Proteomic High Affinity Zn2+ Trafficking: Where Does Metallothionein Fit in?
Abstract
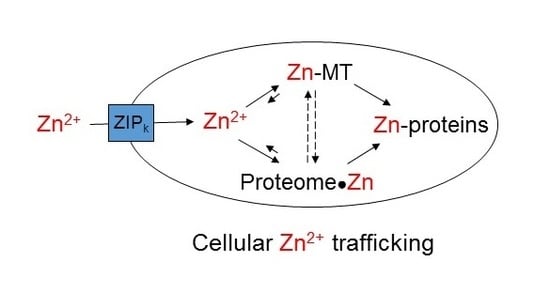
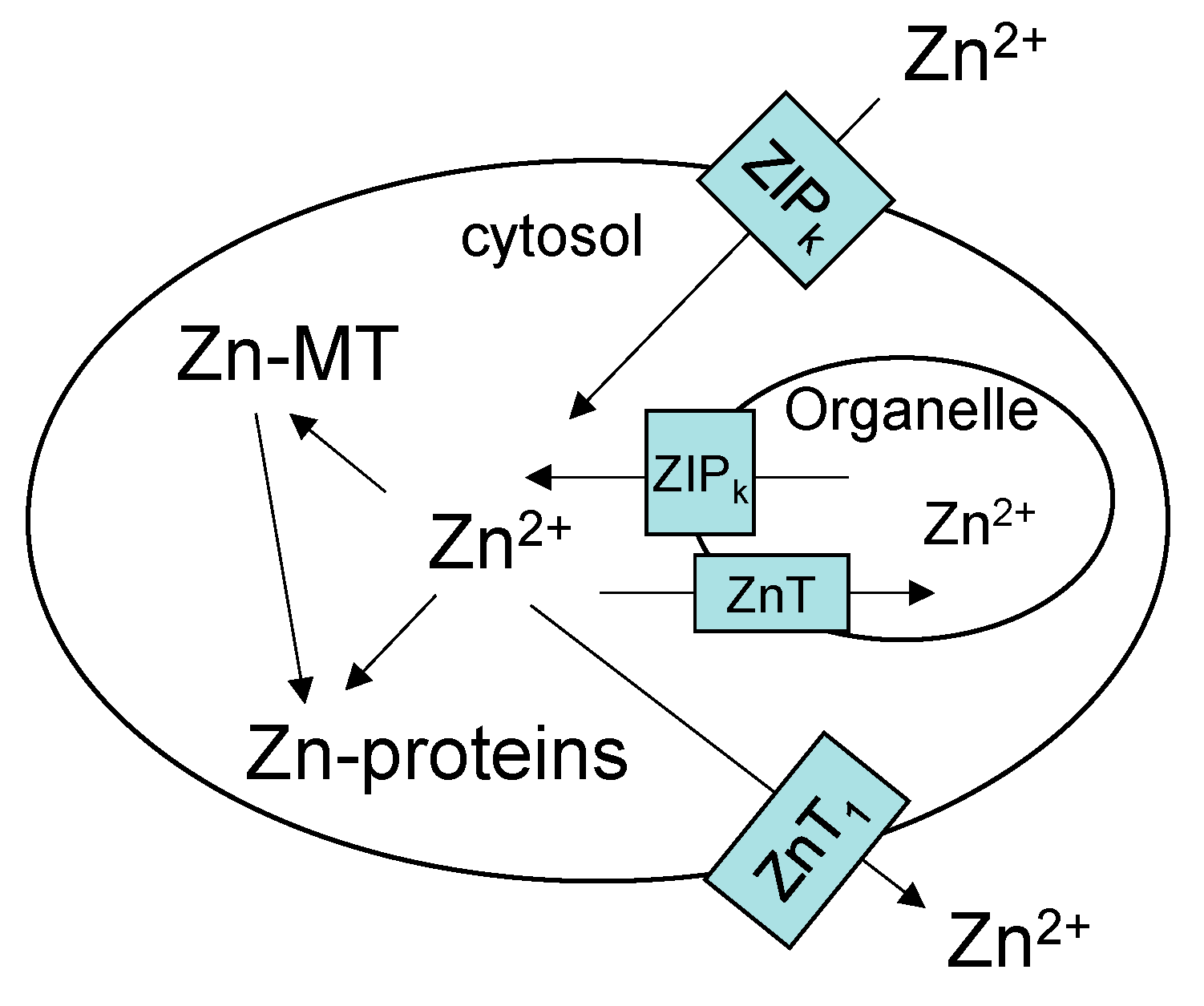
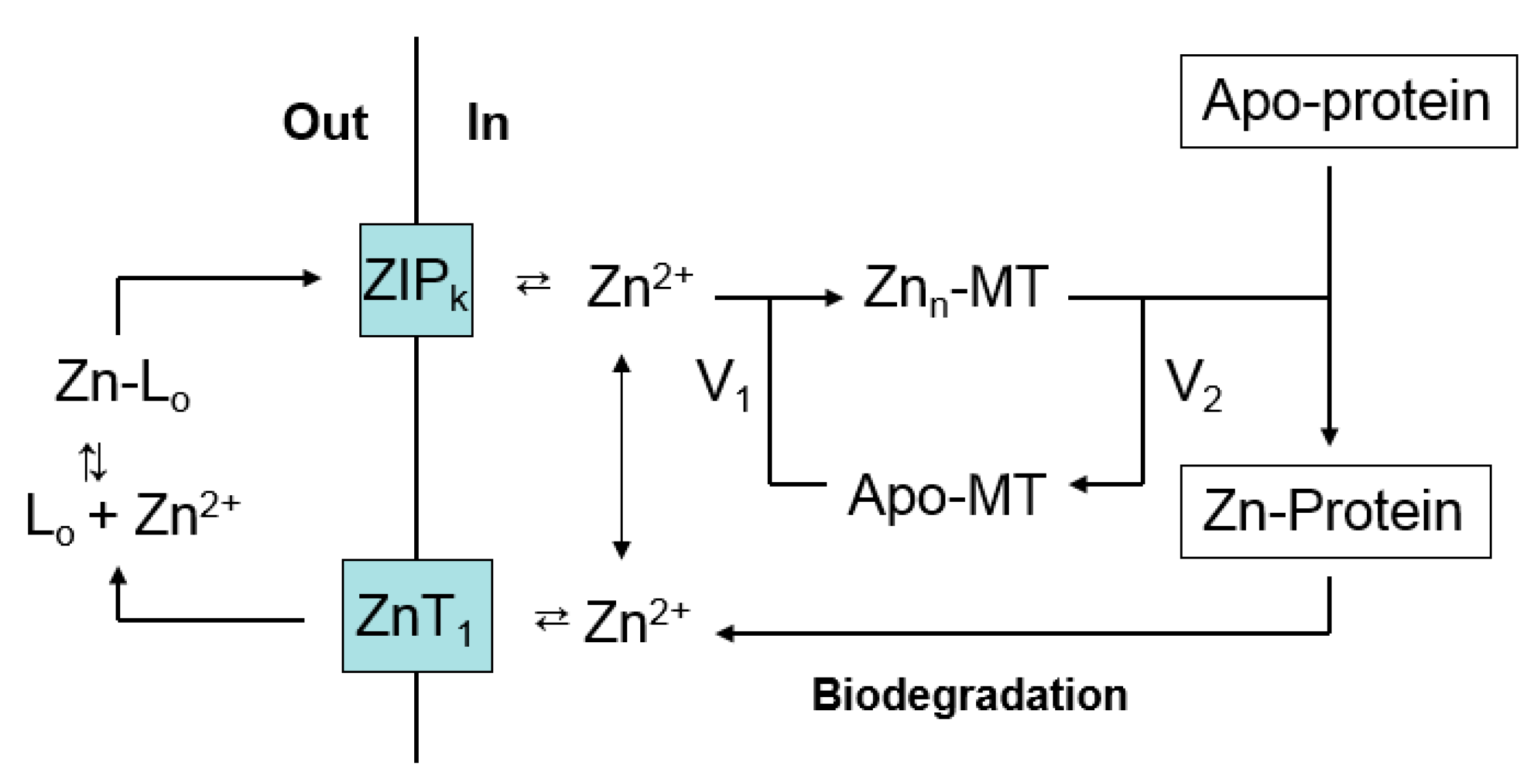
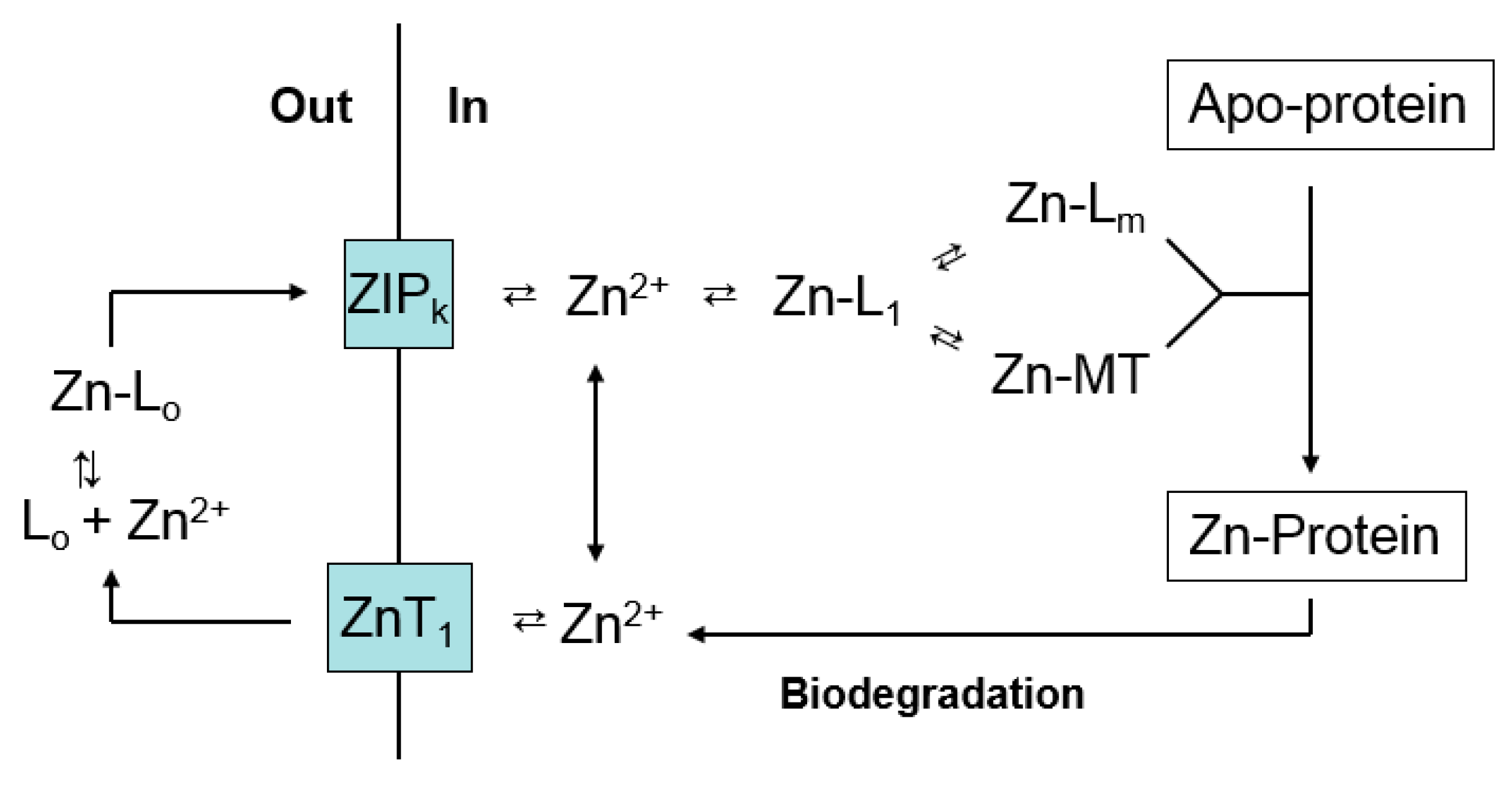
:1. Overview of Zinc Trafficking
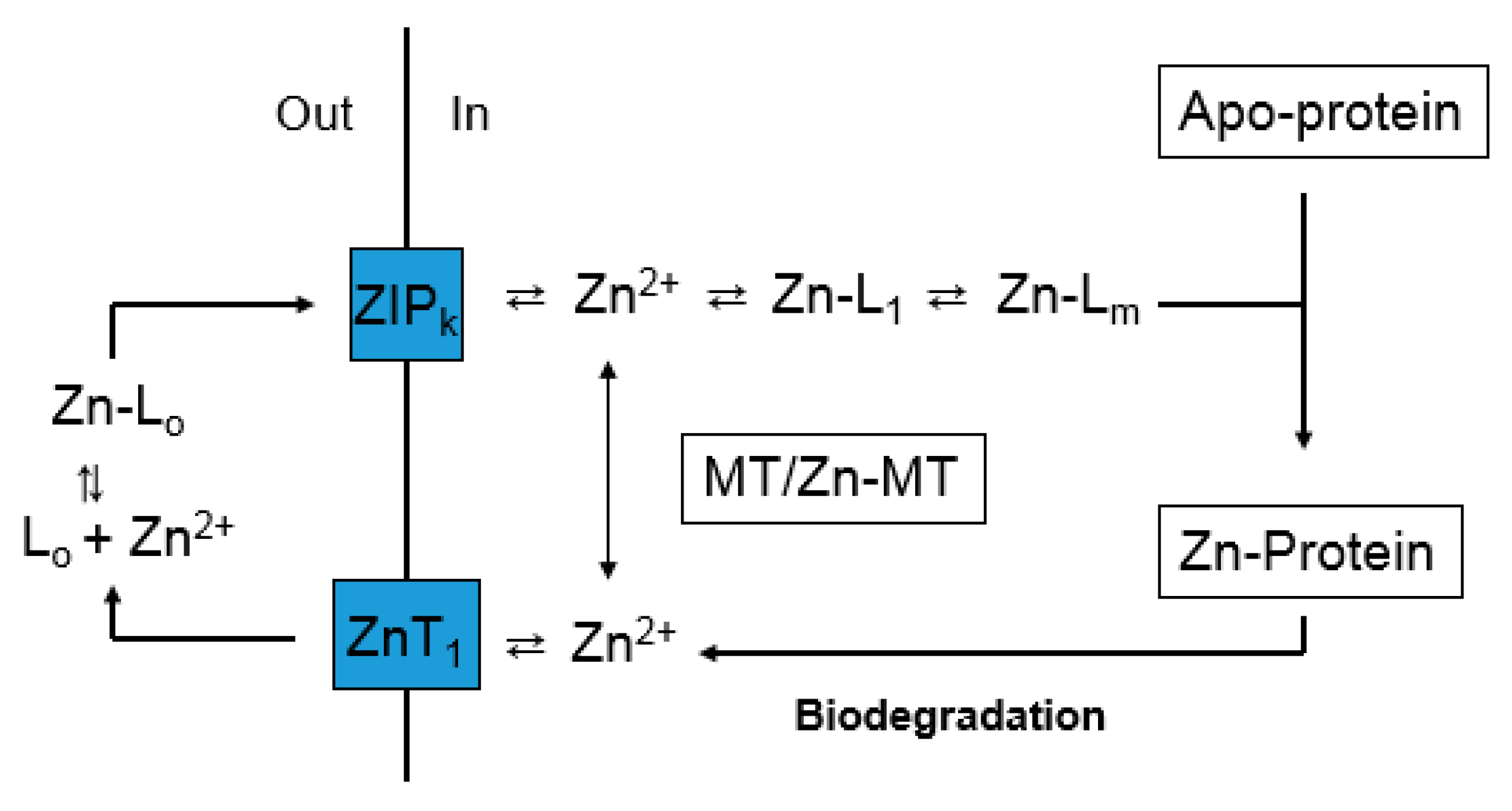
2. Chemical Properties of Metallothionein Related to Zn2+ Trafficking
3. Cellular Zn2+ and Its Milieu
4. Metallothionein and Zinc Trafficking—The Biological Context
5. Zinc Trafficking in Metallothionein-Null Organisms and Cells
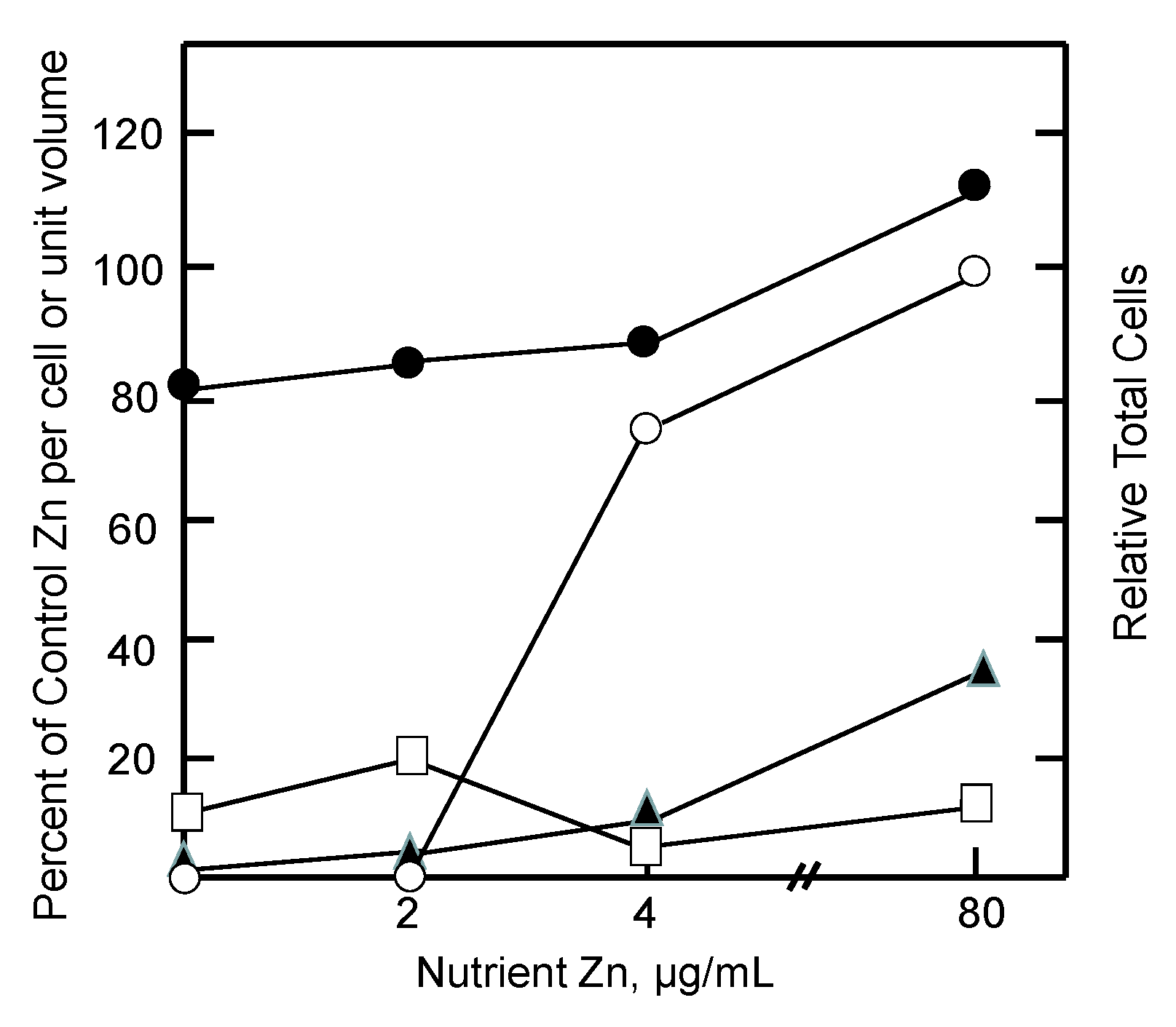
6. Metallothionein, Cell Proliferation, and Zinc Deficiency
7. Metal-Unsaturated Metallothionein and the Trafficking of Zinc
8. Oxidized Metallothionein and the Trafficking of Zn2+
9. Metallothionein Gene Expression
10. Interaction of Metallothionein and the Proteome during Zinc Trafficking
11. Emerging Technology to Address Zinc Trafficking
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Zinc through the three domains of life. J. Proteome Res. 2006, 5, 3173–3178. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.; Karim, M.; Petering, D. Zinc proteomics. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tepaamorndech, S. The SLC30 family of zinc transporters—A review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 2013, 34, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kambe, T. The functions of metallothionein and ZIP and ZnT transporters: An overview and perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 2011, 3, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, T.; Ohrvik, H.; Thiele, D.J. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. Biophys. Acta 2012, 1823, 1580–1593. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009, 109, 4682–4707. [Google Scholar] [CrossRef] [PubMed]
- Sussulini, A.; Becker, J.S. Combination of PAGE and LA-ICP-MS as an analytical workflow in metallomics: State of the art, new quantification strategies, advantages and limitations. Metallomics 2011, 3, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Sigel, A.; Sigel, H.; Sigel, R.O. Metallothioneins and related chelators. In Metal Ions in Life Sciences; RSC Publishing: Cambridge, UK, 2009; Volume 5. [Google Scholar]
- Grzywacz, A.; Gdula-Argasińska, J.; Muszyńska, B.; Tyszka-Czochara, M.; Librowski, T.; Opoka, W. Metal responsive transcription factor 1 (MTF-1) regulates zinc dependent cellular processes at the molecular level. Acta Biochim. Pol. 2015, 62, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Aras, M.A.; Aizenman, E. Redox regulation of intracellular zinc: Molecular signaling in the life and death of neurons. Antioxid. Redox Signal. 2011, 15, 2249–2263. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Deagen, J.T.; Whanger, P.D.; Weswig, P.H. Biological Function of Metallothionein. V. Its induction in rats by various stresses. Am. J. Physiol. 1978, 234, E282–E285. [Google Scholar] [PubMed]
