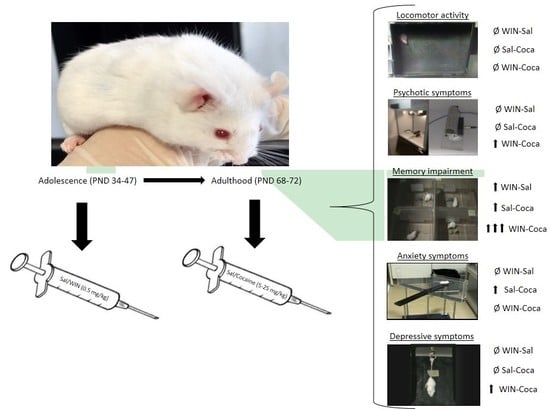
Adolescent Exposure to the Synthetic Cannabinoid WIN 55212-2 Modifies Cocaine Withdrawal Symptoms in Adult Mice
Abstract
:1. Introduction
2. Results
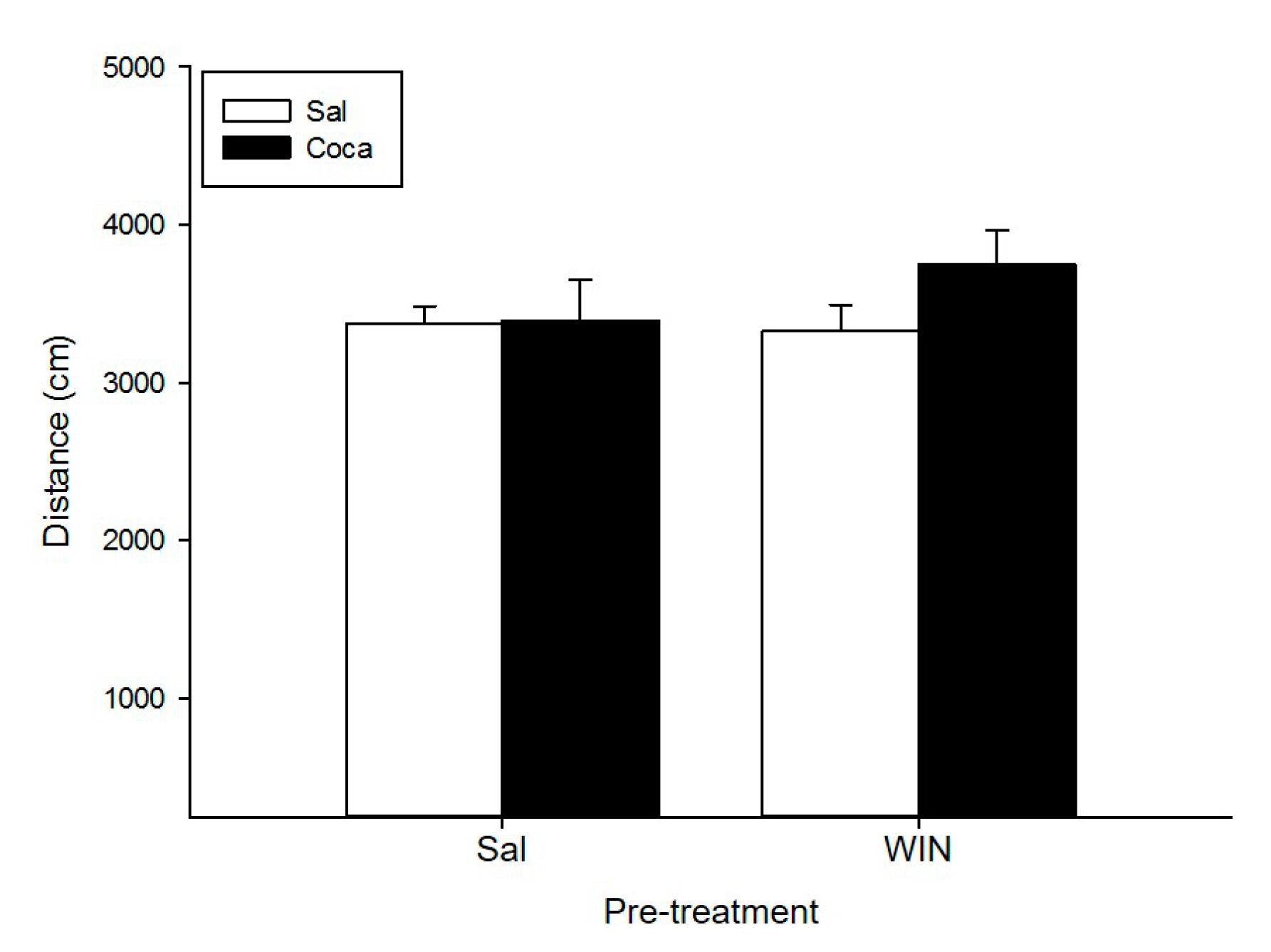
2.1. Open Field
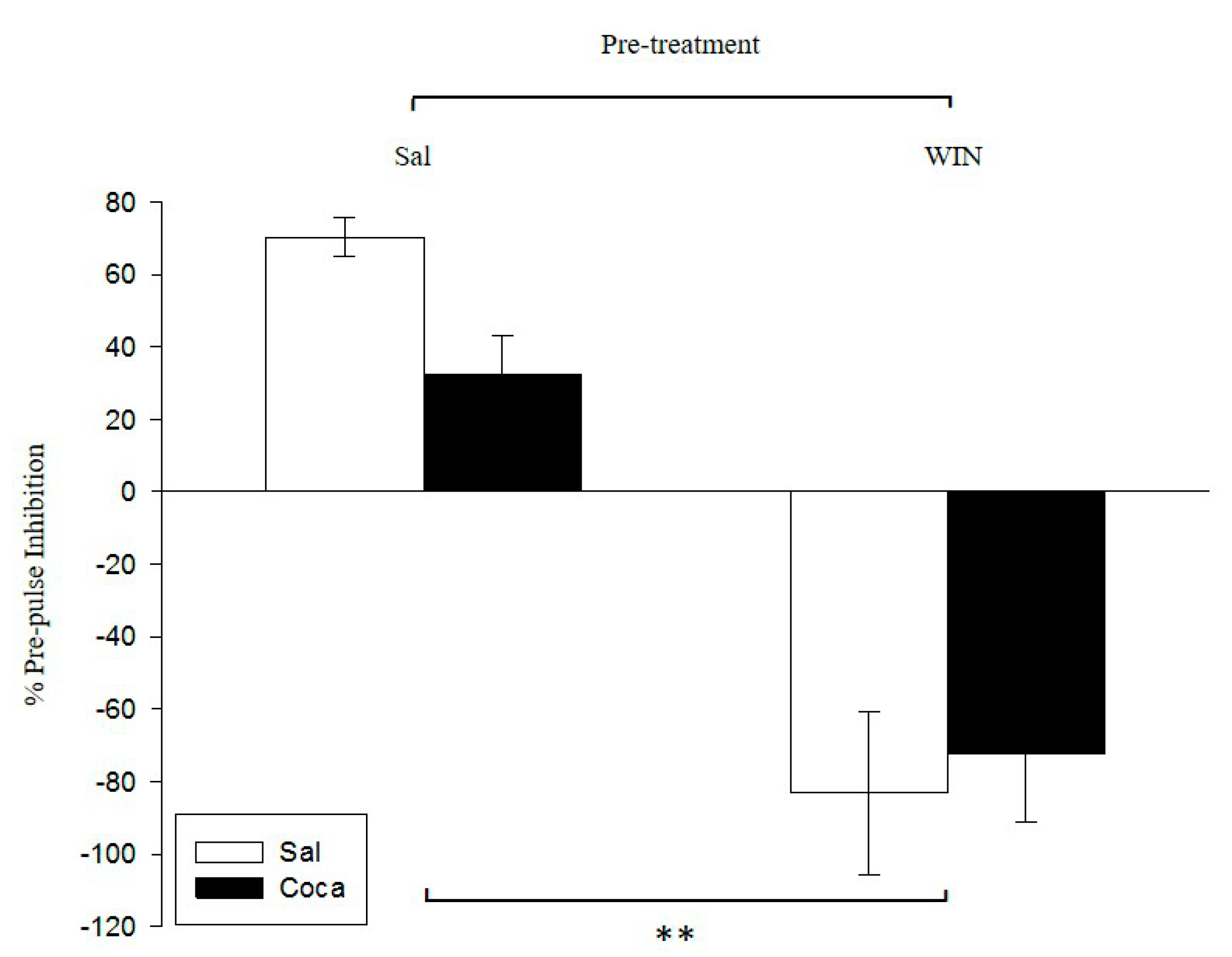
2.2. Pre-Pulse Inhibition
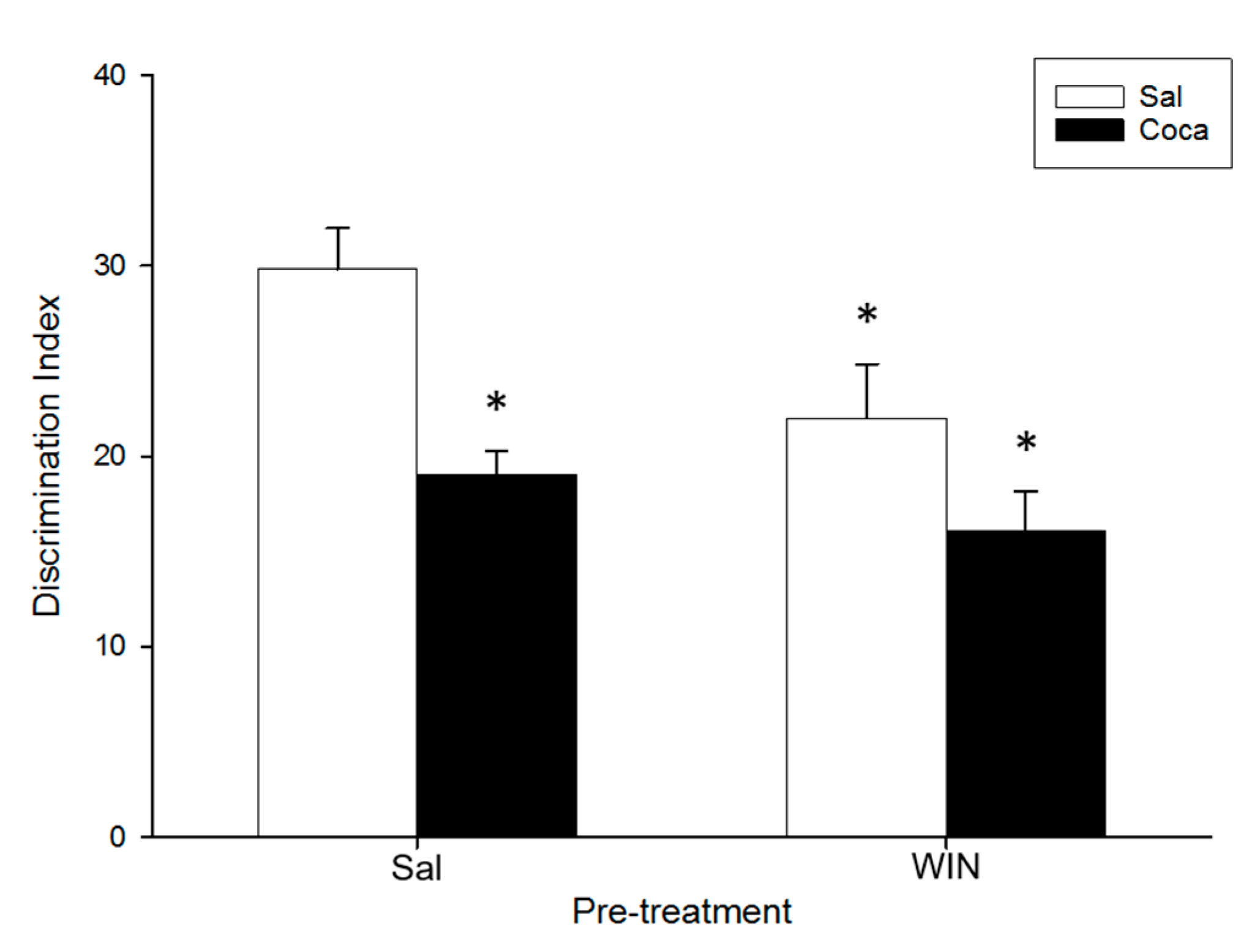
2.3. Object Recognition
2.4. Elevated Plus Maze
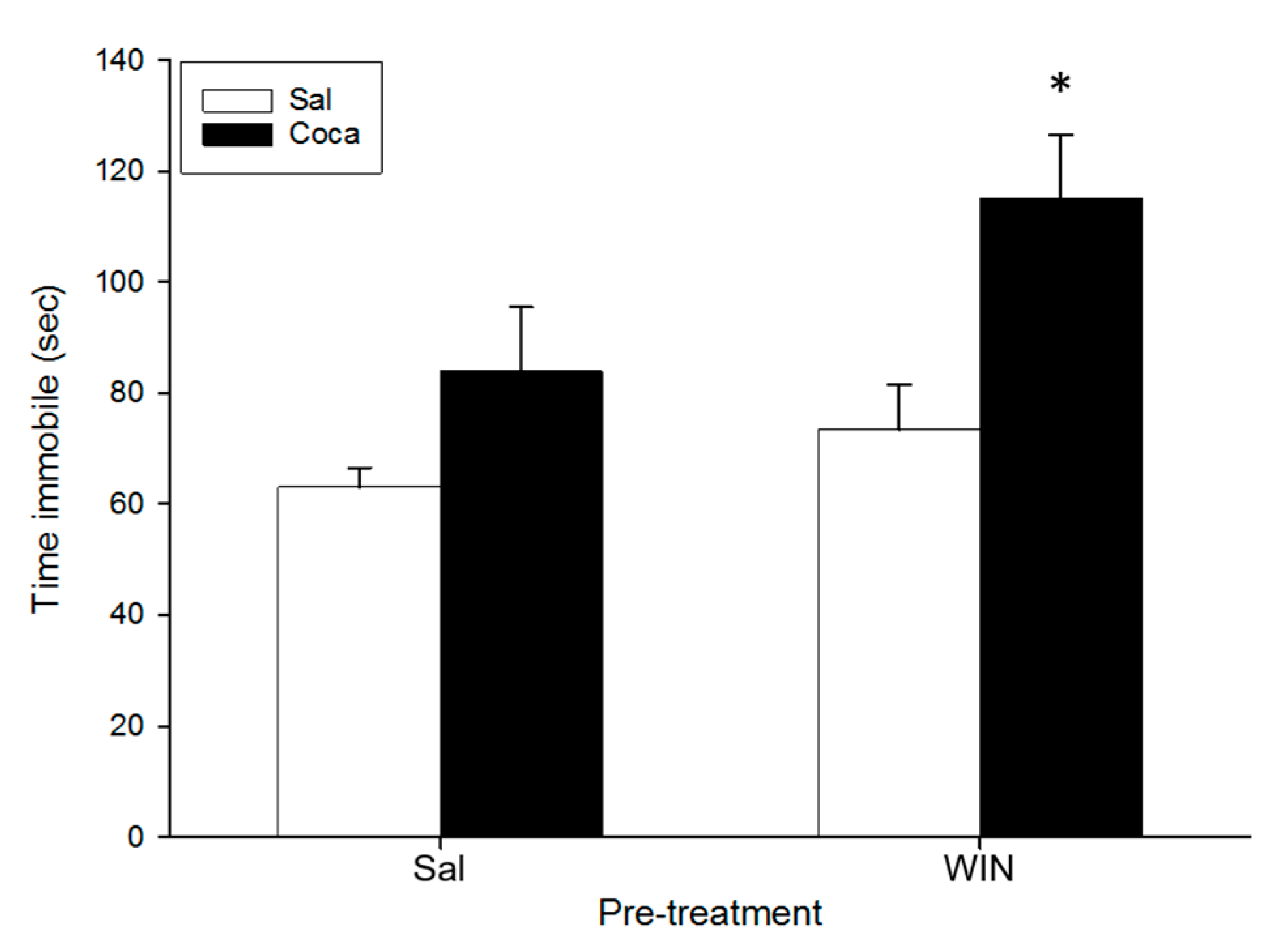
2.5. Tail Suspension Test
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Experimental Design
4.4. Behavioral Testing
4.4.1. Open Field
4.4.2. Pre-Pulse Inhibition
4.4.3. Object Recognition
4.4.4. Elevated Plus Maze
4.4.5. Tail Suspension
4.5. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Substance Abuse Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings; DHHS: Rockville, MD, USA, 2011. [Google Scholar]
- Lichtman, A.H.; Martin, B.R. Δ9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology 1996, 126, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- EMCDDA-European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2016: Trends and Developments; Publications Office of the European Union: Luxembourg, 2016; ISBN 978-92-9168-890-6. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid receptor ligands: Clinical and neuropharmacological considerations, relevant to future drug discovery and development. Expert Opin. Investig. Drugs 2000, 9, 1553–1571. [Google Scholar] [CrossRef] [PubMed]
- Sagheddu, C.; Melis, M. Individual differences and vulnerability to drug addiction: A focus on the endocannabinoid system. CNS Neurol. Disord. Drug Targets 2015, 14, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ahmad, T.; Loureiro, M.; Zunder, J.; Laviolette, S.R. The role of cannabinoid transmission in emotional memory formation: Implications for addiction and schizophrenia. Front. Psychiatry 2014, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, B.; Miller, M.L.; Hurd, Y.L. Cannabis use during adolescent development: Susceptibility to psychiatric illness. Front. Psychiatry 2013, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Hayatbakhsh, M.R.; Najman, J.M.; Jamrozik, K.; Mamun, A.A.; Alati, R.; Bor, W. Cannabis and anxiety and depression in young adults: A large prospective study. J. Am. Acad. Child. Adolesc. Psychiatry 2007, 46, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.H.; Caspi, A.; Ambler, A.; Harrington, H.; Houts, R.; Keefe, R.S.; McDonald, K.; Ward, A.; Poulton, R.; Moffitt, T.E. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. USA 2012, 109, E2657–E2664. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Krebs, M.O.; le Pen, G.; Jay, T.M. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front. Neurosci. 2014, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Realini, N.; Rubino, T.; Parolaro, D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol. Res. 2009, 60, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Rushlow, W.J.; Laviolette, S.R. What can rats tell us about adolescent cannabis exposure? Insights from preclinical research. Can. J. Psychiatry 2016, 61, 328–334. [Google Scholar] [PubMed]
