The Co-Expression Pattern of p63 and HDAC1: A Potential Way to Disclose Stem Cells in Interfollicular Epidermis
Abstract
:1. Introduction
2. Results
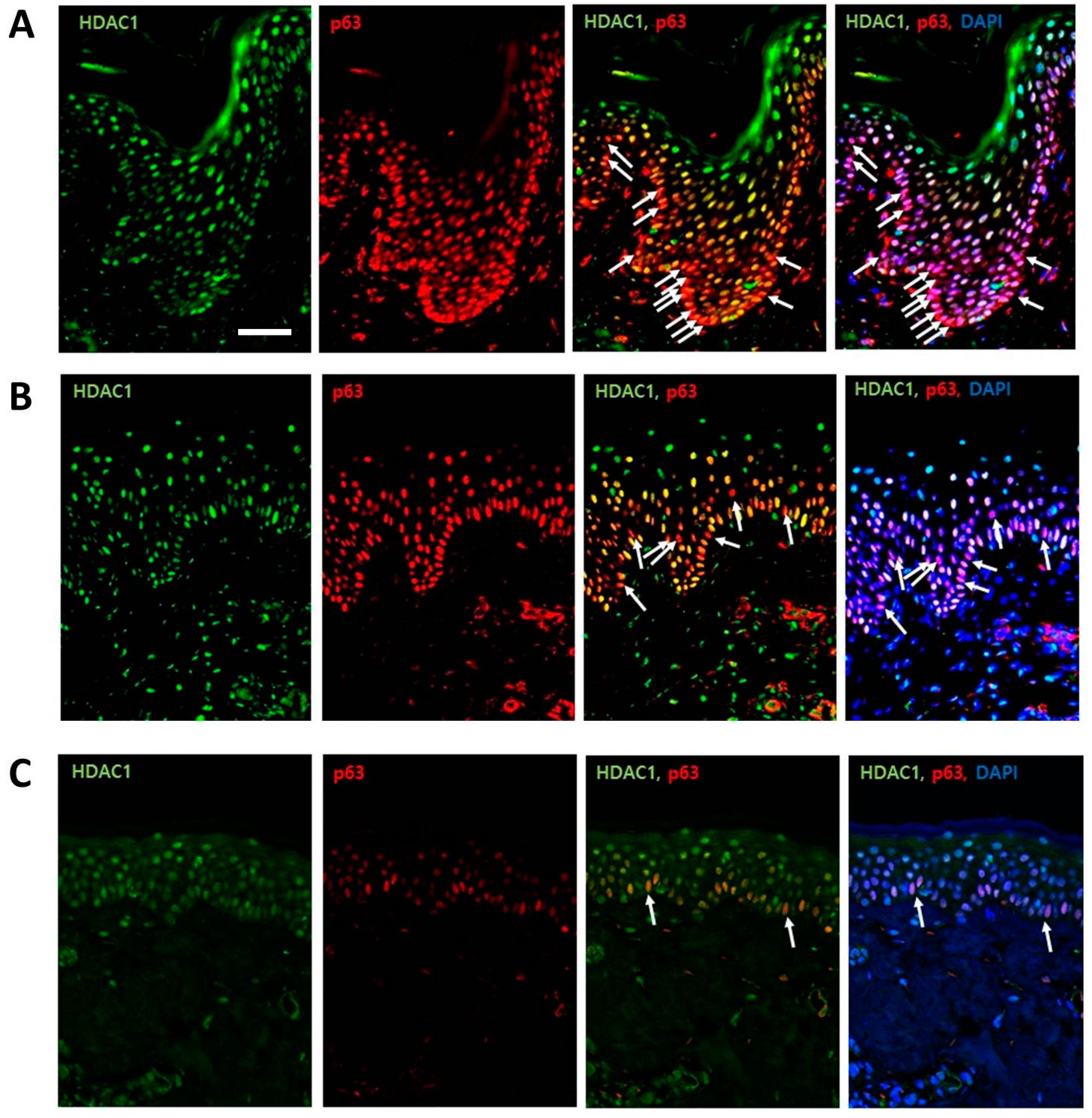
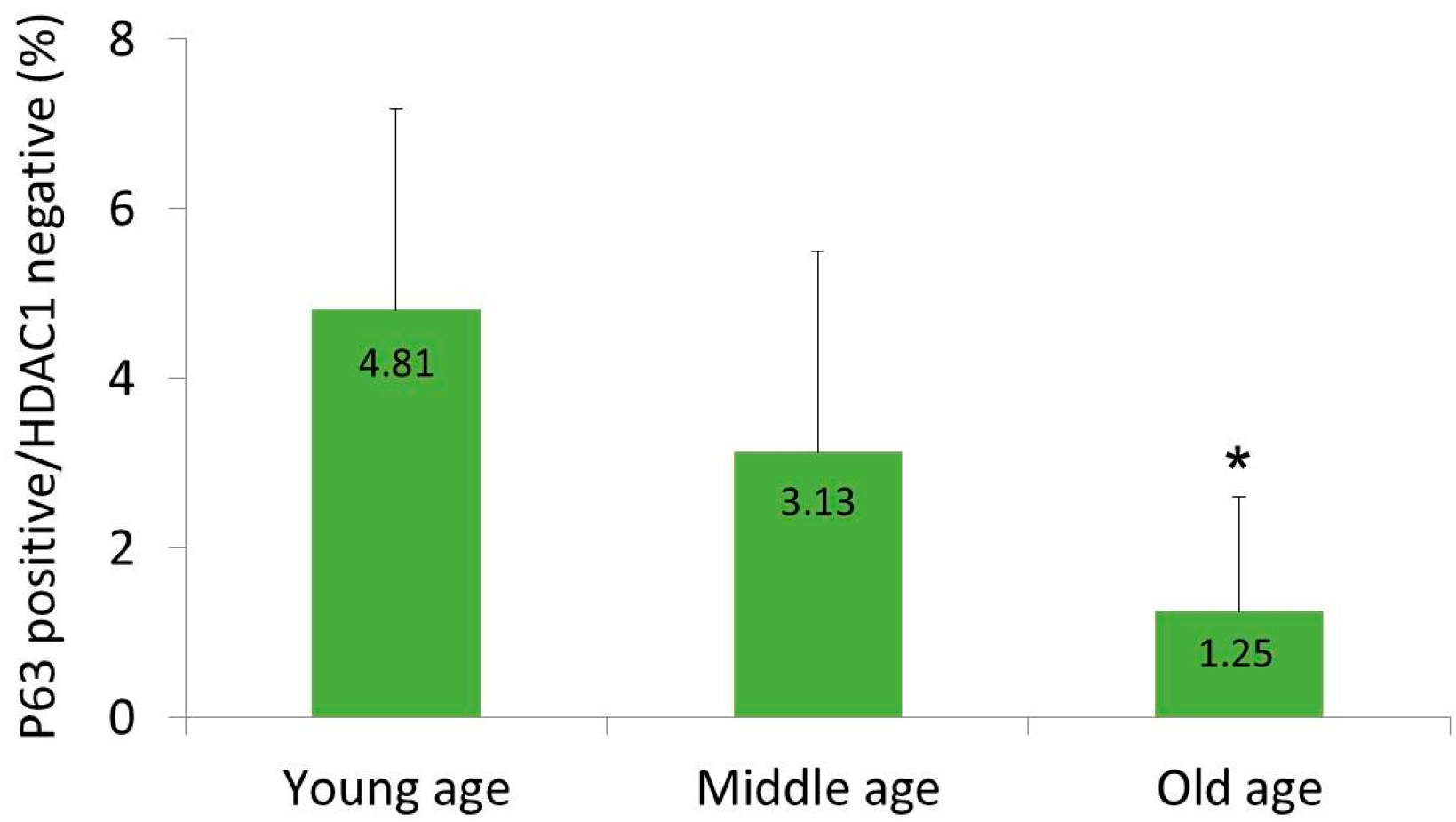
2.1. HDAC1 and p63 Expressions According in Normal Human Skins in Different Age Groups
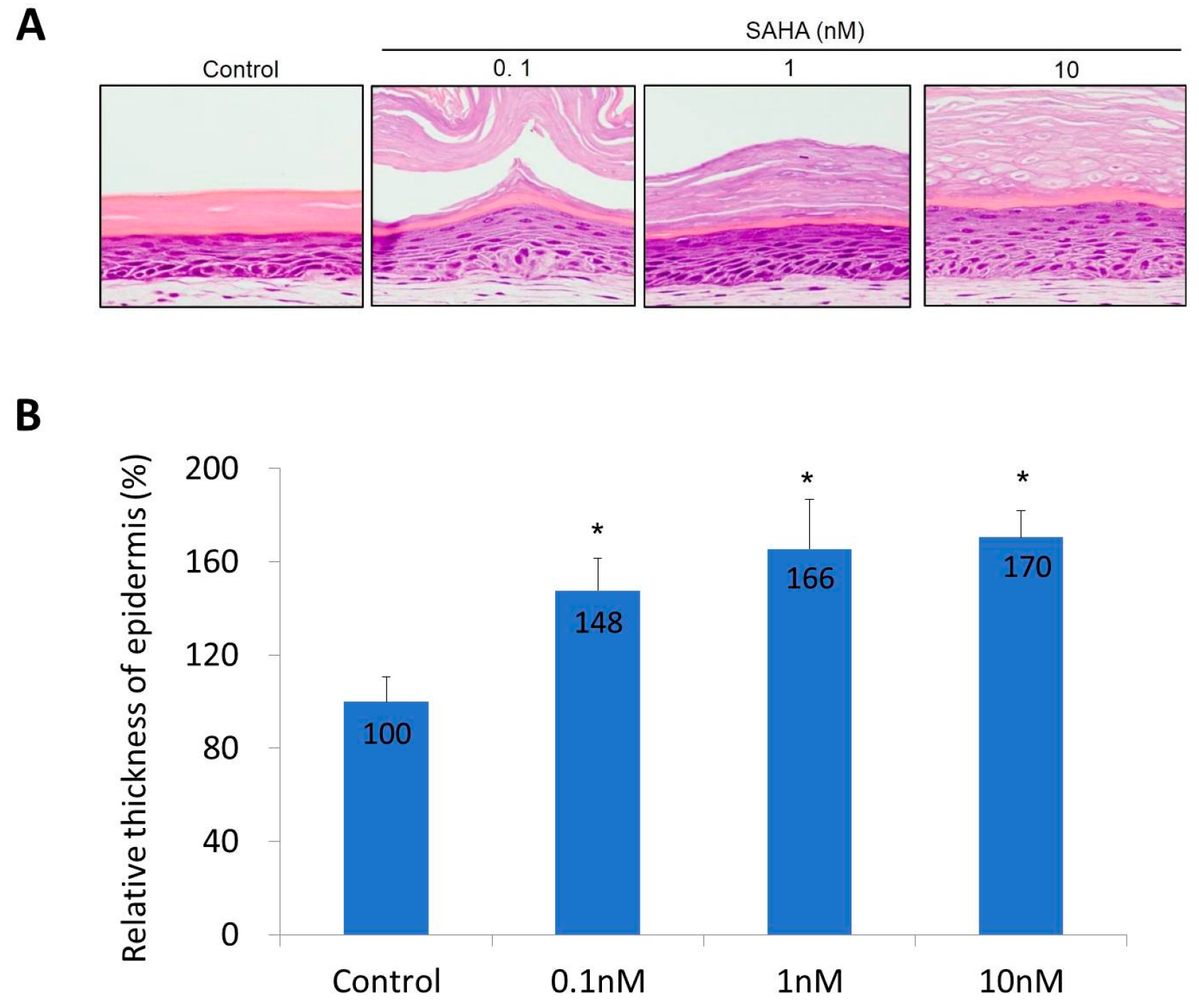
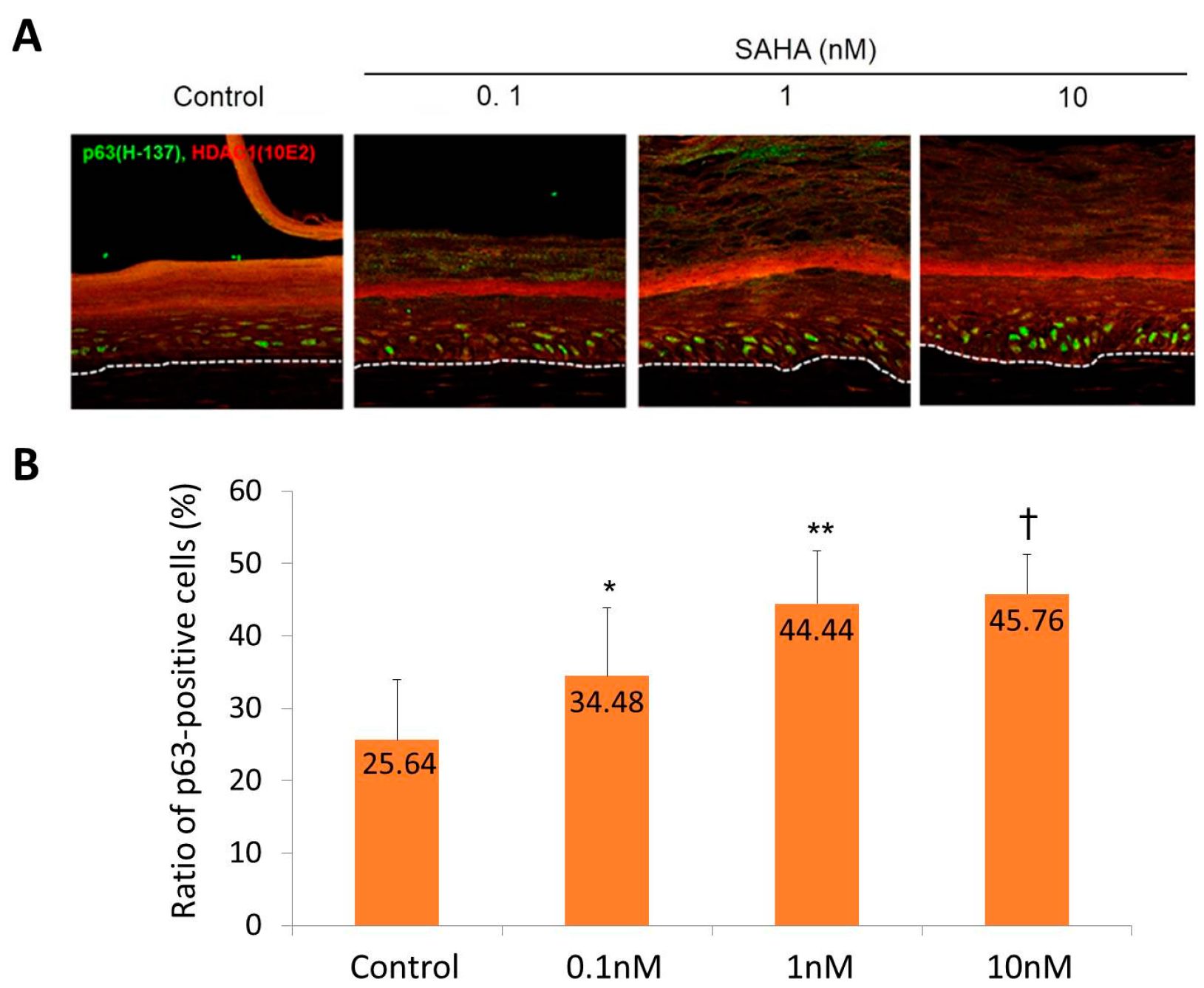
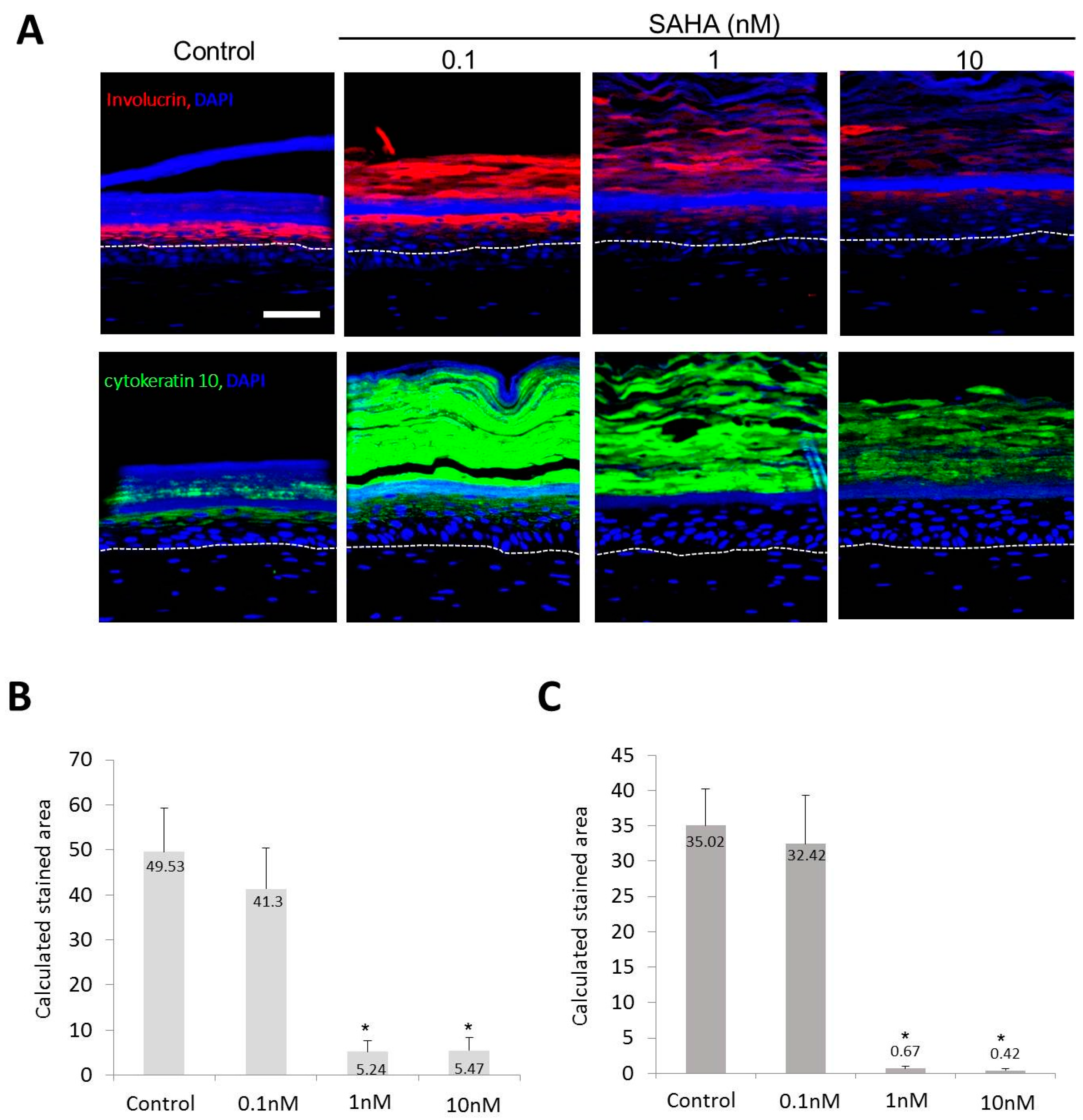
2.2. Reconstruction of the Skin Equivalent and the Effect of Suberoylanilohydroxamic Acid
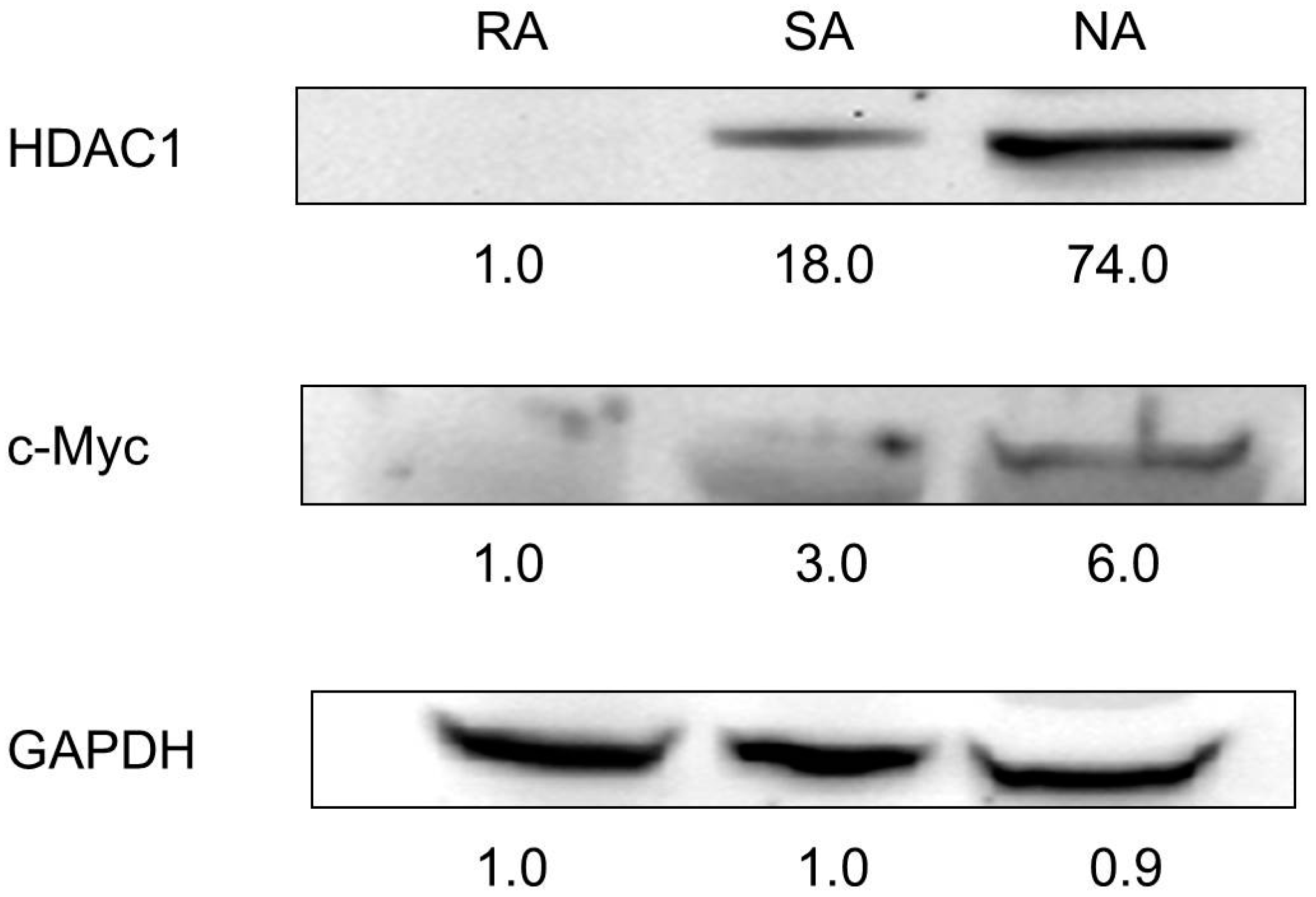
2.3. HDAC1 Expression in Rapidly Adhering Keratinocytes to Type IV Collagen
3. Discussion
4. Experimental Section
4.1. Human Samples
4.2. Immunohistochemical Staining of Human Samples
4.3. Cell Culture
4.4. Culture of Skin Equivalent and SAHA Treatment
4.5. Immunohistochemistry of Skin Equivalent (SE) and Image Analysis
4.6. Isolation of Primary Epidermal Cells
