Tear Film Steroid Profiling in Dry Eye Disease by Liquid Chromatography Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Sensitivity, Linearity, Imprecision, and Recovery
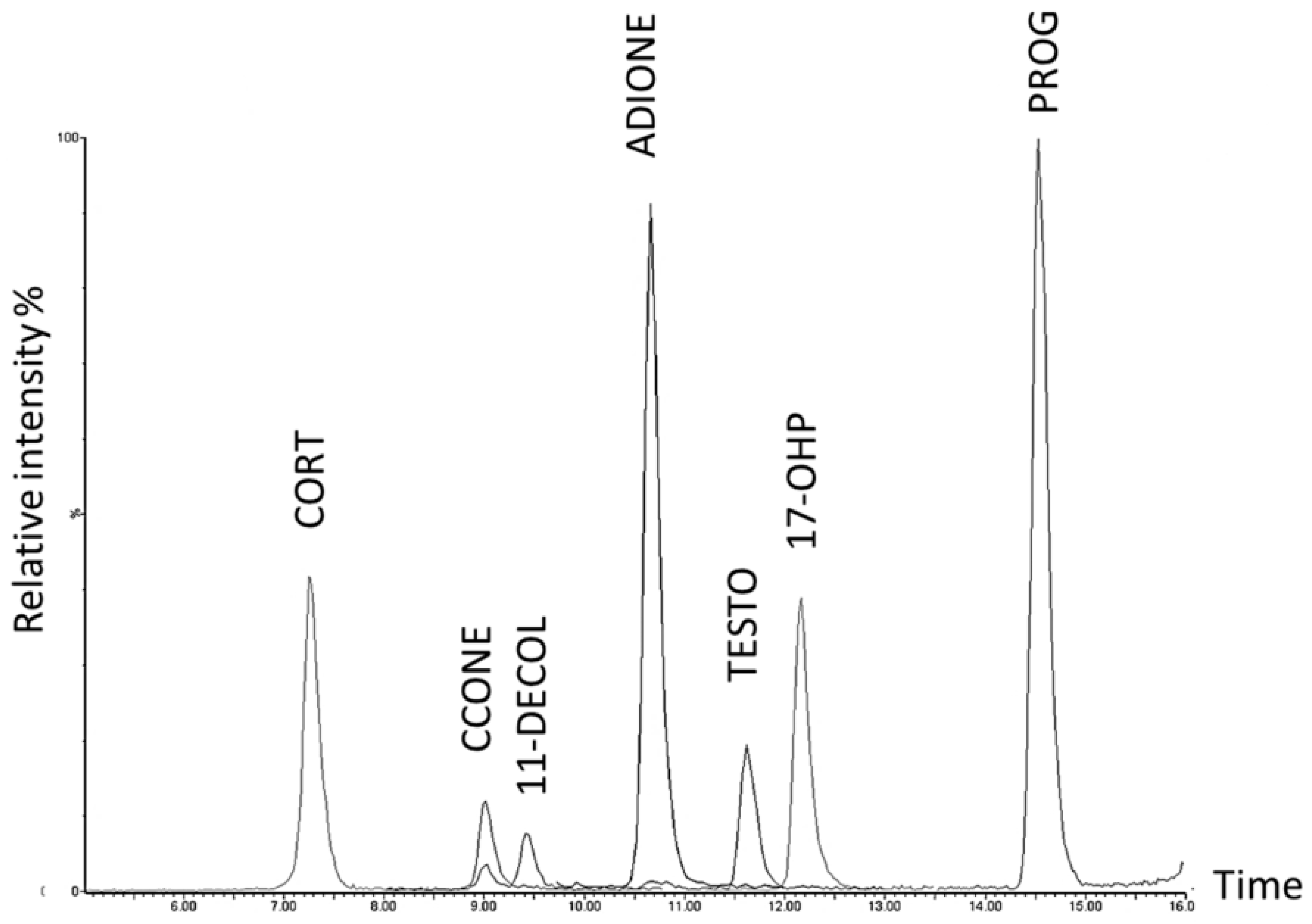
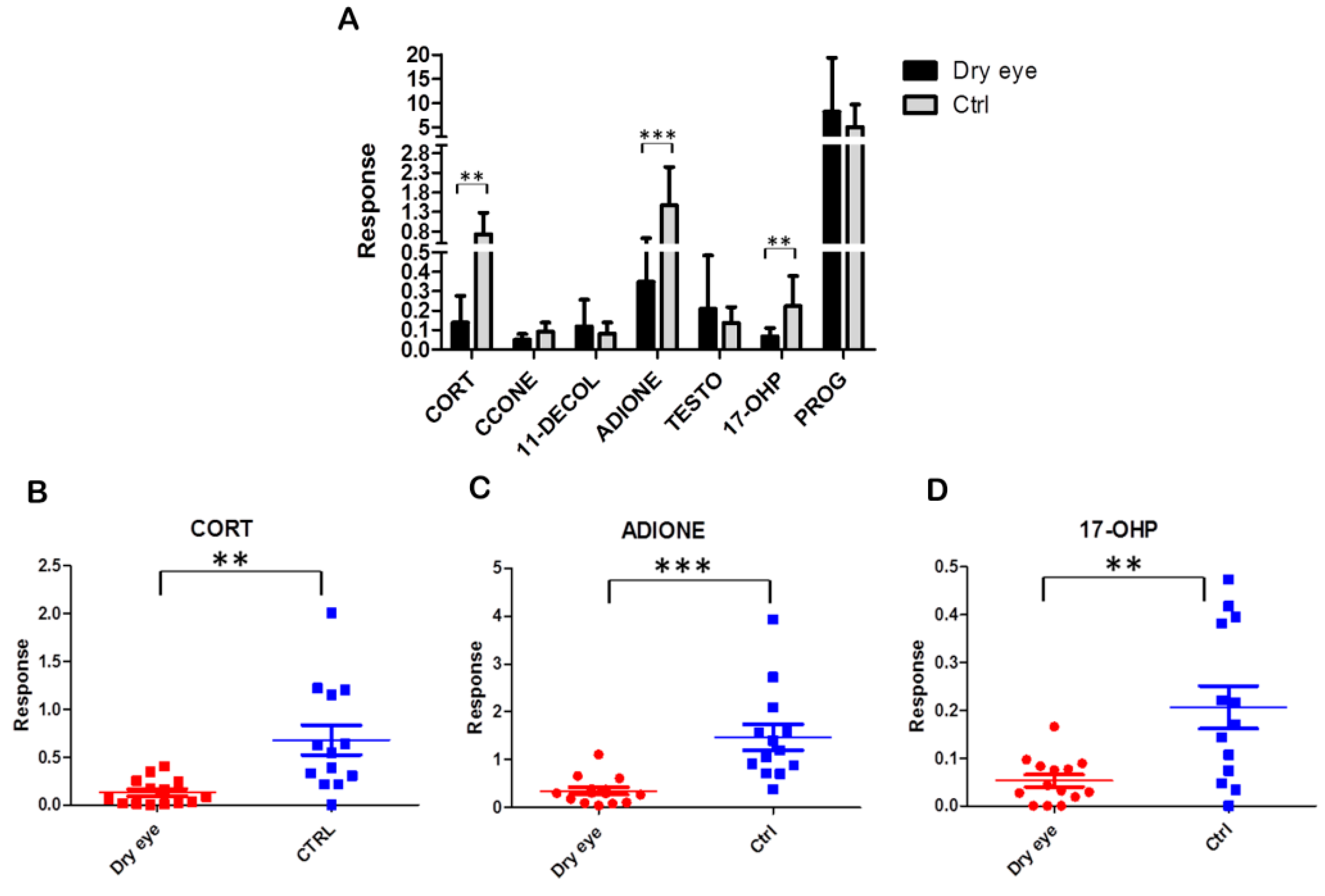
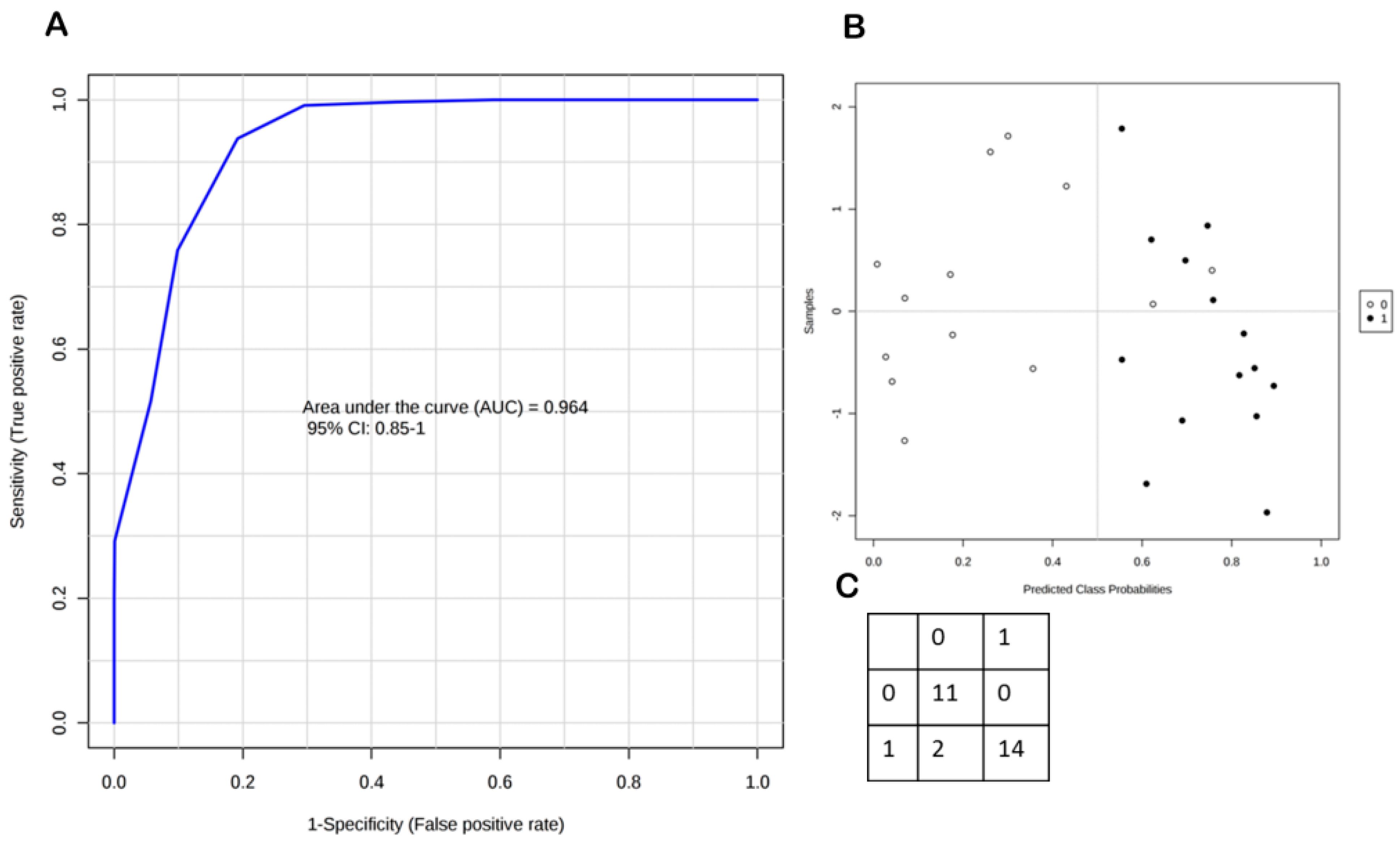
2.2. Steroid Profiling of Dry Eye and Healthy Control Tear Samples by LC-MS/MS Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Samples
4.3. Materials
4.4. Preparation of Standard Solutions, Calibrators and Quality Controls
4.5. Sample Preparation
4.6. LC-MS/MS Analysis
4.7. Quantification
4.8. Assay Performance Characteristics
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, X.; Zhao, P.; Wang, G.; Zhao, X. The effects of estrogen and androgen on tear secretion and matrix metalloproteinase-2 expression in lacrimal glands of ovariectomized rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Anonymous. The epidemiology of dry eye disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 93–107. [Google Scholar]
- Marcozzi, G.; Liberati, V.; Madia, F.; Centofanti, M.; de Feo, G. Age- and gender-related differences in human lacrimal fluid peroxidase activity. Ophthalmologica 2003, 217, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, C.; Caruso, S.; Napolitano, G.; Malaguarnera, G.; Cicinelli, M.V.; Amato, R.; Reibaldi, M.; Incarbone, G.; Bucolo, C.; Drago, F.; et al. Low levels of 17-β-oestradiol, oestrone and testosterone correlate with severe evaporative dysfunctional tear syndrome in postmenopausal women: A case-control study. Br. J. Ophthalmol. 2014, 98, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Mathers, W.D.; Stovall, D.; Lane, J.A.; Zimmerman, M.B.; Johnson, S. Menopause and tear function: The influence of prolactin and sex hormones on human tear production. Cornea 1998, 17, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.A.; Rocha, E.M.; Ullman, M.D.; Krenzer, K.L.; Gao, J.; Toda, I.; Dana, M.R.; Bazzinotti, D.; da Silveira, L.A.; Wickham, L.A. Androgen regulation of the meibomian gland. Adv. Exp. Med. Biol. 1998, 438, 327–331. [Google Scholar] [PubMed]
