., III; Petering, D.H.; Garvey, J.; Kraker, A.J. Basal metallothionein in tumors: Widespread presence of apoprotein. J. Inorg. Biochem. 1994, 54, 91–105. [Google Scholar] [CrossRef]
- Maret, W. Molecular aspects of zinc signals. In Zinc Signals in Cellular Functions and Disorders; Fukuda, T., Kambe, T., Eds.; Springer: Tokyo, Japan, 2014; pp. 7–26. [Google Scholar]
- Taylor, K.M.; Kille, P.; Hogstrand, C. Protein kinase CK2 opens the gate for zinc signaling. Cell Cycle 2012, 11, 1863–1864. [Google Scholar] [CrossRef] [PubMed]
- Hardyman, J.E.; Tyson, J.; Jackson, K.A.; Aldridge, C.; Cockell, S.J.; Wakeling, L.A.; Valentine, R.A.; Ford, D. Zinc sensing by metal-responsive transcription factor 1 (MTF1) controls metallothionein and ZnT1 expression to buffer the sensitivity of the transcriptome response to zinc. Metallomics 2016, 8, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y. Metal-binding properties of metallothionein in extracellular fluids and its role in cadmium-exposed rats. In Biological Roles of Metallothionein; Foulkes, E.C., Ed.; Elsevier: Amsterdam, The Netherlands, 1982; pp. 27–35. [Google Scholar]
- Cousins, R.J. Metallothionein synthesis and degradation: Relationship to cadmium metabolism. Environ. Health Perspect. 1979, 28, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Cousins, R.J. Metallothionein—Aspects related to copper and zinc metabolism. J. Inherit. Metab. Dis. 1983, 6 (Suppl. 1), 15–21. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R.; Richards, V. Human foetal liver contains both zinc- and copper-rich forms of metallothionein. J. Biol. Chem. 1980, 255, 5380–5383. [Google Scholar] [PubMed]
- Chen, P.; Onana, P.; Shaw, C.F., III; Petering, D.H. Characterization of calf liver Cu, Zn-metallothionein: Naturally variable Cu and Zn stoichiometries. Biochem. J. 1996, 317, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Stockert, R.J.; Morell, A.G.; Sternlieb, I. Purification of canine hepatic lysosomal copper-metallothionein. Methods Enzymol. 1991, 205, 286–291. [Google Scholar] [PubMed]
- Bremner, I. Involvement of metallothionein in hepatic metabolism of copper. J. Nutr. 1987, 117, 19–29. [Google Scholar] [PubMed]
- Krauter, B.; Nagel, W.; Hartmann, H.J.; Weser, U. Copper-thionein in melanoma. Biochim. Biophys. Acta 1989, 1013, 212–217. [Google Scholar] [CrossRef]
- Kelly, E.J.; Palmiter, R.D. A murine model of Menkes disease reveals a physiological function of metallothionein. Nat. Genet. 1996, 13, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Irving, H.M.N.H.; Williams, R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- Rae, T.D.; Schmidt, P.J.; Pufahl, R.A.; Culotta, V.C.; O’Halloran, T.V. Undetectable free copper: The requirement of copper chaperone for superoxide dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Bethin, K.E.; Cimato, T.R.; Ettinger, M.J. Copper binding to mouse liver S-adenosylhomocysteine hydrolase and the effects of copper on its levels. J. Biol. Chem. 1995, 270, 20703–20711. [Google Scholar] [CrossRef] [PubMed]