- Petering, D.H.; Loftsgaarden, J.; Schneider, J.; Fowler, B. Metabolism of cadmium, zinc and copper in the rat kidney: The role of metallothionein and other binding sites. Environ. Health Perspect. 1984, 54, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.K.; Lee, D.K.; Ravindra, R.; Lichtlen, P.; Sirito, M.; Sawadogo, M.; Schaffner, W. The transcription factors MTF-1 and USF1 cooperate to regulate mouse metallothionein-I expression in response to the essential metal zinc in visceral endoderm cells during early development. EMBO J. 2001, 20, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.Ø.; Larsen, A.; Stoltenberg, M.; Penkowa, M. The Role of metallothionein in oncogenesis and cancer prognosis. Prog. Histochem. Cytochem. 2009, 44, 29–64. [Google Scholar] [CrossRef] [PubMed]
- Robbins, A.H.; McRee, D.E.; Williamson, M.; Collett, S.A.; Xuong, N.H.; Furey, W.F.; Wang, B.C.; Stout, C.D. Refined crystal structure of Cd, Zn metallothionein at 2.0 A resolution. J. Mol. Biol. 1991, 221, 1269–1293. [Google Scholar] [PubMed]
- Petering, D.H.; Krezoski, S.; Tabatabai, N.M. Metallothionein toxicology: Metal ion trafficking and Cellular protection. In Metallothioneins and Related Chelators: Metal Ions in Life Sciences; Sigel, A., Sigel, H., Sigel, R.O., Eds.; RSC Publishing: Cambridge, UK, 2009; Volume 5, pp. 353–397. [Google Scholar]
- Quesada, A.R.; Byrnes, R.W.; Krezoski, S.O.; Petering, D.H. Direct reaction of H2O2 with sulfhydryl groups in HL-60 cells: Zinc-metallothionein and other sites. Arch. Biochem. Biophys. 1996, 334, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.S.; Kuo, S.M.; Woo, E.S.; Pitt, B.R. The protein thiol metallothionein as an antioxidant and protectant against antineoplastic drugs. Chem. Biol. Interact. 1998, 111, 255–262. [Google Scholar] [CrossRef]
- Otvos, J.D.; Armitage, I.M. Structure of the metal clusters in rabbit liver metallothionein. Proc. Natl. Acad. Sci. USA 1980, 77, 7094–7098. [Google Scholar] [CrossRef] [PubMed]
- Nielson, K.B.; Winge, D.R. Order of metal binding in metallothionein. J. Biol. Chem. 1983, 258, 13063–13069. [Google Scholar] [PubMed]
- Ejnik, J.; Robinson, J.; Zhu, J.; Försterling, H.; Shaw, C.F.; Petering, D.H. Folding pathway of Apo-metallothionein induced by Zn2+, Cd2+ and Co2+. J. Inorg. Biochem. 2002, 88, 144–152. [Google Scholar] [CrossRef]
- Li, T.Y.; Kraker, A.J.; Shaw, C.F., III; Petering, D.H. Ligand substitution reactions of metallothioneins with EDTA and Apo-carbonic anhydrase. Proc. Natl. Acad. Sci. USA 1980, 77, 6334–6338. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Minkel, D.T.; Shaw, C.F., III; Petering, D.H. On the reactivity of metallothioneins with 5,5′-dithiobis-(2-nitrobenzoic acid). Biochem. J. 1981, 193, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.F., III; Savas, M.M.; Petering, D.H. Ligand substitution and sulfhydryl reactivity of metallothionein. Methods Enzymol. 1991, 205, 401–414. [Google Scholar] [PubMed]
- Gan, T.; Munoz, A.; Shaw, C.F., III; Petering, D.H. Reaction of 111Cd7-metallothionein with EDTA. A Reappraisal. J. Biol. Chem. 1995, 270, 5339–5345. [Google Scholar] [CrossRef] [PubMed]
- Petering, D.H.; Shaw, C.F., III. Stability constants and related equilibrium properties of metallothioneins. Methods Enzymol. 1991, 205, 475–484. [Google Scholar] [PubMed]