- Gleason, K.A.; Birnbaum, S.G.; Shukla, A.; Ghose, S. Susceptibility of the adolescent brain to cannabinoids: Long-term hippocampal effects and relevance to schizophrenia. Transl. Psychiatry 2012, 2, e199. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Roig, J.; Benito, E.; Agis-Balboa, R.C.; Piscitelli, F.; Hoyer-Fender, S.; di Marzo, V.; Havemann-Reinecke, U. Chronic exposure to cannabinoids during adolescence causes long-lasting behavioral deficits in adult mice. Addict. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Koch, M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 2003, 28, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Wegener, N.; Koch, M. Behavioural disturbances and altered Fos protein expression in adult rats after chronic pubertal cannabinoid treatment. Brain Res. 2009, 1253, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Bini, V.; Frau, R.; Devoto, P.; Pardu, A.; Fan, Y.; Solbrig, M.V. Juvenile cannabinoid treatment induces frontostriatal gliogenesis in Lewis rats. Eur. Neuropsychopharmacol. 2014, 24, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.V.; Guimarães, F.S.; Grace, A. Effects of pubertal cannabinoid administration on attentional set-shifting and dopaminergic hyper-responsivity in a developmental disruption model of schizophrenia. Int. J. Neuropsychopharmacol. 2014, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Bambico, F.R.; Nguyen, N.T.; Katz, N.; Gobbi, G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol. Dis. 2010, 37, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Raver, S.M.; Keller, A. Permanent suppression of cortical oscillations in mice after adolescent exposure to cannabinoids: Receptor mechanisms. Neuropharmacology 2014, 86, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, Z.J.; Chergui, K. Induction of cannabinoid- and N-methyl-d-aspartate receptor-mediated long-term depression in the nucleus accumbens and dorsolateral striatum is region and age dependent. Int. J. Neuropsychopharmacol. 2015, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Abush, H.; Akirav, I. Short- and long-term cognitive effects of chronic cannabinoids administration in late-adolescence rats. PLoS ONE 2012, 7, e31731. [Google Scholar] [CrossRef] [PubMed]