- Vašák, M.; Kägi, J.H.R. Metallothioneins. In Encyclopedia of Inorganic Chemistry; King, R.B., Ed.; John Wiley & Sons: New York, NY, USA, 1994; pp. 2229–2241. [Google Scholar]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Kozyreva, T.; Zovo, K.; Palumaa, P. Affinity gradients drive copper to cellular destinations. Science 2010, 465, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.S.; Howells, C.; Eaton, E.D.; Shabala, L.; Zovo, K.; Palumaa, P.; Sillard, R.; Woodhouse, A.; Bennett, W.R.; Ray, S.; et al. The native copper- and zinc-binding protein metallothionein blocks copper-mediated Aβ aggregation and toxicity in rat cortical neurons. PLoS ONE 2010, 5, e12030. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum copper to zinc ratio: Relationship with aging and health status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.; Sonois, V.; Delaine, T.; Guilloreau, L.; Gillet, A.; Teissié, J.; Faller, P.; Vašák, M. Metal swap between Zn7-metallothionein-3 and amyloid-β-Cu protects against amyloid-β toxicity. Nat. Chem. Biol. 2008, 4, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Wezynfeld, N.E.; Stefaniak, E.; Stachucy, K.; Drozd, A.; Płonka, D.; Drew, S.C.; Krężel, A.; Bal, W. Resistance of Cu(Aβ4-16) to copper capture by metallothionein-3 supports a function for the Aβ4–42 peptide as a synaptic Cu(II) scavenger. Angew. Chem. Int. Ed. Engl. 2016, 55, 8235–8238. [Google Scholar] [CrossRef] [PubMed]
- Roschitzki, B.; Vašák, M. A distinct Cu4-thiolate cluster of human metallothionein-3 is located in the N-terminal domain. J. Biol. Inorg. Chem. 2002, 7, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Presta, A.; Green, A.R.; Zelazowski, A.; Stillman, M.J. Formation of a continuum of copper(I) thiolate stoichiometric species. Eur. J. Biochem. 1995, 227, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Pountney, D.L.; Schauwecker, I.; Zarn, J.; Vašák, M. Formation of mammalian Cu8-metallothionein in vitro: Evidence for the existence of two Cu(I)4-thiolate clusters. Biochemistry 1994, 33, 9699–9705. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.S.; Irvine, G.W.; Wong, D.L.; Hartwig, A.; Stillman, M.J. Stepwise copper(I) binding to metallothionein: A mixed cooperative and non-cooperative mechanism for all 20 copper ions. Metallomics 2017, 9, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Dolderer, B.; Echner, H.; Beck, A.; Hartmann, H.