- Namdarghanbari, M.A.; Meeusen, J.; Bachowski, G.; Giebel, N.; Johnson, J.; Petering, D.H. Reaction of the zinc sensor FluoZin-3 with Zn7-metallothionein: Inquiry into the existence of a proposed weak binding site. J. Inorg. Biochem. 2010, 104, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Pinter, T.B.; Stillman, M.J. The zinc balance: Competitive zinc metalation of carbonic anhydrase and metallothionein 1A. Biochemistry 2014, 53, 6276–6285. [Google Scholar] [CrossRef] [PubMed]
- Kochańczyk, T.; Drozd, A.; Krężel, A. Relationship between the architecture of zinc coordination and zinc binding affinity in proteins—Insights into zinc regulation. Metallomics 2015, 7, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, L.L.; Krebs, J.F.; Paterno, S.A.; Fierke, C.A. Engineering a cysteine ligand into the zinc binding site of human carbonic anhydrase II. Biochemistry 1993, 32, 9896–9900. [Google Scholar] [CrossRef] [PubMed]
- Udom, A.O.; Brady, F.O. Reactivation in vitro of zinc-requiring Apo-enzymes by rat liver zinc-thionein. Biochem. J. 1980, 187, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zaia, J.; Fabris, D.; Wei, D.; Karpel, R.L.; Fenselau, C. Monitoring metal ion flux in reactions of metallothionein and drug-modified metallothionein by electrospray mass spectrometry. Protein Sci. 1998, 7, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Roesijadi, G.; Bogumil, R.; Vasák, M.; Kägi, J.H. Modulation of DNA binding of a tramtrack zinc finger peptide by the metallothionein-thionein conjugate pair. J. Biol. Chem. 1998, 273, 17425–17432. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Maret, W.; Vallee, B.L. Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 3489–3494. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.J.; Maret, W.; Vallee, B.L. The glutathione redox couple modulates zinc transfer from metallothionein to zinc-depleted sorbitol dehydrogenase. Proc. Natl. Acad. Sci. USA 1998, 95, 3483–3488. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Maret, W. Dual nanomolar and picomolar Zn(II) binding properties of metallothionein. J. Am. Chem. Soc. 2007, 129, 10911–10921. [Google Scholar] [CrossRef] [PubMed]
- Vasák, M.; Kägi, J.H. Metal thiolate clusters in Cobalt(II)-metallothionein. Proc. Natl. Acad. Sci. USA 1981, 78, 6709–6713. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, P.M.; You, C.; Dallinger, R.; Gruber, C.; Brouwer, M.; Kägi, J.H.; Hunziker, P.E. Electrospray ionization mass spectrometry of zinc, cadmium, and copper metallothioneins: Evidence for metal-binding cooperativity. Protein Sci. 2000, 9, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.E.; Summers, K.L.; Stillman, M.J. Noncooperative metalation of metallothionein 1A and its isolated domains with zinc. Biochemistry 2012, 51, 6690–6700. [Google Scholar] [CrossRef] [PubMed]
