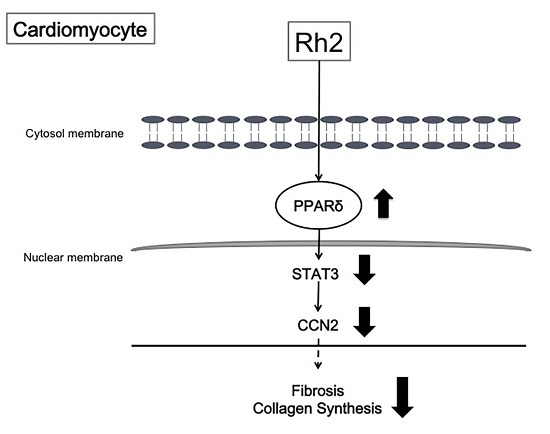
Ginsenoside Rh2 Improves Cardiac Fibrosis via PPARδ–STAT3 Signaling in Type 1-Like Diabetic Rats
Abstract
:1. Introduction
2. Results
2.1. Changes in Blood Glucose Levels and Cardiac Fibrotic Parameters in Rats
2.2. Changes in Heart Tissues
2.3. Effect of Rh2 on Cardiac Performance in Anesthetized Diabetic Rats
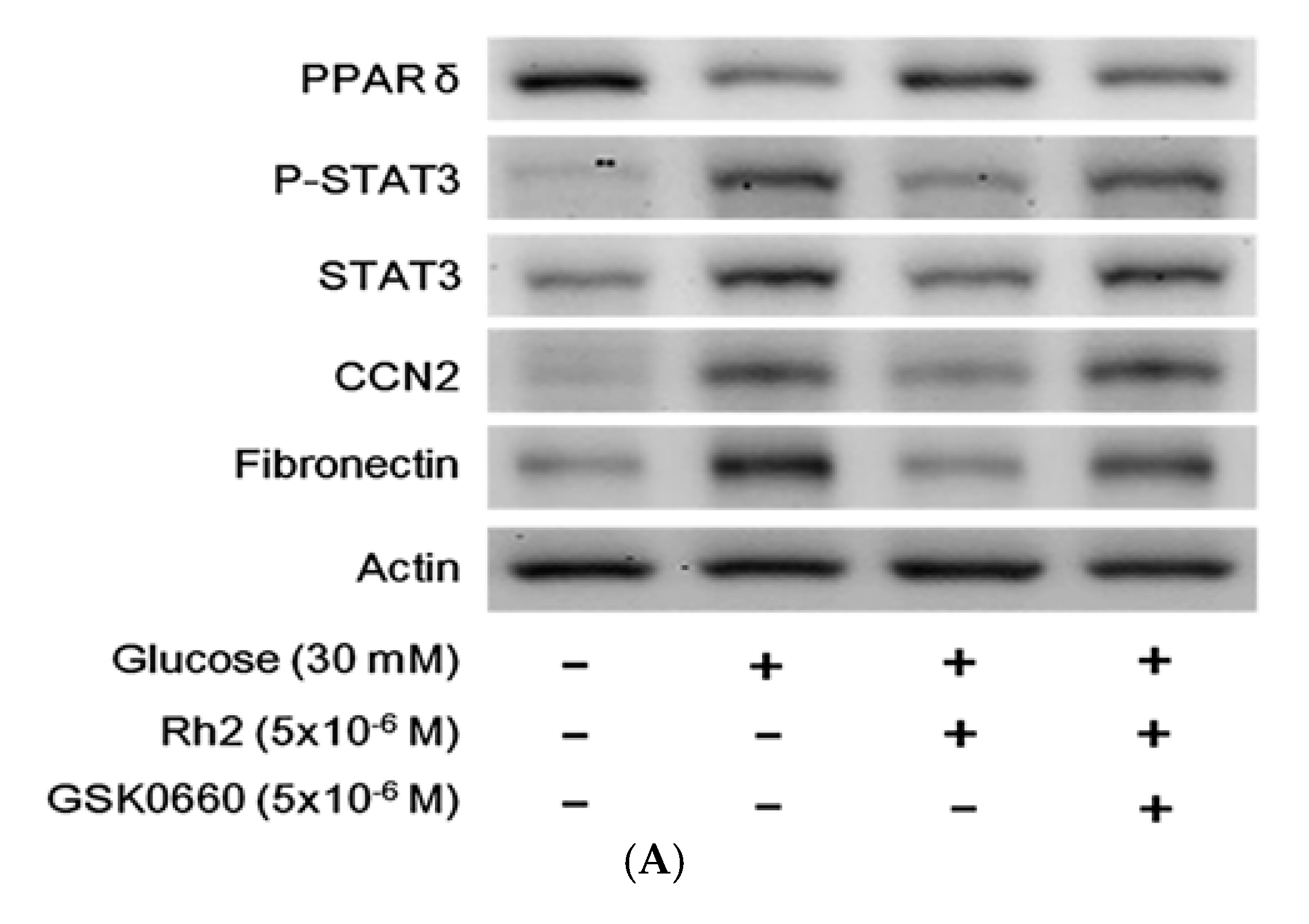
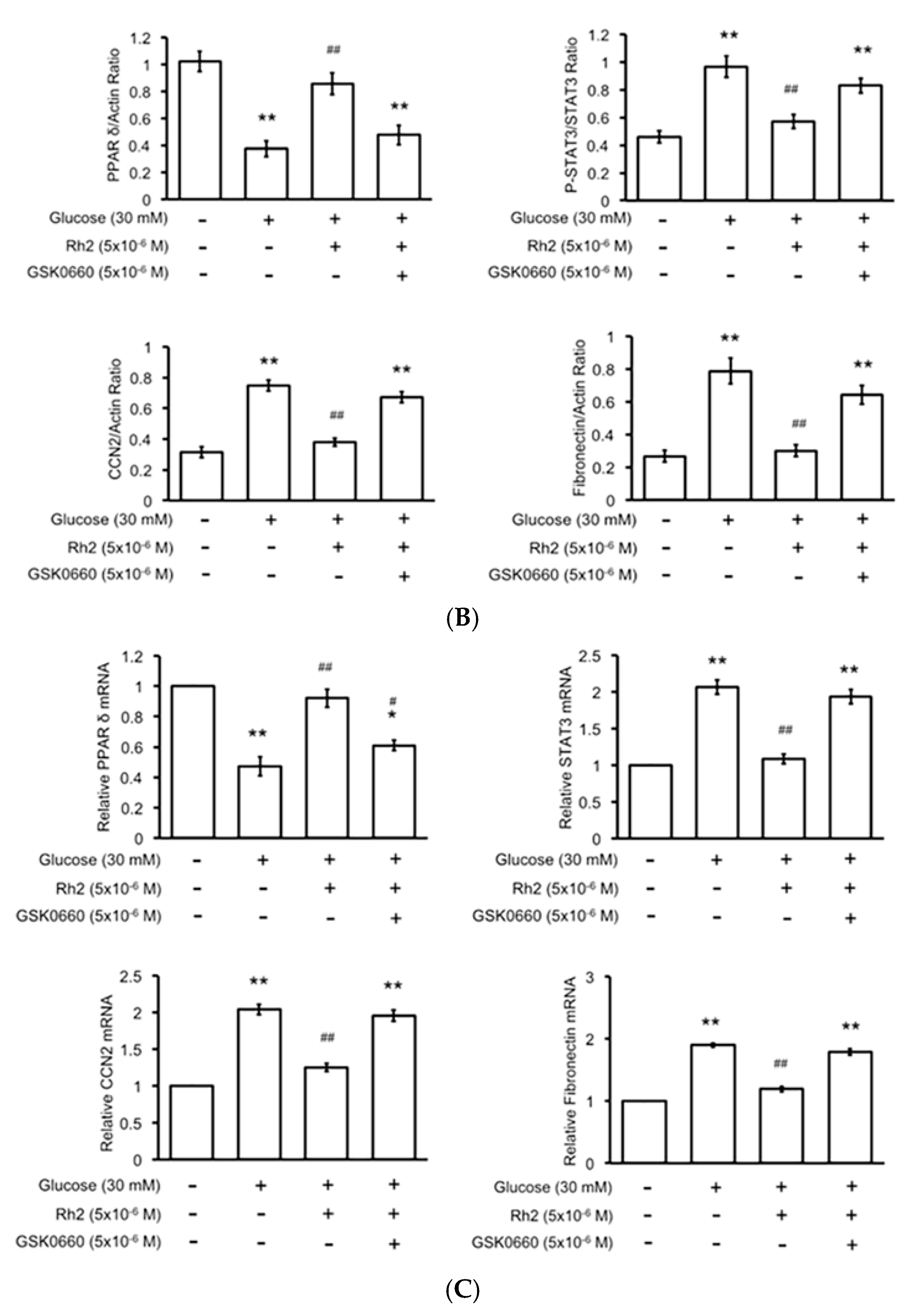
2.4. Effects of Rh2 on Expression Levels of PPAR and Fibrotic Signals in Streptozotocin-Induced Type-1 Diabetic Rat (STZ-Diabetic Rat) Hearts
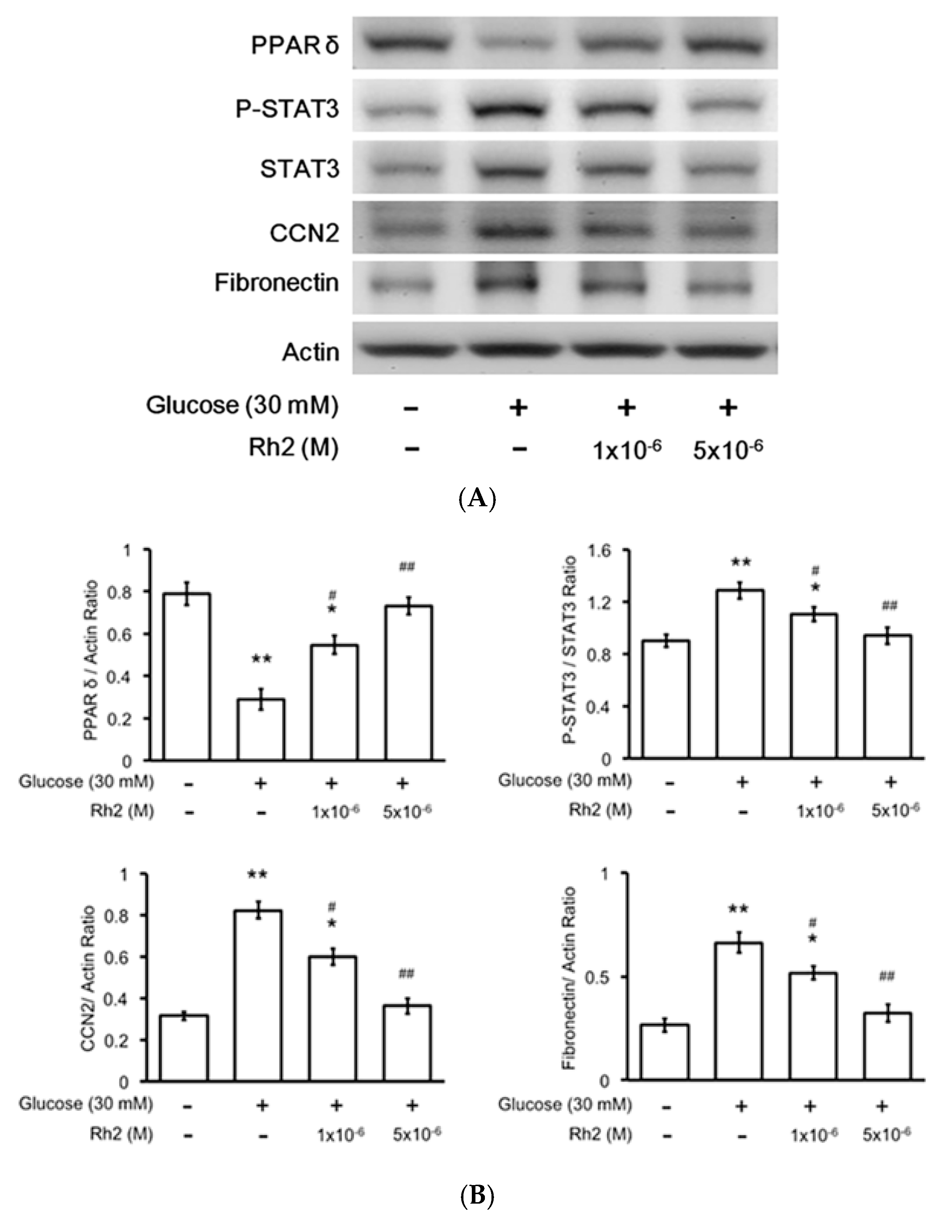
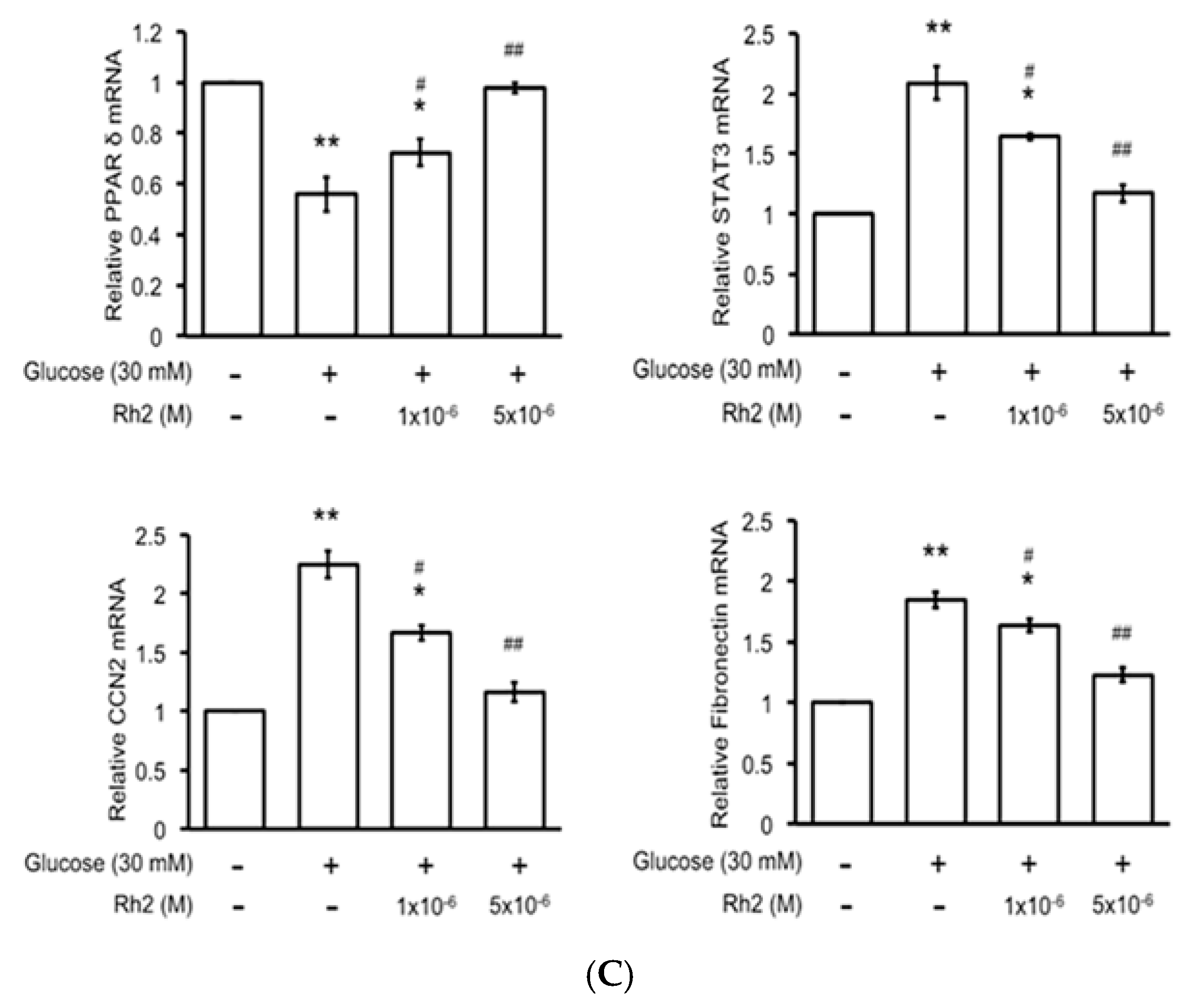
2.5. Changes in Expression Levels of Peroxisome Proliferator-Activated Receptor (PPAR) and Fibrotic Signals by Hyperglycemia Are Reversed by Rh2 in H9c2 Cardiomyocytes
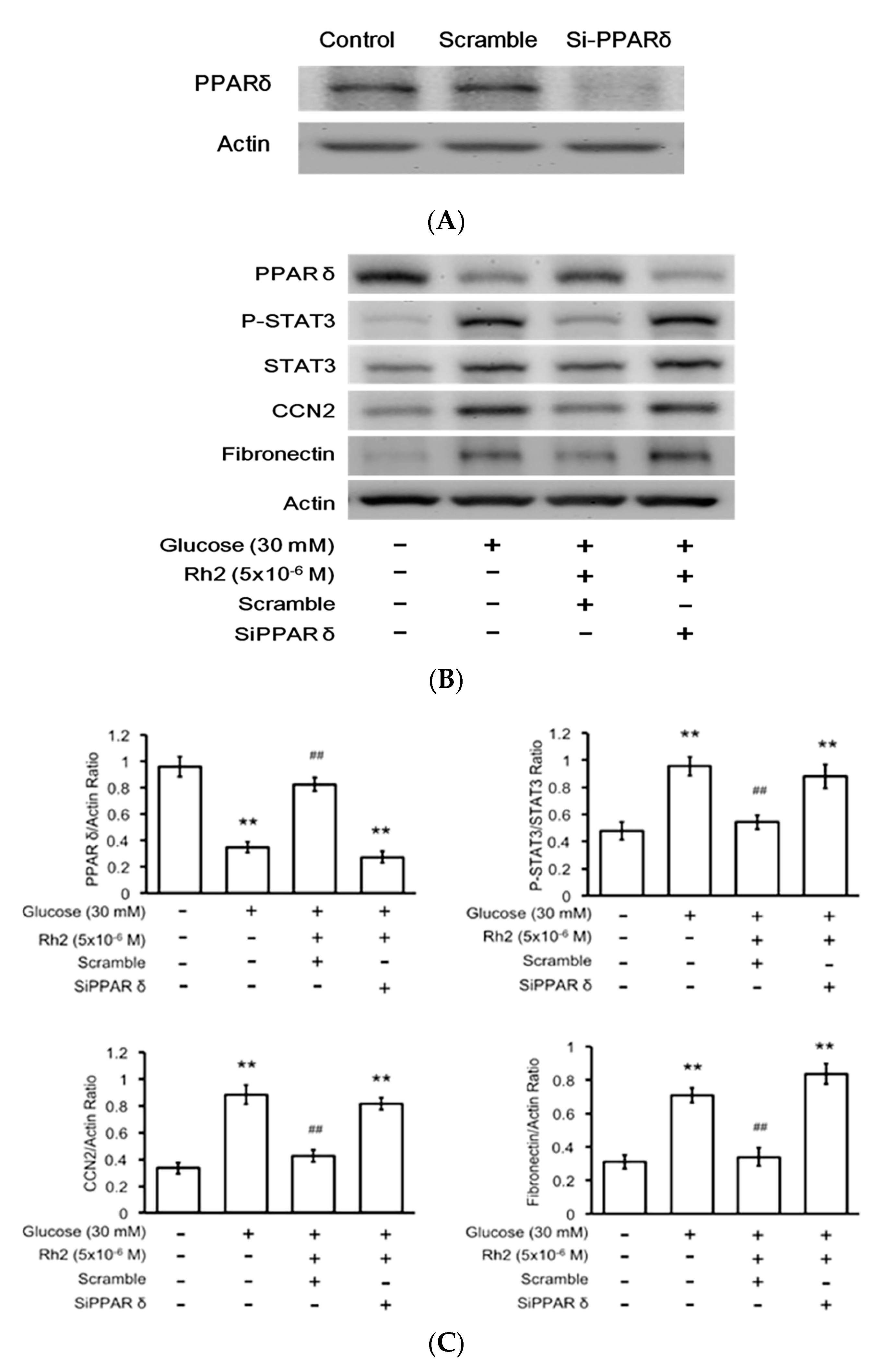
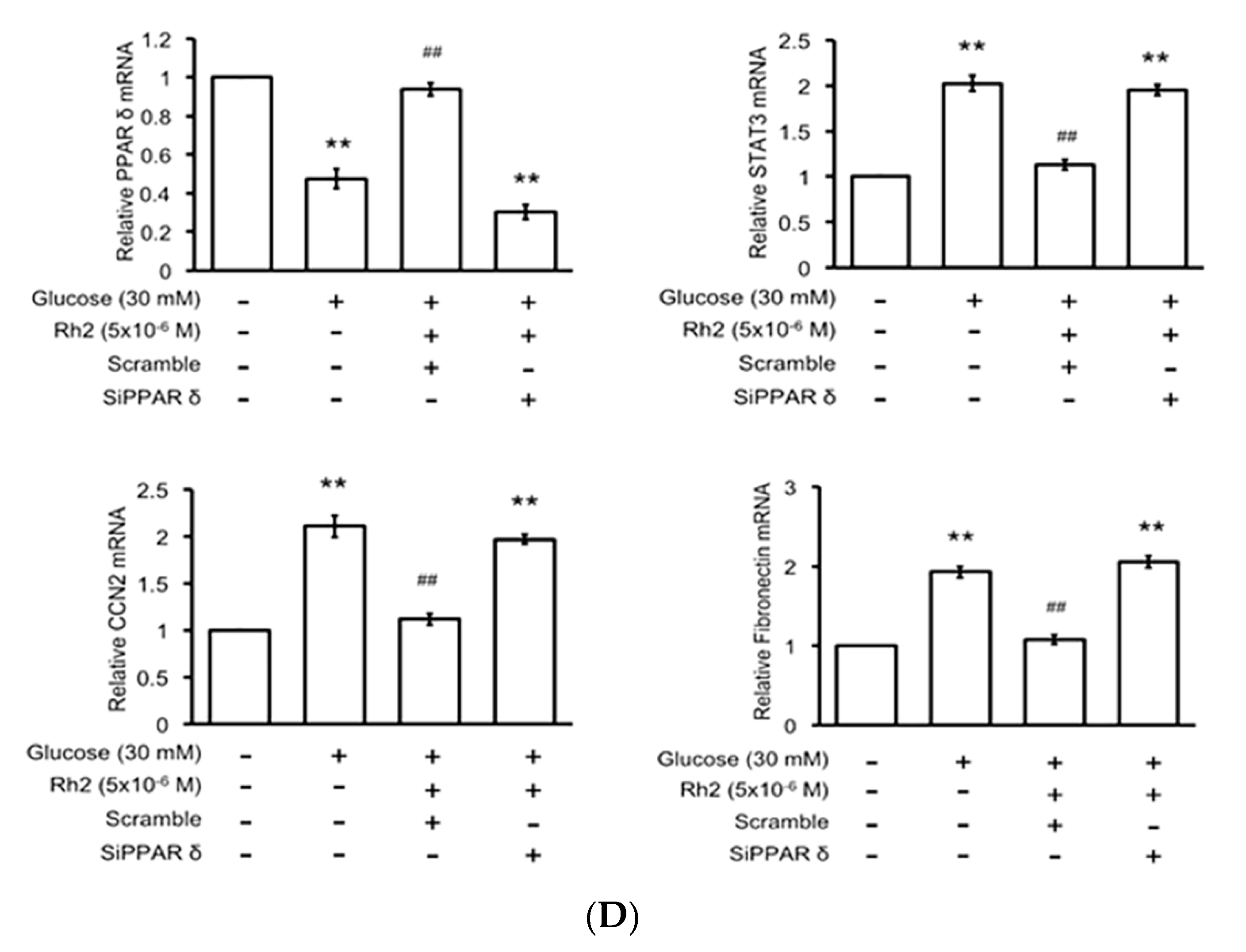
2.6. Effects of Rh2 on Fibrosis-Related Gene Expression Were Impaired by siRNA Specific for PPAR in Cardiomyocytes
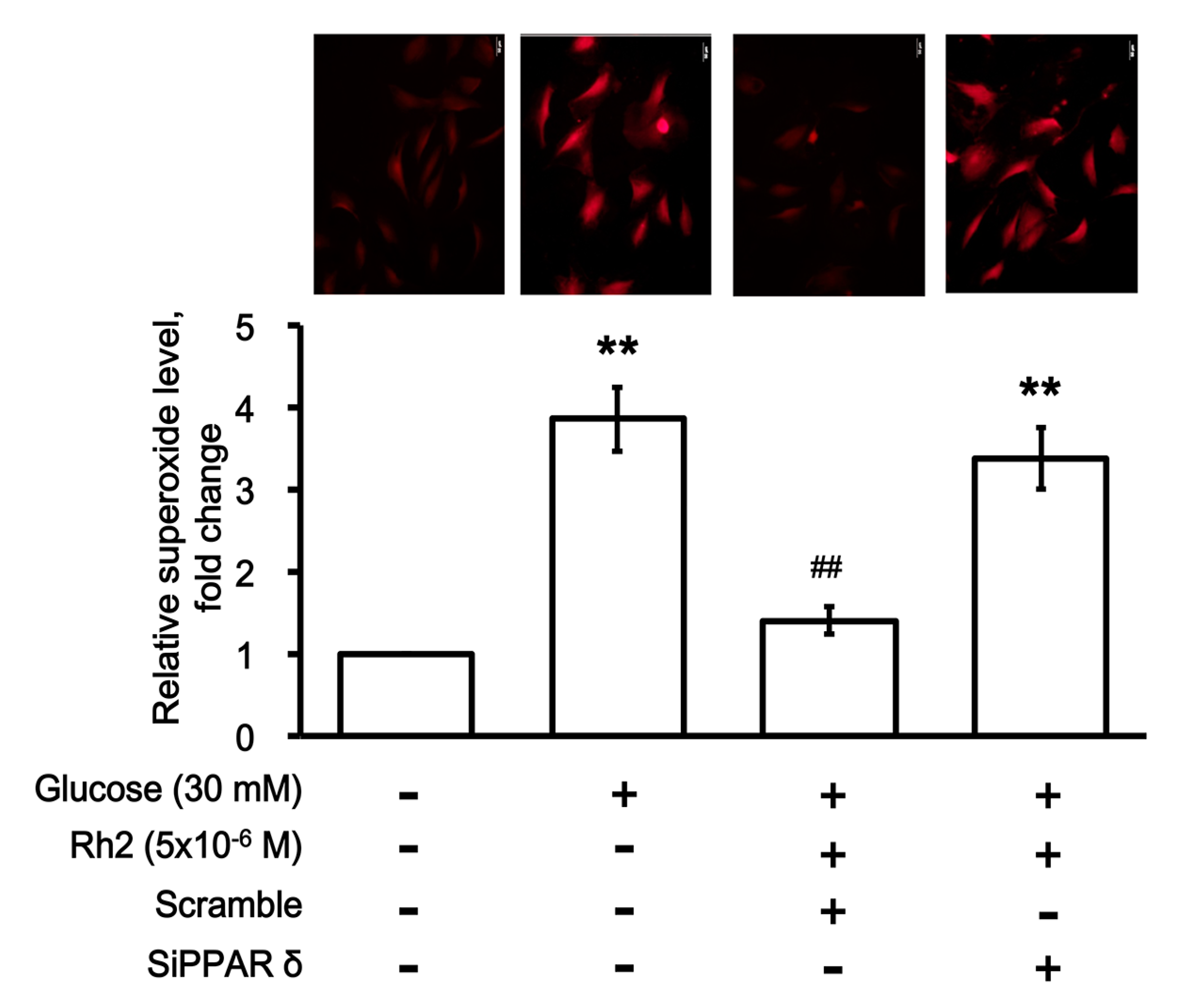
2.7. Effects of Rh2 on Oxidative Damage Were Impaired by siRNA Specific for PPAR in Cardiomyocytes