- Kirschmann, E.K.; Pollock, M.W.; Nagarajan, V.; Torregrossa, M.M. Effects of adolescent cannabinoid self-administration in rats on addiction-related behaviors and working memory. Neuropsychopharmacology 2017, 42, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Cass, D.K.; Flores-Barrera, E.; Thomases, D.R.; Vital, W.F.; Caballero, A.; Tseng, K.Y. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol. Psychiatry 2014, 19, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Candelaria-Cook, F.T.; Hamilton, D.A. Chronic cannabinoid agonist (WIN 55,212–2) exposure alters hippocampal dentate gyrus spine density in adult rats. Brain Res. 2014, 1542, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, L.; Hall, W.; Lynskey, M. The relationship between cannabis use and other substance use in the general population. Drug Alcohol Depend. 2001, 64, 319–327. [Google Scholar] [CrossRef]
- Kandel, D.B.; Yamaguchi, K.; Klein, L.C. Testing the Gateway Hypothesis. Addiction 2006, 101, 470–472. [Google Scholar] [PubMed]
- Kandel, D.; Kandel, E. The Gateway Hypothesis of substance abuse: Developmental, biological and societal perspectives. Acta Paediatr. 2015, 104, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Pistis, M.; Perra, S.; Pillolla, G.; Melis, M.; Muntoni, A.L.; Gessa, G.L. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol. Psychiatry 2004, 56, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ellgren, M.; Hurd, Y.; Franck, J. Amphetamine effects on dopamine levels and behavior following cannabinoid exposure during adolescence. Eur. J. Pharmacol. 2004, 497, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arias, M.; Manzanedo, C.; Roger-Sánchez, C.; do Couto, B.R.; Aguilar, M.A.; Miñarro, J. Effect of adolescent exposure to WIN 55212–2 on the acquisition and reinstatement of MDMA-induced conditioned place preference. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.J.; Johnson, N.L.; Schenk, M.E.; Byrnes, E.M. Cannabinoid exposure in adolescent female rats induces transgenerational effects on morphine conditioned place preference in male offspring. J. Psychopharmacol. 2012, 26, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Vassoler, F.M.; Johnson, N.L.; Byrnes, E.M. Female adolescent exposure to cannabinoids causes transgenerational effects on morphine sensitization in female offspring in the absence of in utero exposure. J. Psychopharmacol. 2013, 27, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Arias, F.; Szerman, N.; Vega, P.; Mesias, B.; Basurte, I.; Morant, C.; Ochoa, E.; Poyo, F.; Babin, F. Cocaine abuse or dependency and other psychiatric disorders. Madrid study on dual pathology. Rev. Psiquiatr. Salud. Ment. 2013, 6, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Arias, F.; Szerman, N.; Vega, P.; Mesias, B.; Basurte, I.; Morant, C.; Ochoa, E.; Poyo, F.; Babin, F. Abuse or dependence on cannabis and other psychiatric disorders. Madrid study on dual pathology prevalence. Actas Esp. Psiquiatr. 2013, 41, 122–129. [Google Scholar] [PubMed]