- Scott, G.; Yiu, S.C.; Wasilewski, D.; Song, J.; Smith, R.E. Combined esterified estrogen and methyltestosterone treatment for dry eye syndrome in postmenopausal women. Am. J. Ophthalmol. 2005, 139, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Wickham, L.A.; da Silveira, L.A.; Krenzer, K.L.; Yu, F.S.; Toda, I.; Sullivan, B.D.; Sullivan, D.A. Identification of androgen receptor protein and 5α-reductase mRNA in human ocular tissues. Br. J. Ophthalmol. 2000, 84, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; Bucci, S.; Agnifili, L.; Fasanella, V.; D’Aguanno, S.; Mastropasqua, A.; Ciancaglini, M.; Mastropasqua, L.; di Ilio, C.; Sacchetta, P.; et al. Differential protein expression in tears of patients with primary open angle and pseudoexfoliative glaucoma. Mol. Biosyst. 2012, 8, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; Agnifili, L.; Fasanella, V.; D’Aguanno, S.; Mastropasqua, R.; di Ilio, C.; Sacchetta, P.; Urbani, A.; del Boccio, P. Shotgun proteomics reveals specific modulated protein patterns in tears of patients with primary open angle glaucoma naive to therapy. Mol. Biosyst. 2013, 9, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Salvisberg, C.; Tajouri, N.; Hainard, A.; Burkhard, P.R.; Lalive, P.H.; Turck, N. Exploring the human tear fluid: Discovery of new biomarkers in multiple sclerosis. Proteom. Clin. Appl. 2014, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Calton, L.; Brown, H.A.; Gillingwater, S.; Wallace, A.M.; Petrucci, F.; Ciavardelli, D.; Urbani, A.; Sacchetta, P.; Morris, M. Confirmation of congenital adrenal hyperplasia by adrenal steroid profiling of filter paper dried blood samples using ultra-performance liquid chromatography-tandem mass spectrometry. Clin. Chem. Lab. Med. 2011, 49, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Sirolli, V.; Rossi, C.; di