-J.; Weser, U.; Luchinat, C.; del Bianco, C. Coordination of three and four Cu(I) to the α- and β-domain of vertebrate Zn-metallothionein-1, respectively, induces significant structural changes. FEBS J. 2007, 274, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Vašák, M.; Kägi, J.H. Spectroscopic properties of metallothionein. In Metal Ions in Biological Systems; Sigel, H., Ed.; Marcel Dekker: New York, NY, USA, 1983; Volume 15, pp. 213–273. [Google Scholar]
- Li, T.-Y.; Kraker, A.J.; Shaw, C.F.; Petering, D.H. Ligand substitution reactions of metallothioneins with EDTA and apo-carbonic anhydrase. Proc. Natl. Acad. Sci. USA 1980, 77, 6334–6338. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Petering, D.H.; Shaw, C.F. Structure-reactivity relationships of metallothionein, a unique metal-binding protein. Comments Inorg. Chem. 1989, 9, 1–35. [Google Scholar] [CrossRef]
- Namdarghanbari, M.A.; Meeusen, J.; Bachowski, G.; Giebel, N.; Johnson, J.; Petering, D.H. Reaction of the zinc sensor FluoZin-3 with Zn7-metallothionein: Inquiry into existence of proposed weak binding site. J. Inorg. Biochem. 2010, 104, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Rodriguez, A.R. Electrochemical behavior of metallothioneins and related molecules. Part III: Metallothionein. Electroanalysis 1995, 7, 674–680. [Google Scholar] [CrossRef]
- Hasler, D.W.; Jensen, L.T.; Zerbe, O.; Winge, D.T.; Vašák, M. Effect of the two conserved prolines of human growth inhibitory factor (metallothionein-3) on its biological activity and structure fluctuations: Comparison with mutant protein. Biochemistry 2000, 39, 14567–14575. [Google Scholar] [CrossRef] [PubMed]
- Noszal, B. Group constant: A measure of submolecular basicity. J. Phys. Chem. 1986, 90, 4104–4110. [Google Scholar] [CrossRef]
- Dorčák, V.; Krężel, A. Correlation of acid–base chemistry of phytochelatin PC2 with its coordination properties towards the toxic metal ion Cd(II). Dalton Trans. 2003, 2253–2259. [Google Scholar] [CrossRef]
- Hao, Q.; Hong, S.-H.; Maret, W. Domain-specific fluorescence resonance energy transfer (FRET) sensors of metallothionein/thionein. Protein Eng. Des. Sel. 2005, 18, 255–263. [Google Scholar]
- Rigby-Duncan, K.E.; Stillman, M.J. Metal-dependent protein folding: Metallation of metallothionein. J. Inorg. Biochem. 2006, 100, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Foloppe, N.; Nilson, L. The glutaredoxin −C–P–Y–C– motif: Influence of peripherial residues. Structure 2004, 12, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Pande, J.; Vašák, M.; Kägi, J.H.R. Interaction of lysine residues with metal thiolate clusters in metallothionein. Biochemistry 1985, 24, 6717–6722. [Google Scholar] [CrossRef] [PubMed]