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boehnke, K.; Falkowska-Hansen, B.; Stark, H.J.; Boukamp, P. Stem cells of the human epidermis and their niche: Composition and function in epidermal regeneration and carcinogenesis. Carcinogenesis 2012, 33, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.M.; Prowse, D.M. Distinct epidermal stem cell compartments are maintained by independent niche microenvironments. Stem Cell Rev. 2006, 2, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in wound healing: A comprehensive review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Lo Celso, C.; Silva-Vargas, V. Epidermal stem cells: An update. Curr. Opin. Genet. Dev. 2006, 16, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E. Skin stem cells: Rising to the surface. J. Cell Biol. 2008, 180, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Levy, V.; Lindon, C.; Harfe, B.D.; Morgan, B.A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell 2005, 9, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Liu, Y.; Yang, Z.; Nguyen, J.; Liang, F.; Morris, R.J.; Cotsarelis, G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005, 11, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Byun, S.Y.; Kwon, S.H.; Park, K.C. Niche interactions in epidermal stem cells. World J. Stem Cells 2015, 7, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Jensen, K.B. Epidermal stem cell diversity and quiescence. EMBO Mol. Med. 2009, 1, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Fuchs, E. Stem cells of the skin epithelium. Proc. Natl. Acad. Sci. USA 2003, 100, 11830–11835. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Fuchs, E. Stem cells in the skin: Waste not, Wnt not. Genes Dev. 2003, 17, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Janes, S.M.; Lowell, S.; Hutter, C. Epidermal stem cells. J. Pathol. 2002, 197, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Mascre, G.; Dekoninck, S.; Drogat, B.; Youssef, K.K.; Brohee, S.; Sotiropoulou, P.A.; Simons, B.D.; Blanpain, C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012, 489, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Garza, L.A.; Yang, C.C.; Zhao, T.; Blatt, H.B.; Lee, M.; He, H.; Stanton, D.C.; Carrasco, L.; Spiegel, J.H.; Tobias, J.W.; et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J. Clin. Investig. 2011, 121, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Terunuma, A.; Tock, C.L.; Radonovich, M.F.; Pise-Masison, C.A.; Hopping, S.B.; Brady, J.N.; Udey, M.C.; Vogel, J.C. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J. Clin. Investig. 2006, 116, 249–260. [Google Scholar] [CrossRef] [PubMed]
- LeBoeuf, M.; Terrell, A.; Trivedi, S.; Sinha, S.; Epstein, J.A.; Olson, E.N.; Morrisey, E.E.; Millar, S.E. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev. Cell 2010, 19, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Koster, M.I.; Kim, S.; Roop, D.R. P63 deficiency: A failure of lineage commitment or stem cell maintenance? J. Investig. Dermatol. Symp. Proc. 2005, 10, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Lechler, T.; Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005, 437, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; de Luca, M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef] [PubMed]
- Candi, E.; Amelio, I.; Agostini, M.; Melino, G. MicroRNAs and p63 in epithelial stemness. Cell Death Differ. 2015, 22, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhang, T.; Qiao, L.; Du, J.; Li, S.; Zhao, H.; Wang, F.; Huang, Q.; Meng, W.; Zhu, H.; et al. TLR7-expressing cells comprise an interfollicular epidermal stem cell population in murine epidermis. Sci. Rep. 2014, 4, 5831. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Morris, R.J.; Kaur, P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. USA 2000, 97, 10960–10965. [Google Scholar] [CrossRef] [PubMed]
- Ruthenburg, A.J.; Li, H.; Patel, D.J.; Allis, C.D. Multivalent engagement of chromatin modifications by linked binding modules. Nat. Rev. Mol. Cell Biol. 2007, 8, 983–994. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.J.; Pajak, A.; Balazsi, K.; Dagnino, L. Ca2+ and BMP-6 signaling regulate E2F during epidermal keratinocyte differentiation. J. Biol. Chem. 2001, 276, 23531–23538. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Cho, H.J.; Choi, H.R.; Kwon, S.B.; Park, K.C. Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell. Mol. Life Sci. 2004, 61, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Laurikkala, J.; Mikkola, M.L.; James, M.; Tummers, M.; Mills, A.A.; Thesleff, I. p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development 2006, 133, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mahendran, A.; Yao, Y.; Ngo, L.; Venta-Perez, G.; Choy, M.L.; Kim, N.; Ham, W.S.; Breslow, R.; Marks, P.A. Development of a histone deacetylase 6 inhibitor and its biological effects. Proc. Natl. Acad. Sci. USA 2013, 110, 15704–15709. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Harper, S.; Watt, F.M. Stem cell patterning and fate in human epidermis. Cell 1995, 80, 83–93. [Google Scholar] [CrossRef]
- Stingl, J.; Eirew, P.; Ricketson, I.; Shackleton, M.; Vaillant, F.; Choi, D.; Li, H.I.; Eaves, C.J. Purification and unique properties of mammary epithelial stem cells. Nature 2006, 439, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Wagers, A.J.; Weissman, I.L. Differential expression of α2 integrin separates long-term and short-term reconstituting Lin-/loThy1.1loc-kit+ Sca-1+ hematopoietic stem cells. Stem Cells 2006, 24, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Calenic, B.; Greabu, M.; Caruntu, C.; Tanase, C.; Battino, M. Oral keratinocyte stem/progenitor cells: Specific markers, molecular signaling pathways and potential uses. Periodontology 2000 2015, 69, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Dokmanovic, M.; Clarke, C.; Marks, P.A. Histone deacetylase inhibitors: Overview and perspectives. Mol. Cancer Res. 2007, 5, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M. The stem cell compartment in human interfollicular epidermis. J. Dermatol. Sci. 2002, 28, 173–180. [Google Scholar] [CrossRef]
- Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential In Vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 1274–1278. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-W.; Choi, H.-R.; Nam, K.-M.; Lee, H.-S.; Kim, S.-A.; Joe, H.-J.; Kazumi, T.; Park, K.-C. The Co-Expression Pattern of p63 and HDAC1: A Potential Way to Disclose Stem Cells in Interfollicular Epidermis. Int. J. Mol. Sci. 2017, 18, 1360. https://doi.org/10.3390/ijms18071360
Shin J-W, Choi H-R, Nam K-M, Lee H-S, Kim S-A, Joe H-J, Kazumi T, Park K-C. The Co-Expression Pattern of p63 and HDAC1: A Potential Way to Disclose Stem Cells in Interfollicular Epidermis. International Journal of Molecular Sciences. 2017; 18(7):1360. https://doi.org/10.3390/ijms18071360
Chicago/Turabian StyleShin, Jung-Won, Hye-Ryung Choi, Kyung-Mi Nam, Hyun-Sun Lee, Sung-Ae Kim, Hyun-Jae Joe, Toyama Kazumi, and Kyoung-Chan Park. 2017. "The Co-Expression Pattern of p63 and HDAC1: A Potential Way to Disclose Stem Cells in Interfollicular Epidermis" International Journal of Molecular Sciences 18, no. 7: 1360. https://doi.org/10.3390/ijms18071360