Castelnuovo, A.; Felaco, P.; Amoroso, L.; Zucchelli, M.; Ciavardelli, D.; di Ilio, C.; Sacchetta, P.; Bernardini, S.; et al. Toward personalized hemodialysis by low molecular weight amino-containing compounds: Future perspective of patient metabolic fingerprint. Blood Transf. Trasf. Sangue 2012, 10, s78–s88. [Google Scholar]
- Pieragostino, D.; D’Alessandro, M.; di Ioia, M.; Rossi, C.; Zucchelli, M.; Urbani, A.; di Ilio, C.; Lugaresi, A.; Sacchetta, P.; del Boccio, P. An integrated metabolomics approach for the research of new cerebrospinal fluid biomarkers of multiple sclerosis. Mol. Biosyst. 2015, 11, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Marzano, V.; Santini, S.; Rossi, C.; Zucchelli, M.; D’Alessandro, A.; Marchetti, C.; Mingardi, M.; Stagni, V.; Barila, D.; Urbani, A. Proteomic profiling of ATM kinase proficient and deficient cell lines upon blockage of proteasome activity. J. Proteom. 2012, 75, 4632–4646. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, C.T.; Bansal, S.; Booth, B.; DeStefano, A.J.; Rose, M.J.; Sailstad, J.; Shah, V.P.; Skelly, J.P.; Swann, P.G.; Weiner, R. Quantitative bioanalytical methods validation and implementation: Best practices for chromatographic and ligand binding assays. Pharm. Res. 2007, 24, 1962–1973. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [PubMed]
- Azcarate, P.M.; Venincasa, V.D.; Feuer, W.; Stanczyk, F.; Schally, A.V.; Galor, A. Androgen deficiency and dry eye syndrome in the aging male. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5046–5053. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Feuer, W.; Lee, D.J.; Florez, H.; Carter, D.; Pouyeh, B.; Prunty, W.J.; Perez, V.L. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am. J. Ophthalmol. 2011, 152, 377–384.e2. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Calton, L.; Hammond, G.; Brown, H.A.; Wallace, A.M.; Sacchetta, P.; Morris, M. Serum steroid profiling for congenital adrenal hyperplasia using liquid chromatography-tandem mass spectrometry. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Vogeser, M.; Seger, C. A decade of HPLC-MS/MS in the routine clinical laboratory—Goals for further developments. Clin. Biochem. 2008, 41, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.C.; Mendu, D.R.; Ramanathan, L.V.; Pessin, M.S.; Carlow, D.C. Sensitive simultaneous quantitation of testosterone and estradiol in serum by LC-MS/MS without derivatization and comparison with the CDC HoSt program. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1048, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Heussner, K.; Rauh, M.; Cordasic, N.; Menendez-Castro, C.; Huebner, H.; Ruebner, M.; Schmidt, M.; Hartner, A.; Rascher, W.; Fahlbusch, F.B. Adhesive blood microsampling systems for steroid measurement via LC-MS/MS in the rat. Steroids 2017, 120, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mezzullo, M.; Fazzini, A.; Gambineri, A.; di Dalmazi, G.; Mazza, R.; Pelusi, C.; Vicennati, V.; Pasquali, R.; Pagotto, U.; Fanelli, F. Parallel diurnal fluctuation of testosterone, androstenedione, dehydroepiandrosterone and 17OHprogesterone as assessed in serum and saliva: Validation of a novel liquid chromatography-tandem mass spectrometry method for salivary steroid profiling. Clin. Chem. Lab. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Naldi, A.C.; Fayad, P.B.; Prevost, M.; Sauve, S. Analysis of steroid hormones and their conjugated forms in water and urine by on-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry. Chem. Cent. J. 2016, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Mathian, B.; Millot, F.; Patricot, M.C.; Mathieu, E.; Queyrel, N.; Lacroix, I.; Somma-Delpero, C.; Boudou, P. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin. Chem. 2003, 49, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Hysi, P.G.; Hammond, C.J. A Metabolome-Wide Study of Dry Eye Disease Reveals Serum Androgens as Biomarkers. Ophthalmology 2017, 124, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, A.; Valentini, G.; di Martino, G.; DaPonte, A.; de Bellis, A.; Iacono, G. Influence of testosterone therapy on clinical and immunological features of autoimmune diseases associated with Klinefelter’s syndrome. J. Clin. Endocrinol. Metab. 1987, 64, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Truong, S.; Cole, N.; Stapleton, F.; Golebiowski, B. Sex hormones and the dry eye. Clin. Exp. Optom. 2014, 97, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.A.; Sullivan, B.D.; Evans, J.E.; Schirra, F.; Yamagami, H.; Liu, M.; Richards, S.M.; Suzuki, T.; Schaumberg, D.A.; Sullivan, R.M.; et al. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann. N. Y. Acad. Sci. 2002, 966, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Kunicki, M.; Radowicki, S. Androgen and 17-hydroxyprogesterone concentrations in blood serum versus menstrual patterns in women with polycystic ovary syndrome (PCOS). Ginekol. Polska 2010, 81, 745–749. [Google Scholar]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
| Analyte | Concentration Range (ng/mL) | Calibration Function (y = b1(±SD)x + b0(±SD)) a,b | R2 |
|---|---|---|---|
| CORT | 0.3–20.08 | y = 0.396(±0.04)x + 0.026(±0.160) | 0.996 |
| 11-DECOL | 0.05–1.00 | y = 3.041(±0.942)x + 0.077(±0.052) | 0.994 |
| ADIONE | 0.05–2.91 | y = 9.492(±0.584)x + 0.204(±0.054) | 0.994 |
| TESTO | 0.02–1.00 | y = 9.276 (±2.446)x + 0.171(±0.103) | 0.993 |
| 17-OHP | 0.05–4.20 | y = 4.372 (±1.218)x − 0.053(±0.045) | 0.993 |
| PROG | 0.05–4.20 | y = 11.015 (±1.789)x + 0.279(±0.182) | 0.994 |
| Intra-Assay | Inter-Assay | Recovery % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | QC Level | Mean Response | SD | %CV | Mean Response | SD | %CV | Mean | SD |
| CORT | QC1 | 0.13 | 0.02 | 13.47 | 0.14 | 0.01 | 8.43 | 50.12 | 13.18 |
| QC2 | 0.52 | 0.01 | 2.35 | 0.52 | 0.03 | 5.12 | 33.12 | 0.73 | |