- Romero-Isart, N.; Oliva, B.; Vašák, M. Influence of NH–Sγ bonding interactions on the structure and dynamics of metallothioneins. J. Mol. Model. 2010, 16, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Sénèque, O.; Latour, J.M. Coordination properties of zinc finger peptides revisited: Ligand competition studies reveal higher affinities for zinc and cobalt. J. Am. Chem. Soc. 2010, 132, 17760–17774. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, A.; Kochańczyk, T.; Miłoch, A.; Krężel, A. Method for accurate determination of dissociation constants of optical ratiometric systems: Chemical probes, genetically encoded sensors, and interacting molecules. Anal. Chem. 2013, 85, 11479–11486. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-J.; Maret, W.; Vallee, B.L. The glutathione redox couple modulates zinc transfer from metallothionein to zinc-depleted sorbitol dehydrogenase. Proc. Natl. Acad. Sci. USA 1998, 95, 3483–3488. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Maret, W.; Vallee, B.L. Control of zinc transfer between thionein, metallothionein, and zinc protein. Proc. Natl. Acad. Sci. USA 1998, 95, 3489–3494. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.E.; Nakamura, K.; Chlebowski, J.F. 65Zn(II), 115mCd(II), 60Co(II), and Mg(II) binding to alkaline phosphatase of Escherichia coli. Structural and functional effects. J. Biol. Chem. 1983, 258, 386–395. [Google Scholar] [PubMed]
- Yu, X.; Wojciechowski, M.; Fenselau, C. Assessment of metals in reconstituted metallothioneins by electrospray mass spectrometry. Anal. Chem. 1993, 65, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Zaia, J.; Fabris, D.; Wei, D.; Karpel, R.L.; Fenselau, C. Monitoring metal ion flux in reactions of metallothionein and drug-modified metallothionein by electrospray mass spectrometry. Protein Sci. 1998, 7, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, P.M.; You, C.; Dallinger, R.; Gruber, C.; Brouwer, M.; Kägi, J.H.; Hunziker, P.E. Electrospray ionization mass spectrometry of zinc, cadmium, and copper metallothioneins: Evidence for metal-binding cooperativity. Protein Sci. 2000, 9, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Palumaa, P.; Tammiste, I.; Kruusela, K.; Kangur, L.; Jörnvall, H.; Sillard, R. Metal binding of metallothionein-3 versus metallothionein-2: Lower affinity and higher plasticity. Biochim. Biophys. Acta 2005, 1747, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.E.L.; Summers, K.L.; Stillman, M.J. Noncooperative metalation of metallothionein 1A and its isolated domains with zinc. Biochemistry 2012, 51, 6690–6700. [Google Scholar] [CrossRef] [PubMed]