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Statement
4.2. Diabetic Rat Model
4.3. Treatment Protocols
4.4. Materials
4.5. Hemodynamic Measurements
4.6. Determination of Cardiac Weight Index
4.7. Histology of the Myocardial Tissues
4.8. Cell Culture and Treatment
4.9. Small Interfering RNA (SiRNA)
4.10. Western Blot Analysis
4.11. Real-Time Reverse-Transcription-Polymerase Chain Reaction
- collagen I (α1) F: 5′-CTGGCAAGAACGGAGATGAT-3’;
- collagen I (α1) R: 5′-AATCCACGAGCACCCTGA-3′;
- collagen III (α1) F: 5′-CAGGAAACCCAGGAGAAAGG-3′;
- collagen III (α1) R: 5′-CCAGCTACTCCTATAGGTCCTG-3′;
- PPARδ R: 5′-TTCCATGACTGACCCCACTT-3′;
- PPARδ L: 5′-GAGGACAAACCCACGGTAAA-3′;
- β-actin F: 5′-CTAAGGCCAACCGTGAAAAG-3′;
- β-actin R: 5′-GCCTGGATGGCTACGTACA-3′.
4.12. Identification of Intracellular Superoxide Levels
4.13. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CCN2 | Connective tissue growth factor |
| DCM | Diabetic cardiomyopathy |
| DHE | Dihydroergotamine |
| ECM | Extracellular matrix |
| HW/BW | Ratio, heart weight and body weight ratio |
| STAT3 | Signal transducer and activator of transcription 3 |