- Pinter, T.B.; Stillman, M.J. Putting the pieces into place: Properties of intact zinc metallothionein 1A determined from interaction of its isolated domains with carbonic anhydrase. Biochem. J. 2015, 471, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Petering, D.H.; Shaw, C.F. Structure-reactivity relationships of metallothionein, a unique metal binding protein. Comments Inorg. Chem. 1989, 9, 1–35. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006, 11, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Eide, D.J. Zinc Transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 2006, 1763, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Petering, D.H.; Kothinti, R.; Meeusen, J.; Rana, U. Cellular inorganic chemistry concepts and examples. In Cellular and Molecular Biology of Metals; Zalups, R.K., Koropatnick, J., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2010; Chapter 1; pp. 1–35. [Google Scholar]
- Karim, M.R.; Petering, D.H. Newport green, a fluorescent sensor of weakly bound cellular Zn2+: Competition with proteome for Zn2+. Metallomics 2016, 8, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Hao, Q.; Maret, W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007, 463, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Petering, D.H. Detection of Zn2+ release in nitric oxide treated cells and proteome: Dependence on fluorescent sensor and proteomic sulfhydryl groups. Metallomics 2016, 9, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.J.; Ahner, B.A. Determination of stability constants of Cu(I), Cd(II) & Zn(II) complexes with thiols using fluorescent probes. J. Inorg. Biochem. 2013, 128, 12–23. [Google Scholar]
- Namdarghanbari, M.A.; Bertling, J.; Krezoski, S.; Petering, D.H. Toxic metal proteomics: Reaction of the mammalian zinc proteome with Cd2+. J. Inorg. Biochem. 2014, 136, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Bozym, R.A.; Chimienti, F.; Giblin, L.J.; Gross, G.W.; Korichneva, I.; Li, Y.; Libert, S.; Maret, W.; Parviz, M.; Frederickson, C.J.; et al. Free zinc ions outside a narrow concentration range are toxic to a variety of Cells in vitro. Exp. Biol. Med. 2010, 235, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Pluth, M.D.; Tomat, E.; Lippard, S.J. Biochemistry of mobile zinc and nitric oxide revealed by fluorescent sensors. Annu. Rev. Biochem. 2011, 80, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics 2010, 2, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Simons, T.J. Intracellular free zinc and zinc buffering in human red blood cells. J. Membr. Biol. 1991, 123, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bozym, R.A.; Thompson, R.B.; Stoddard, A.K.; Fierke, C.A. Intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem. Biol. 2006, 1, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kagi, J.H.; Vallee, B.L. Metallothionein: A cadmium- and zinc-containing protein from equine renal cortex. J. Biol. Chem. 1960, 235, 3460–3465. [Google Scholar] [PubMed]
- Chen, R.W.; Vasey, E.J.; Whanger, P.D. Accumulation and depletion of zinc in rat liver and kidney metallothioneins. J. Nutr. 1977, 107, 805–813. [Google Scholar] [PubMed]
- Klein, D.; Scholz, P.; Drasch, G.A.; Müller-Höcker, J.; Summer, K.H. Metallothionein, copper and zinc in fetal and neonatal human liver: Changes during development. Toxicol. Lett. 1991, 56, 61–67. [Google Scholar] [CrossRef]
- Munger, K.; Germann, U.A.; Beltramini, M.; Niedermann, D.; Baitella-Eberlej, G.; Kagi, J.H.R.; Lerch, K. (Cu,Zn)-metallothioneins from fetal bovine liver: chemical and spectroscopic properties. J. Biol. Chem. 1985, 260, 10032–10038. [Google Scholar] [PubMed]