- EMCDDA-European Monitoring Centre for Drugs and Drug Addiction. European Drug Report: Trends and Developments; Publications Office of the European Union: Luxembourg, 2014; ISBN 978-92-9168-694-0. [Google Scholar] [CrossRef]
- Rodríguez-Arias, M.; Roger-Sánchez, C.; Vilanova, I.; Revert, N.; Manzanedo, C.; Miñarro, J.; Aguilar, M.A. Effects of cannabinoid exposure during adolescence on the conditioned rewarding effects of WIN 55212-2 and cocaine in mice: Influence of the novelty-seeking trait. Neural Plast. 2016, 2016, 6481862. [Google Scholar] [CrossRef] [PubMed]
- Deroche-Gamonet, V.; Piazza, P.V. Psychobiology of cocaine addiction: Contribution of a multi-symptomatic animal model of loss of control. Neuropharmacology 2014, 76, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Craige, C.P.; Lewandowski, S.; Kirby, L.G.; Unterwald, E.M. Dorsal raphe 5-HT2c receptor and GABA networks regulate anxiety produced by cocaine withdrawal. Neuropharmacology 2015, 93, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Dalley, J.W.; Lääne, K.; Pena, Y.; Theobald, D.E.; Everitt, B.J.; Robbins, T.W. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology 2005, 182, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Perrine, S.A.; Sheikh, I.S.; Nwaneshiudu, C.A.; Schroeder, J.A.; Unterwald, E.M. Withdrawal from chronic administration of cocaine decreases Δ opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology 2008, 54, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Martin, N.L.; Cotes, R.O. Cocaine-induced psychotic disorders: Presentation, mechanism, and management. J. Dual Diagn. 2014, 10, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Schoenbaum, G.; Saddoris, M.P.; Ramus, S.J.; Shaham, Y.; Setlow, B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur. J. Neurosci. 2004, 19, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Mateos-García, A.; Roger-Sánchez, C.; Rodriguez-Arias, M.; Miñarro, J.; Aguilar, M.A.; Manzanedo, C.; Arenas, M.C. Higher sensitivity to the conditioned rewarding effects of cocaine and MDMA in High-Novelty-Seekers mice exposed to a cocaine binge during adolescence. Psychopharmacology 2015, 232, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Manzanedo, C.; Aguilar, M.A.; Rodríguez-Arias, M.; Navarro, M.; Miñarro, J. Cannabinoid agonist-induced sensitisation to morphine place preference in mice. Neuroreport 2004, 15, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Manzanedo, C.; Rodríguez-Arias, M.; Daza-Losada, M.; Maldonado, C.; Aguilar, M.A.; Miñarro, J. Effect of the CB1 cannabinoid agonist WIN 55212-2 on the acquisition and reinstatement of MDMA-induced conditioned place preference in mice. Behav. Brain Funct. 