| STZ | Streptozotocin |
References
- Bauters, C.; Lamblin, N.; Mc Fadden, E.P.; Van Belle, E.; Millaire, A.; de Groote, P. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc. Diabetol. 2003, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Solang, L.; Malmberg, K.; Ryden, L. Diabetes mellitus and congestive heart failure. Further knowledge needed. Eur. Heart J. 1999, 20, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; McMullen, J.R.; Julius, T.L.; Tan, J.W.; Love, J.E.; Cemerlang, N.; Kiriazis, H.; Du, X.J.; Ritchie, R.H. Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes 2010, 59, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Falcao-Pires, I.; Leite-Moreira, A.F. Diabetic cardiomyopathy: Understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail. Rev. 2012, 17, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Mair, M.; Blaas, L.; Osterreicher, C.H.; Casanova, E.; Eferl, R. Jak-stat signaling in hepatic fibrosis. Front. Biosci. 2011, 16, 2794–2811. [Google Scholar] [CrossRef]
- Radhakrishnan, S.S.; Blalock, T.D.; Robinson, P.M.; Secker, G.; Daniels, J.; Grotendorst, G.R.; Schultz, G.S. Effect of connective tissue growth factor on protein kinase expression and activity in human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8076–8085. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, T.; Du, G.; Zhao, Q.; Ma, L.; Zhu, J. The impact of 1,25-dihydroxyvitamin D3 on the expression of connective tissue growth factor and transforming growth factor-β1 in the myocardium of rats with diabetes. Diabetes Res. Clin. Pract. 2014, 104, 226–233. [Google Scholar] [CrossRef] [PubMed]
- White, E.S.; Baralle, F.E.; Muro, A.F. New insights into form and function of fibronectin splice variants. J. Pathol. 2008, 216, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Meyer, C.; Li, J.; Nadalin, S.; Konigsrainer, A.; Weng, H.; Dooley, S.; ten Dijke, P. Transforming growth factor-β (TGF-β)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J. Biol. Chem. 2013, 288, 30708–30719. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, K.E.; Breen, E.P.; Gallagher, H.C.; Buggy, D.J.; Hurley, J.P. Understanding Stat3 signaling in cardiac ischemia. Basic Res. Cardiol. 2016, 111, 27. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Cheng, J.T.; Chen, Z.C. Telmisartan improves cardiac fibrosis in diabetes through peroxisome proliferator activated receptor δ (PPARδ): From bedside to bench. Cardiovasc. Diabetol. 2016, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ku, S. Finding and producing probiotic glycosylases for the biocatalysis of ginsenosides: A mini review. Molecules 2016, 21, 645. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Chen, K. Roles and mechanisms of ginsenoside in cardiovascular diseases: Progress and perspectives. Sci. China Life Sci. 2016, 59, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Choo, M.K.; Oh, J.K.; Ryu, J.H.; Kim, D.H. Ginsenoside Rh2 reduces ischemic brain injury in rats. Biol. Pharm. Bull. 2004, 27, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, J.S. Effect of ginsenoside Rh-2 via activation of caspase-3 and Bcl-2-insensitive pathway in ovarian cancer cells. Physiol. Res. 2016, 65, 1031–1037. [Google Scholar] [PubMed]
- Li, B.; Zhao, J.; Wang, C.Z.; Searle, J.; He, T.C.; Yuan, C.S.; Du, W. Ginsenoside rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011, 301, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, P.; Gou, H.; Zhang, J.; Zhu, M.; Wang, Z.H.; Tian, J.W.; Jiang, Y.T.; Fu, F.H. Cardioprotective effects of 20(s)-ginsenoside Rh2 against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid. Based Complement. Altern. Med. 2012, 2012, 506214. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Liu, C.F.; Tsai, T.H.; Chen, Y.L.; Chang, H.W.; Tsai, C.Y.; Leu, S.; Zhen, Y.Y.; Chai, H.T.; Chung, S.Y.; et al. Effect of obesity reduction on preservation of heart function and attenuation of left ventricular remodeling, oxidative stress and inflammation in obese mice. J. Trans. Med. 2012, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Chan, P.; Chen, L.J.; Chang, C.K.; Liu, Z.; Lin, J.W. Merit of ginseng in the treatment of heart failure in type 1-like diabetic rats. BioMed Res. Int. 2014, 2014, 484161. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.T.; Huang, C.C.; Liu, I.M.; Tzeng, T.F.; Chang, C.J. Novel mechanism for plasma glucose-lowering action of metformin in streptozotocin-induced diabetic rats. Diabetes 2006, 55, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Yu, B.C.; Chen, L.J.; Cheng, K.C.; Lin, H.J.; Cheng, J.T. Characterization