- Zhu, J.; Meeusen, J.; Krezoski, S.; Petering, D.H. Reactivity of Zn-, Cd-, and Apo-metallothionein with nitric oxide compounds: In vitro and cellular comparison. Chem. Res. Toxicol. 2010, 23, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.E.; Choo, K.H.A. Targeting and Germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc. Natl. Acad. Sci. USA 1993, 90, 8088–8092. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, J.; Choo, K.H.; Michalska, A.E.; Klaassen, C.D. Metallothionein-I and -II knock-out mice are sensitive to cadmium-induced liver mRNA expression of c-Jun and p53. Toxicol. Appl. Pharmacol. 1996, 136, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Eddins, D.; Petro, A.; Pollard, N.; Freedman, J.H.; Levin, E.D. Mercury-induced cognitive impairment in metallothionein-1/2 null mice. Neurotoxicol. Teratol. 2008, 30, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.S.; Kondo, Y.; Dellapiazza, D.; Michalska, A.E.; Choo, K.H.; Pitt, B.R. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J. Biol. Chem. 1995, 270, 5506–5510. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Woo, E.S.; Michalska, A.E.; Choo, K.H.; Lazo, J.S. Metallothionein null cells have increased sensitivity to anticancer drugs. Cancer Res. 1995, 55, 2021–2023. [Google Scholar] [PubMed]
- Andrews, G.K.; Geiser, J. Expression of the mouse metallothionein-I and -II genes provides a reproductive advantage during maternal dietary zinc deficiency. J. Nutr. 1999, 129, 1643–1648. [Google Scholar] [PubMed]
- Fong, L.; Tan, K.; Tran, C.; Cool, J.; Scherer, M.A.; Elovaris, R.; Coyle, P.; Foster, B.K.; Rofe, A.M.; Xian, C.J. Interaction of dietary zinc and intracellular binding protein metallothionein in postnatal bone growth. Bone 2009, 44, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Perraut, C.; Pollard, N.; Freedman, J.H. Metallothionein expression and neurocognitive function in mice. Physiol. Behav. 2006, 87, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.H.; Wood, A.M.; Newman, A.M.; Bremner, I.; Choo, K.H.A.; Michalska, A.E.; Duncan, S.; Trayhurn, P. Obesity and hyperleptinemia in metallothionein (-I and -II) null mice. Proc. Natl. Acad. Sci. USA 1998, 95, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, A.B.; Agrawal, K.C. Antisense down-regulation of metallothoinein induces growth arrest and apoptosis in human breast carcinoma cells. Cancer Gene Ther. 1997, 4, 199–207. [Google Scholar] [PubMed]
- Lim, D.; Jocelyn, K.M.; Yip, G.W.; Bay, B.H. Silencing the metallothionein-2A gene inhibits cell cycle progression from G1- to S-phase involving ATM and cdc25A signaling in breast cancer cells. Cancer Lett. 2009, 276, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Tekur, S.; Ho, S.M. Ribozyme-mediated downregulation of human metallothionein II(a) Induces apoptosis in human prostate and ovarian cancer cell lines. Mol. Carcinog. 2002, 33, 44–55. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [PubMed]