2010, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Daza-Losada, M.; Miñarro, J.; Aguilar, M.A.; Valverde, O.; Rodríguez-Arias, M. Acute blockade of CB1 receptor leads to reinstatement of MDMA-induced conditioned place preference. Pharmacol. Biochem. Behav. 2011, 100, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.A.; Hogan, J.B.; Benson, K.A.; Shehata, C.W.; Landauer, M.R. Behavioral effects of vehicles: DMSO, ethanol, Tween-20, Tween-80, and emulphor-620. Pharmacol. Biochem. Behav. 1995, 50, 521–526. [Google Scholar] [CrossRef]
- Maldonado, R. Study of cannabinoid dependence in animals. Pharmacol. Ther. 2002, 95, 153–164. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Wojcieszak, J. Spice/K2 drugs—More than innocent substitutes for marijuana. Int. J. Neuropsychopharmacol. 2014, 17, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Ksir, C.; Hart, C.L. Cannabis and psychosis: A critical overview of the relationship. Psychiatry Rep. 2016, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.U.; Efferen, T.R.; Duncan, E.J.; Rotrosen, J. Prepulse inhibition of the acoustic startle response in cocaine-withdrawn rats. Pharmacol. Biochem. Behav. 2001, 68, 753–759. [Google Scholar] [CrossRef]
- Murphy, C.A.; di Iorio, L.; Feldon, J. Effects of psychostimulant withdrawal on latent inhibition of conditioned active avoidance and prepulse inhibition of the acoustic startle response. Psychopharmacology 2001, 156, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, J.C.; Aguilar, M.A.; Giménez-Gómez, P.; Miñarro, J.; Rodríguez-Arias, M. Adolescent but not adult ethanol binge drinking modulates cocaine withdrawal symptoms in mice. PLoS ONE 2017, 12, e0172956. [Google Scholar] [CrossRef] [PubMed]
- Raver, S.M.; Haughwout, S.P.; Keller, A. Adolescent cannabinoid exposure permanently suppresses cortical oscillations in adult mice. Neuropsychopharmacology 2013, 38, 2338–2347. [Google Scholar] [CrossRef] [PubMed]
- O’Tuathaigh, C.M.; Hryniewiecka, M.; Behan, A.; Tighe, O.; Coughlan, C.; Desbonnet, L.; Cannon, M.; Karayiorgou, M.; Gogos, J.A.; Cotter, D.R.; et al. Chronic adolescent exposure to Δ-9-tetrahydrocannabinol in COMT mutant mice: Impact on psychosis-related and other phenotypes. Neuropsychopharmacology 2010, 35, 2262–2273. [Google Scholar] [CrossRef] [PubMed]
- Cadoni, C.; Simola, N.; Espa, E.; Fenu, S.; Di Chiara, G. Strain dependence of adolescent Cannabis influence on heroin reward and mesolimbic dopamine transmission in adult Lewis and Fischer 344 rats. Addict. Biol. 2015, 20, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Briand, L.A.; Gross, J.P.; Robinson, T.E. Impaired object recognition following prolonged withdrawal from extended-access cocaine self-administration. Neuroscience 2008, 155, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Morisot, N.; le Moine, C.; Millan, M.J.; Contarino, A. CRF1 receptor-deficiency reduces recognition memory deficits and vulnerability to stress induced by cocaine withdrawal. Int. J. Neuropsychopharmacol. 