- Pinter, T.B.J.; Stillman, M.J. The zinc balance: Competitive zinc metalation of carbonic anhydrase and metallothionein 1A. Biochemistry 2014, 53, 6276–6285. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, L.L.; Krebs, J.F.; Paterno, S.A.; Fierke, C.A. Engineering a cysteine ligand into the zinc binding site of human carbonic anhydrase II. Biochemistry 1993, 32, 9896–9900. [Google Scholar] [CrossRef] [PubMed]
- Mattapalli, H.; Monteith, W.B.; Burns, C.S.; Danell, A.S. Zinc deposition during ESI-MS analysis of peptide-zinc-complexes. J. Am. Soc. Mass Spectrom. 2009, 20, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Kostyukevich, Y.; Kononikhin, A.; Popov, I.; Indeykina, M.; Kozin, S.A.; Makarov, A.A.; Nikolaev, E. Supermetallization of peptides and proteins during electrospray ionization. J. Mass Spectrom. 2015, 50, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.R.; Zhou, Z.L.; Qian, W.J.; Kennedy, R. Detection and imaging of zinc secretion from pancreatic β-cells using a new fluorescent zinc indicator. J. Am. Chem. Soc. 2002, 124, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, I.; Krężel, A.; Goch, W.; Zhukov, I.; Paczkowska, I.; Bal, W. Revised stability constant, spectroscopic properties and binding mode of Zn(II) to FluoZin-3, the most common zinc probe in life sciences. J. Inorg. Biochem. 2016, 161, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.C.; Shami Shah, A.; DeSilva, S.; Gleaton, A.; Su, A.; Goundie, B.; Croteau, M.L.; Stevenson, M.J.; Wilcox, D.E.; Austin, R.N. Thermodynamics of Pb(II) and Zn(II) binding to MT-3, a neurologically important metallothionein. Metallomics 2016, 8, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Krężel, A.; Hao, Q.; Maret, W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007, 463, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Nakajima, K. Development of a high sensitivity ELISA for the assay of metallothionein. Curr. Pharm. Biotechnol. 2013, 14, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Werynska, B.; Pula, B.; Muszczynska-Bernhard, B.; Gomulkiewicz, A.; Piotrowska, A.; Prus, R.; Podhorska-Okolow, M.; Jankowska, R.; Dziegiel, P. Metallothionein 1F and 2A overexpression predicts poor outcome of non-small cell lung cancer patients. Exp. Mol. Pathol. 2013, 94, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Shabb, J.B.; Muhonen, W.W.; Mehus, A.A. Quantitation of human metallothionein isoforms in cells, tissues, and cerebrospinal fluid by mass spectrometry. Methods Enzymol. 2017, 586, 413–431. [Google Scholar] [PubMed]
- Coufalíková, K.; Benešová, I.; Vaculovič, T.; Kanický, V.; Preisler, J. LC coupled to ESI, MALDI and ICP MS—A multiple hyphenation for metalloproteomic studies. Anal. Chim. Acta 2017, 968, 58–65. [Google Scholar] [CrossRef] [PubMed]