- Petering, H.G.; Buskirk, H.H.; Crim, J.A. The effect of dietary mineral supplements of the rat on the antitumor activity of 3-ethoxy-2-oxobutyraldehyde bis(thiosemicarbazone). Cancer Res. 1967, 27, 1115–1121. [Google Scholar] [PubMed]
- Minkel, D.T.; Dolhun, P.J.; Calhoun, B.L.; Saryan, L.A.; Petering, D.H. Zinc deficiency and growth of ehrlich ascites tumor. Cancer Res. 1979, 39, 2451–2456. [Google Scholar] [PubMed]
- Saryan, L.A.; Minkel, D.T.; Dolhun, P.J.; Calhoun, B.L.; Wielgus, S.; Schaller, M.; Petering, D.H. Effects of zinc deficiency on cellular processes and morphology in ehrlich ascites tumor cells. Cancer Res. 1979, 39, 2457–2465. [Google Scholar] [PubMed]
- Mita, M.; Imura, N.; Kumazawa, Y.; Himeno, S. Suppressed proliferative response of spleen T cells from metallothionein null mice. Microbiol. Immunol. 2002, 46, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.R.; Mara, T.W.; Cherian, M.G. Impaired hepatic regeneration in metallothionein-I/II knockout mice after partial hepatectomy. Exp. Biol. Med. 2005, 230, 61–67. [Google Scholar]
- Kraker, A.J.; Petering, D.H. Tumor-host zinc metabolism: The central role of metallothionein. Biol. Trace Elem. Res. 1983, 5, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Krezoski, S.K.; Villalobos, J.; Shaw, C.F., III; Petering, D.H. Kinetic lability of zinc bound to metallothionein in ehrlich cells. Biochem. J. 1988, 255, 483–491. [Google Scholar] [PubMed]
- Kraker, A.J.; Krakower, G.; Shaw, C.F., III; Petering, D.H.; Garvey, J.S. Zinc metabolism in ehrlich cells: Properties of a metallothionein-like zinc-binding protein. Cancer Res. 1988, 48, 3381–3388. [Google Scholar] [PubMed]
- Suhy, D.A.; Simon, K.D.; Linzer, D.I.; O’Halloran, T.V. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J. Biol. Chem. 1999, 274, 9183–9192. [Google Scholar] [CrossRef] [PubMed]
- Langmade, S.J.; Ravindra, R.; Daniels, P.J.; Andrews, G.K. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J. Biol. Chem. 2000, 275, 34803–34809. [Google Scholar] [CrossRef] [PubMed]
- Braun, W.; Vasák, M.; Robbins, A.H.; Stout, C.D.; Wagner, G.; Kägi, J.H.; Wüthrich, K. Comparison of the NMR solution structure and the X-ray crystal structure of Rat metallothionein-2. Proc. Natl. Acad. Sci. USA 1992, 89, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, A.; Shaw, C.F., III; Petering, D.H.; Garvey, J.; Kraker, A.J. Basal metallothionein in tumors: widespread presence of apoprotein. J. Inorg. Biochem. 1994, 54, 91–105. [Google Scholar] [CrossRef]
- Yang, Y.; Maret, W.; Vallee, B.L. Differential fluorescence labeling of cysteinyl clusters uncovers high tissue levels of thionein. Proc. Natl. Acad. Sci. USA 2001, 98, 5556–5559. [Google Scholar] [CrossRef] [PubMed]
- Petering, D.H.; Zhu, J.; Krezoski, S.; Meeusen, J.; Kiekenbush, C.; Krull, S.; Specher, T.; Dughish, M. Apo-metallothionein emerging as a major player in the cellular activities of metallothionein. Exp. Biol. Med. 2006, 231, 1528–1534. [Google Scholar] [CrossRef]
- Petering, D.H.; Quesada, A.; Dughish, M.; Eisch, S.; Gan, T.; Lemkuil, D.; Pattanaik, A.; Byrnes, R.W.; Savas, M.; Whelan, H.; et al. Metallothionein in tumor and host: intersections of zinc metabolism, the stress response, and tumor therapy. In Metallothionein III, Biological Roles and Medical Implications; Suzuki, K., Imura, N., Kimura, M., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1993; pp. 329–346. [Google Scholar]