2014, 17, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Stopponi, S.; Soverchia, L.; Ubaldi, M.; Cippitelli, A.; Serpelloni, G.; Ciccocioppo, R. Chronic THC during adolescence increases the vulnerability to stress-induced relapse to heroin seeking in adult rats. Eur. Neuropsychopharmacol. 2014, 24, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.J.; Magalhães, A.; Melo, P.; de Sousa, L.; Tavares, M.A.; Monteiro, P.R.; Summavielle, T. Long-term effects of chronic cocaine exposure throughout adolescence on anxiety and stress responsivity in a Wistar rat model. Neuroscience 2014, 277, 343–355. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Citó Mdo, C.; da Silva, F.C.; Silva, M.I.; Moura, B.A.; Macêdo, D.S.; Woods, D.J.; Fonteles, M.M.; de Vasconcelos, S.M.; de Sousa, F.C. Reversal of cocaine withdrawal-induced anxiety by ondansetron, buspirone and propranolol. Behav. Brain Res. 2012, 231, 116–123. [Google Scholar] [PubMed]
- El Hage, C.; Rappeneau, V.; Etievant, A.; Morel, A.L.; Scarna, H.; Zimmer, L.; Bérod, A. Enhanced anxiety observed in cocaine withdrawn rats is associated with altered reactivity of the dorsomedial prefrontal cortex. PLoS ONE 2012, 7, e43535. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.J.; Pearson, L.S.; Buccafusco, J.J. Effect of the use-dependent, nicotinic receptor antagonist BTMPS in the forced swim test and elevated plus maze after cocaine discontinuation in rats. Neurosci. Lett. 2010, 474, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Kupferschmidt, D.A.; Newman, A.E.; Boonstra, R.; Erb, S. Antagonism of cannabinoid 1 receptors reverses the anxiety-like behavior induced by central injections of corticotropin-releasing factor and cocaine withdrawal. Neuroscience 2012, 204, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lejuez, C.W.; Zvolensky, M.J.; Daughters, S.B.; Bornovalova, M.A.; Paulson, A.; Tull, M.T.; Ettinger, K.; Otto, M.W. Anxiety sensitivity: A unique predictor of dropout among inner-city heroin and crack/cocaine users in residential substance use treatment. Behav. Res. Ther. 2008, 46, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Valzachi, M.C.; Teodorov, E.; Marcourakis, T.; Bailey, A.; Camarini, R. Enhancement of behavioral sensitization, anxiety-like behavior, and hippocampal and frontal cortical CREB levels following cocaine abstinence in mice exposed to cocaine during adolescence. PLoS ONE 2013, 8, e78317. [Google Scholar] [CrossRef] [PubMed]