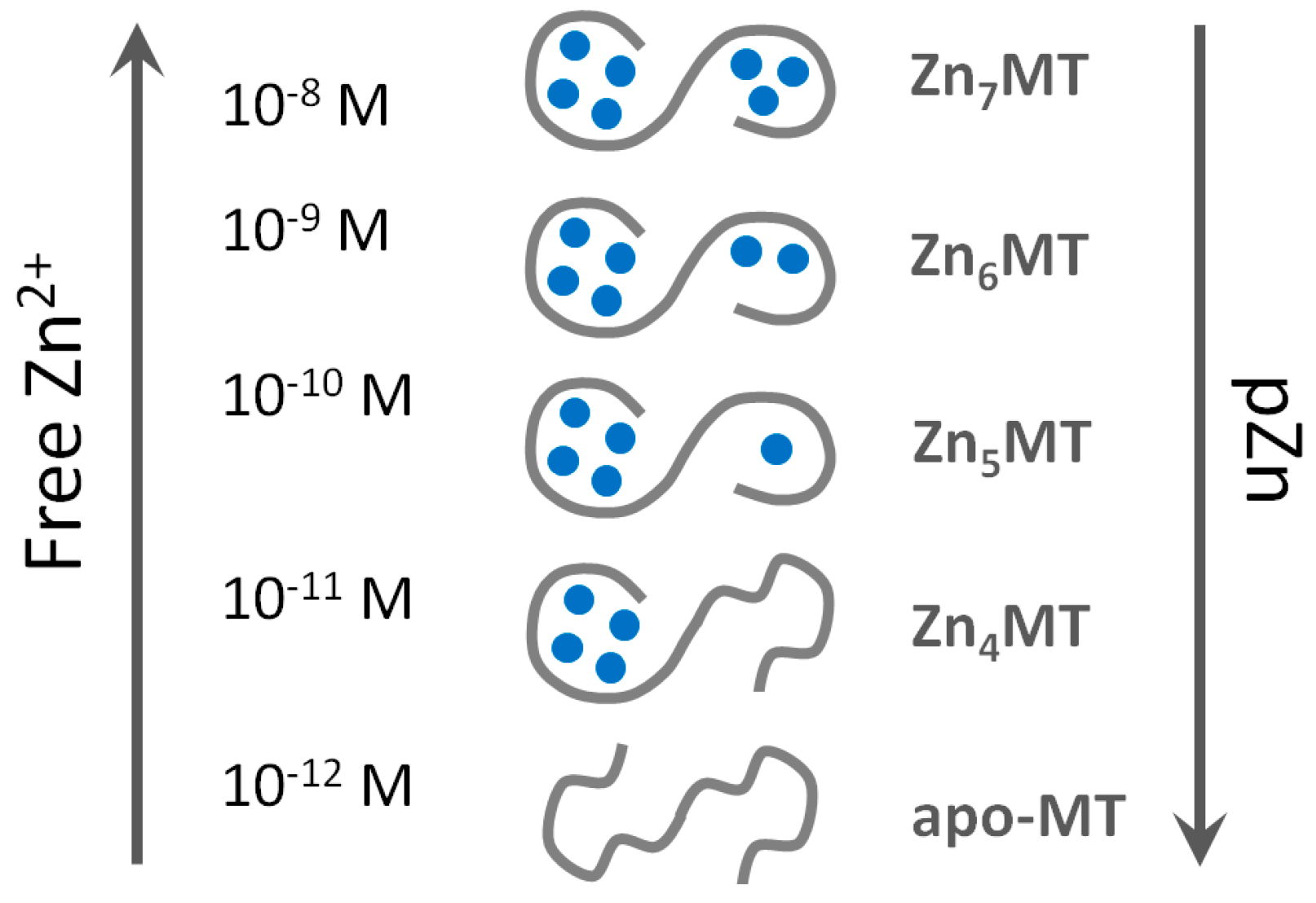
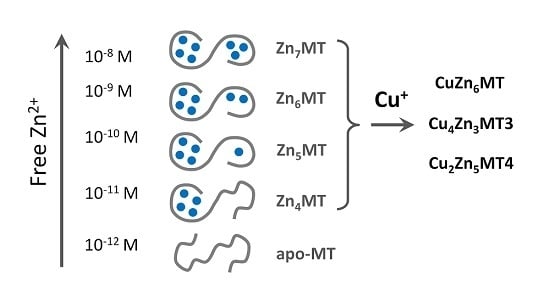
| Formula | Source | Reference |
|---|---|---|
| Zn, Cd, Cu | horse kidney | [1] |
| Cd0.2Cu0.1Zn6.7MT | human liver | [25] |
| CuZn6MT | human corneal epithelial cells | [26] |
| Zn7MT | same cells, induction with Zn2+ | [26] |
| Cu4Zn3MT3 | human brain | [5] |
| Cu2Zn5MT4 | mouse tongue * | [6] |
| Cd2Zn5MT2 | rat liver, induction with Cd2+ | [9] |
| Cd3Cu3ZnMT2 | mouse liver, induction with Cd2+ | [27] |
| Cd4CuZn2MT1 | mouse liver, induction with Cd2+ | [27] |
| Cd6CuMT1/MT2 | mouse liver, induction with Cd2+ | [27] |
| MT Isoform | Number of Amino Acids | UniProt Name | Entry Number | Annotation Score | Protein Existence * | Variants |
|---|---|---|---|---|---|---|
| MT1A | 61 | MT1A_HUMAN | P04731 | 5 | PL | T27 → N, K51 → R |
| MT1B | 61 | MT1B_HUMAN | P07438 | 4 | PIH | - |
| MT1E | 61 | MT1E_HUMAN | P04732 | 5 | PL | - |
| MT1F | 61 | MT1F_HUMAN | P04733 | 5 | PL | - |
| MT1G | 62 | MT1G_HUMAN | P13640 | 5 | PL | A10 deletion (isoform 2) |
| MT1H | 61 | MT1H_HUMAN | P80294 | 5 | PL | - |
| MT1L (MT1R) | 61 | MT1L_HUMAN | Q93083 | 3 | TL | - |
| MT1K (MT1M) | 61 | MT1M_HUMAN | Q8N339 | 4 | PIH | T20 → K |
| MT1X | 61 | MT1X_HUMAN | P80297 | 5 | PL | - |
| MT2 | 61 | MT2_HUMAN | P02795 | 5 | PL | A42 → V |
| MT3 | 68 | MT3_HUMAN | P25713 | 5 | PL | - |
| MT4 | 62 | Metallothionein 4 | AAI13443.1 | 3 | PIH | 30C → Y, R31 → W, G48 → D |
| Transcription Factor * | Sp1 | MTF1 | EGR1 | GR | RAR | Ikaros | Churchill |
|---|---|---|---|---|---|---|---|
| Zinc Motif | ZnFinger | 6ZnFingers | ZnFinger | ZnTwist | ZnTwist | ZnFinger | ZnFinger/ZnCluster |
| MT1 | |||||||
| A | 5 | 2 | 4 | 1 | 2 | 2 | 1 |
| E | 1 | 1 | 3 | 6 | 1 | 2 | 6 |
| J (pseudogene) | 2 | 2 | 3 | 1 | 3 | 4 | 8 |
| B | 1 | 4 | - | 1 | 5 | 8 | 3 |
| K/M | 4 | 2 | 8 | 2 | 2 | 2 | 9 |
| G | 5 | 2 | - | - | 3 | 6 | 6 |
| F | 8 | 5 | 5 | - | 5 | 6 | 8 |
| H | 2 | 5 | - | 3 | 3 | 7 | 5 |
| X | 3 | 3 | 4 | 2 | 3 | 9 | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krężel, A.; Maret, W. The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism. Int. J. Mol. Sci. 2017, 18, 1237. https://doi.org/10.3390/ijms18061237
Krężel A, Maret W. The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism. International Journal of Molecular Sciences. 2017; 18(6):1237. https://doi.org/10.3390/ijms18061237
Chicago/Turabian StyleKrężel, Artur, and Wolfgang Maret. 2017. "The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism" International Journal of Molecular Sciences 18, no. 6: 1237. https://doi.org/10.3390/ijms18061237