| QC3 | 2.88 | 0.15 | 5.09 | 2.57 | 0.13 | 5.14 | 44.29 | 1.98 | |
| 11-DECOL | QC1 | 0.27 | 0.02 | 7.97 | 0.15 | 0.02 | 12.41 | 46.99 | 6.64 |
| QC2 | 0.67 | 0.01 | 1.63 | 0.72 | 0.06 | 7.83 | 67.45 | 6.67 | |
| QC3 | 2.19 | 0.11 | 4.98 | 2.26 | 0.21 | 9.48 | 58.18 | 2.83 | |
| ADIONE | QC1 | 0.83 | 0.04 | 4.99 | 0.99 | 0.03 | 3.15 | 23.26 | 7.16 |
| QC2 | 2.14 | 0.10 | 4.77 | 2.30 | 0.15 | 6.72 | 22.01 | 2.26 | |
| QC3 | 11.99 | 0.13 | 1.12 | 11.85 | 0.32 | 2.73 | 24.53 | 2.23 | |
| TESTO | QC1 | 0.67 | 0.09 | 13.82 | 0.56 | 0.05 | 8.77 | 19.50 | 2.92 |
| QC2 | 2.14 | 0.31 | 14.67 | 1.92 | 0.09 | 4.51 | 37.46 | 0.48 | |
| QC3 | 6.39 | 0.73 | 11.49 | 5.29 | 0.74 | 13.94 | 44.85 | 5.91 | |
| 17-OHP | QC1 | 0.21 | 0.03 | 15.24 | 0.35 | 0.04 | 10.84 | 22.78 | 9.92 |
| QC2 | 0.78 | 0.07 | 8.86 | 1.04 | 0.06 | 5.99 | 30.61 | 6.49 | |
| QC3 | 6.25 | 0.17 | 2.77 | 6.76 | 0.62 | 9.15 | 50.64 | 2.60 | |
| PROG | QC1 | 0.83 | 0.03 | 3.20 | 1.01 | 0.07 | 6.90 | 39.56 | 4.31 |
| QC2 | 2.26 | 0.21 | 9.54 | 2.95 | 0.19 | 6.70 | 30.60 | 1.92 | |
| QC3 | 13.92 | 0.10 | 0.72 | 14.77 | 1.22 | 8.30 | 40.43 | 7.50 | |
| Analyte | Calibration Levels (ng/mL) | QC Levels (ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | L5 | L6 | L7 | QC1 | QC2 | QC3 | |
| CORT | 0.30 | 0.50 | 1.00 | 1.51 | 3.61 | 8.66 | 20.80 | 0.50 | 1.51 | 8.66 |
| CCONE | 0.05 | 0.10 | 0.20 | 0.29 | 0.70 | 1.68 | 4.03 | 0.10 | 0.29 | 1.68 |
| 11-DECOL | 0.05 | 0.07 | 0.10 | 0.20 | 0.40 | 0.70 | 1.00 | 0.07 | 0.20 | 0.70 |
| ADIONE | 0.05 | 0.08 | 0.10 | 0.20 | 0.49 | 1.17 | 2.91 | 0.08 | 0.20 | 1.17 |
| TESTO | 0.02 | 0.05 | 0.08 | 0.15 | 0.30 | 0.60 | 1.00 | 0.05 | 0.15 | 0.60 |
| 17-OHP | 0.05 | 0.08 | 0.12 | 0.29 | 0.70 | 1.69 | 4.20 | 0.08 | 0.29 | 1.69 |
| PROG | 0.05 | 0.08 | 0.12 | 0.29 | 0.70 | 1.69 | 4.20 | 0.08 | 0.29 | 1.69 |
| MRM Function | Time Window (min) | Analyte | Transitions (m/z) | Cone Volts | Coll Energy (eV) |
|---|---|---|---|---|---|
| 1 | 5.0–8.5 | CORT | 363.2 > 120.8 | 100 | 20 |