- Chartoff, E.; Sawyer, A.; Rachlin, A.; Potter, D.; Pliakas, A.; Carlezon, W.A. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology 2012, 62, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Zilkha, N.; Feigin, E.; Barnea-Ygael, N.; Zangen, A. Induction of depressive-like effects by subchronic exposure to cocaine or heroin in laboratory rats. J. Neurochem. 2014, 130, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, F.F.; O’Leary, O.F.; Cryan, J.F. Activation but not blockade of GABAB receptors during early-life alters anxiety in adulthood in BALB/c mice. Neuropharmacology 2014, 81, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Grey, J.; Terry, P.; Higgs, S. Contrasting effects of different cannabinoid receptor ligands on mouse ingestive behaviour. Behav. Pharmacol. 2012, 23, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Linsenbardt, D.N.; Boehm, S.L. Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: Involvement of the posterior ventral tegmental area, 2nd. Neuroscience 2009, 164, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Black, Y.D.; Maclaren, F.R.; Naydenov, A.V.; Carlezon, W.A., Jr.; Baxter, M.G.; Konradi, C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J. Neurosci. 2006, 26, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Fendt, M.; Koch, M. Translational value of startle modulations. Cell Tissue Res. 2013, 354, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Gobira, P.H.; Ropke, J.; Aguiar, D.C.; Crippa, J.A.; Moreira, F.A. Animal models for predicting the efficacy and side effects of antipsychotic drugs. Rev. Bras. Psiquiatr. 2013, 35. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.B.; Weber, M.; Geyer, M.A. Genetic models of sensorimotor gating: Relevance to neuropsychiatric disorders. Curr. Top. Behav. Neurosci. 2012, 12, 251–318. [Google Scholar] [PubMed]
- Geyer, M.A.; McIlwain, K.L.; Paylor, R. Mouse genetic models for prepulse inhibition: An early review. Mol. Psychiatry 2002, 7, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, Y.; Li, N.; Wu, X.; Wu, Y. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci. Biobehav. Rev. 2009, 33, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Van den Buuse, M. Modeling the positive symptoms of schizophrenia in genetically modified mice: Pharmacology and methodology aspects. Schizophr. Bull. 2010, 36, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Valsamis, B.; Schmid, S. Habituation and prepulse inhibition of acoustic startle in rodents. J. Vis. Exp. 2011, e3446. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Broadbent, N.J.; Squire, L.R.; Clark, R.E. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 14515–14520. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.; Pascual, M.; Rodríguez-Arias, M.; Miñarro, J.; Guerri, C. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav. Immun. 2016, 53, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaça, M.V.; Aguiar, D.C.; Guimarães, F.S. Animal models of anxiety disorders and stress. Rev. Bras. Psiquiatr. 