- Irvine, G.W.; Duncan, K.E.; Gullons, M.; Stillman, M.J. Metalation kinetics of the human α-metallothionein 1A fragment is dependent on the fluxional structure of the Apo-protein. Chemistry 2015, 21, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Irvine, G.W.; Santolini, M.; Stillman, M.J. Selective cysteine modification of metal-free human metallothionein 1A and its isolated domain fragments: Solution structural properties revealed via ESI-MS. Protein Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rana, U.; Kothinti, R.; Meeusen, J.; Tabatabai, N.M.; Krezoski, S.; Petering, D.H. Zinc binding ligands and cellular zinc trafficking: Apo-metallothionein, glutathione, TPEN, proteomic zinc, and Zn-Sp1. J. Inorg. Biochem. 2008, 102, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Ejnik, J.; Muñoz, A.; Gan, T.; Shaw, C.F., III; Petering, D.H. Interprotein metal ion exchange between cadmium-carbonic anhydrase and Apo- or zinc-metallothionein. J. Biol. Inorg. Chem. 1999, 4, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Kothinti, R.; Tabatabai, N.M.; Petering, D.H. Electrophoretic mobility shift assay of zinc finger proteins: competition for Zn2+ bound to Sp1 in protocols including EDTA. J. Inorg. Biochem. 2011, 105, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Maret, W. Catalytic selenols couple the redox cycles of metallothionein and glutathione. Eur. J. Biochem. 2001, 268, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Tachibana, C.; Rahr Winther, J.; Appenzeller-Herzog, C. Intracellular glutathione pools are heterogeneously concentrated. Redox Biol. 2013, 1, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Savas, M.M.; Shaw, C.F., III; Petering, D.H. The oxidation of rabbit liver metallothionein-II by 5,5′-dithiobis(2-nitrobenzoic acid) and glutathione disulfide. J. Inorg. Biochem. 1993, 52, 235–249. [Google Scholar] [CrossRef]
- Hamer, D.H. Metallothionein. Annu. Rev. Biochem. 1986, 55, 913–951. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.W.; Searle, P.F.; Palmiter, R.D. Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. Nature 1985, 317, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Radtke, F.; Heuchel, R.; Georgiev, O.; Hergersberg, M.; Gariglio, M.; Dembic, Z.; Schaffner, W. Cloned Transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO. J. 1993, 12, 1355–1362. [Google Scholar] [PubMed]
- Dalton, T.P.; Bittel, D.; Andrews, G.K. Reversible activation of mouse metal response element-binding transcription factor 1 DNA binding involves zinc interaction with the zinc finger domain. Mol. Cell. Biol. 1997, 17, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Guerrerio, A.L.; Berg, J.M. Metal ion affinities of the zinc finger domains of the metal responsive element-binding transcription factor-1 (MTF1). Biochemistry 2004, 43, 5437–5444. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.V.; Bittel, D.C.; Ravindra, R.; Jiang, H.; Andrews, G.K. Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J. Biol. Chem. 2000, 275, 9377–9784. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.S.; Lazo, J.S. Nucleocytoplasmic functionality of metallothionein. Cancer Res. 1997, 57, 4236–4241. [Google Scholar] [PubMed]