| CORT | 363.2 > 96.9 | 100 | 20 | ||
| 2H3-CORT | 366.2 > 120.8 | 100 | 20 | ||
| 2 | 8.0–10.5 | CCONE | 347.3 > 120.8 | 100 | 18 |
| CCONE | 347.3 > 96.8 | 100 | 20 | ||
| 2H8-CCONE | 355.3 > 124.8 | 100 | 18 | ||
| 3 | 8.0–10.8 | 11-DECOL | 347.3 > 108.8 | 100 | 22 |
| 11-DECOL | 347.3 > 96.8 | 100 | 20 | ||
| 2H5-11-DECOL | 352.3 > 112.8 | 100 | 22 | ||
| 4 | 9.7–12.0 | ADIONE | 287.2 > 96.9 | 100 | 18 |
| ADIONE | 287.2 > 108.8 | 100 | 20 | ||
| 2H5-ADIONE | 292.2 > 99.9 | 100 | 18 | ||
| 5 | 10.5–13.0 | TESTO | 289.2 > 96.8 | 100 | 19 |
| TESTO | 289.2 > 108.8 | 100 | 18 | ||
| 2H5-TESTO | 294.2 > 99.8 | 100 | 19 | ||
| 6 | 11.2–13.5 | 17-OHP | 331.2 > 96.8 | 100 | 21 |
| 17-OHP | 331.2 > 108.9 | 100 | 23 | ||
| 2H8-OHP | 339.2 > 99.8 | 100 | 21 | ||
| 7 | 13.5–16.0 | PROG | 315.2 > 96.9 | 100 | 18 |
| PROG | 315.2 > 108.8 | 100 | 19 | ||
| 2H9-PROG | 324.2 > 99.9 | 100 | 18 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieragostino, D.; Agnifili, L.; Cicalini, I.; Calienno, R.; Zucchelli, M.; Mastropasqua, L.; Sacchetta, P.; Del Boccio, P.; Rossi, C. Tear Film Steroid Profiling in Dry Eye Disease by Liquid Chromatography Tandem Mass Spectrometry. Int. J. Mol. Sci. 2017, 18, 1349. https://doi.org/10.3390/ijms18071349
Pieragostino D, Agnifili L, Cicalini I, Calienno R, Zucchelli M, Mastropasqua L, Sacchetta P, Del Boccio P, Rossi C. Tear Film Steroid Profiling in Dry Eye Disease by Liquid Chromatography Tandem Mass Spectrometry. International Journal of Molecular Sciences. 2017; 18(7):1349. https://doi.org/10.3390/ijms18071349
Chicago/Turabian StylePieragostino, Damiana, Luca Agnifili, Ilaria Cicalini, Roberta Calienno, Mirco Zucchelli, Leonardo Mastropasqua, Paolo Sacchetta, Piero Del Boccio, and Claudia Rossi. 2017. "Tear Film Steroid Profiling in Dry Eye Disease by Liquid Chromatography Tandem Mass Spectrometry" International Journal of Molecular Sciences 18, no. 7: 1349. https://doi.org/10.3390/ijms18071349