2013, 35, S101–S111. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arias, M.; Vaccaro, S.; Arenas, M.C.; Aguilar, M.A.; Miñarro, J. The novelty-seeking phenotype modulates the long-lasting effects of adolescent MDMA exposure. Physiol. Behav. 2015, 141, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Pollak, D.D.; Rey, C.E.; Monje, F.J. Rodent models in depression research: Classical strategies and new directions. Ann. Med. 2010, 42, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef] [PubMed]
- Vaugeois, J.M.; Passera, G.; Zuccaro, F.; Costentin, J. Individual differences in response to imipramine in the mouse tail suspension test. Psychopharmacology 1997, 134, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.R. New desings in analysis of variance. Annu. Rev. Psychol. 1987, 38, 29–60. [Google Scholar] [CrossRef]
| Measurement | Sal-Sal | Sal-Coca | WIN-Sal | WIN-Coca |
|---|---|---|---|---|
| Time OA (s) | 97 ± 4.7 | 23 ± 9.9 ** | 92 ± 24 | 93 ± 28 |
| % time OA | 32 ± 1.6 | 8 ± 1 ** | 30 ± 2.5 | 31 ± 3 |
| OA entries | 23 ± 2.2 | 11 ± 1.7 * | 21 ± 1.1 | 19 ± 2.1 |
| % entries OA | 51 ± 3 | 25 ± 4.1 ** | 51 ± 3.4 | 43 ± 2.3 |
| Time CA (s) | 130 ± 13 | 194 ± 11 ** | 123 ± 13 | 133 ± 13 |
| CA entries | 24 ± 2.6 | 34 ± 2.8 * | 20 ± 2.6 | 24 ± 3.5 |
| Total entries | 47 ± 3.1 | 45 ± 2 | 41 ± 2.5 | 43 ± 3 |
| Time center (s) | 67 ± 9.2 | 78 ± 5.1 | 71 ± 4.9 | 64 ± 5.2 |
| Treatment | Behavioral Testing | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PND | 34–47 | 48–67 | 68–69 | 70–72 | 75–79 | 80 | 81 | 82 | 83 |
| Group | SAL | - | SAL | SAL | SAL | TST | |||
| SAL | COCA 5 | COCA 15 | COCA 25 | OF | PPI | OR | |||
| WIN | SAL | SAL | SAL | PPI | OR | EPM | |||
| WIN | COCA 5 | COCA 15 | COCA 25 | ||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, M.A.; Ledesma, J.C.; Rodríguez-Arias, M.; Penalva, C.; Manzanedo, C.; Miñarro, J.; Arenas, M.C. Adolescent Exposure to the Synthetic Cannabinoid WIN 55212-2 Modifies Cocaine Withdrawal Symptoms in Adult Mice. Int. J. Mol. Sci. 2017, 18, 1326. https://doi.org/10.3390/ijms18061326
Aguilar MA, Ledesma JC, Rodríguez-Arias M, Penalva C, Manzanedo C, Miñarro J, Arenas MC. Adolescent Exposure to the Synthetic Cannabinoid WIN 55212-2 Modifies Cocaine Withdrawal Symptoms in Adult Mice. International Journal of Molecular Sciences. 2017; 18(6):1326. https://doi.org/10.3390/ijms18061326
Chicago/Turabian StyleAguilar, María A., Juan Carlos Ledesma, Marta Rodríguez-Arias, Carles Penalva, Carmen Manzanedo, José Miñarro, and M. Carmen Arenas. 2017. "Adolescent Exposure to the Synthetic Cannabinoid WIN 55212-2 Modifies Cocaine Withdrawal Symptoms in Adult Mice" International Journal of Molecular Sciences 18, no. 6: 1326. https://doi.org/10.3390/ijms18061326