- Bittel, D.; Dalton, T.; Samson, S.L.; Gedamu, L.; Andrews, G.K. The DNA binding activity of metal response element-binding transcription factor-1 is activated in vivo and in vitro by zinc, but not by other transition metals. J. Biol. Chem. 1998, 273, 7127–7133. [Google Scholar] [CrossRef] [PubMed]
- Krepkiy, D.; Försterling, F.H.; Petering, D.H. Interaction of Cd2+ with Zn finger 3 of transcription factor IIIA: Structures and binding to cognate DNA. Chem. Res. Toxicol. 2004, 17, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Georgiev, O.; Hagmann, M.; Günes, C.; Cramer, M.; Faller, P.; Vasák, M.; Schaffner, W. Activity of metal-responsive transcription factor 1 by toxic heavy metals and H2O2 in vitro is modulated by metallothionein. Mol. Cell. Biol. 2003, 23, 8471–8485. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.B.; Wobig, W.J.; Petering, D.H. Native SDS-PAGE: High resolution electrophoretic separation of proteins with retention of native properties including bound metal ions. Metallomics 2014, 6, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, J.W.; Tomasiewicz, H.; Nowakowski, A.; Petering, D.H. TSQ (6-Methoxy-8-p-toluenesulfonamido-quinoline), a common fluorescent sensor for cellular zinc, images zinc proteins. Inorg. Chem. 2011, 50, 7563–7573. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.B.; Petering, D.H. Reactions of the fluorescent sensor, zinquin, with the zinc-proteome: adduct formation and ligand substitution. Inorg. Chem. 2011, 50, 10124–10133. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, J.W.; Nowakowski, A.; Petering, D.H. Reaction of metal-binding ligands with the zinc proteome: Zinc sensors and N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine. Inorg. Chem. 2012, 51, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.B.; Meeusen, J.W.; Menden, H.; Tomasiewicz, H.; Petering, D.H. Chemical-biological properties of zinc sensors TSQ and zinquin: Formation of sensor-Zn-protein adducts versus Zn(sensor)2 complexes. Inorg. Chem. 2015, 54, 11637–11647. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A. Zinc Chemical Biology: The Pursuit of the Intracellular Targets of Zinquin; UWM Digital Commons: Milwaukee, WI, USA; University of Wisconsin Milwaukee: Milwaukee, WI, USA, 2013. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petering, D.H.; Mahim, A. Proteomic High Affinity Zn2+ Trafficking: Where Does Metallothionein Fit in? Int. J. Mol. Sci. 2017, 18, 1289. https://doi.org/10.3390/ijms18061289
Petering DH, Mahim A. Proteomic High Affinity Zn2+ Trafficking: Where Does Metallothionein Fit in? International Journal of Molecular Sciences. 2017; 18(6):1289. https://doi.org/10.3390/ijms18061289
Chicago/Turabian StylePetering, David H., and Afsana Mahim. 2017. "Proteomic High Affinity Zn2+ Trafficking: Where Does Metallothionein Fit in?" International Journal of Molecular Sciences 18, no. 6: 1289. https://doi.org/10.3390/ijms18061289