of the mechanisms of the increase in PPARδ expression induced by digoxin in the heart using the H9c2 cell line. Br. J. Pharmacol. 2011, 163, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, X.; Chen, X.; Chen, L.; Zheng, G.; Zhou, H.; Zhou, X. Anti-fibrosis effect of relaxin and spironolactone combined on isoprenaline-induced myocardial fibrosis in rats via inhibition of endothelial-mesenchymal transition. Cell. Physiol. Biochem. 2017, 41, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Hsu, C.T.; Niu, H.S.; Chang, C.H.; Cheng, J.T.; Shieh, J.M. Lung damage induced by hyperglycemia in diabetic rats: The role of signal transducer and activator of transcription 3 (Stat3). J. Diabetes Complicat. 2016, 30, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Goyal, B.R.; Mehta, A.A. Diabetic cardiomyopathy: Pathophysiological mechanisms and cardiac dysfuntion. Hum. Exp. Toxicol. 2013, 32, 571–590. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac fibrosis: The fibroblast awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Souders, C.A.; Bowers, S.L.; Baudino, T.A. Cardiac fibroblast: The renaissance cell. Circ. Res. 2009, 105, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Tokudome, T.; Horio, T.; Yoshihara, F.; Suga, S.; Kawano, Y.; Kohno, M.; Kangawa, K. Direct effects of high glucose and insulin on protein synthesis in cultured cardiac myocytes and DNA and collagen synthesis in cardiac fibroblasts. Metab. Clin. Exp. 2004, 53, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qi, H.; Wang, Y.; Wu, M.; Cao, Y.; Huang, W.; Li, L.; Ji, Z.; Sun, H. Allicin protects against myocardial apoptosis and fibrosis in streptozotocin-induced diabetic rats. Phytomedicine Int. J. Phytother. Phytopharmacol. 2012, 19, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Chavali, V.; Nandi, S.S.; Singh, S.R.; Mishra, P.K. Generating double knockout mice to model genetic intervention for diabetic cardiomyopathy in humans. Methods Mol. Biol. 2014, 1194, 385–400. [Google Scholar] [PubMed]
- Ma, Y.; Li, H.; Yue, Z.; Guo, J.; Xu, S.; Xu, J.; Jia, Y.; Yu, N.; Zhang, B.; Liu, S.; et al. Cryptotanshinone attenuates cardiac fibrosis via downregulation of Cox-2, Nox-2, and Nox-4. J. Cardiovasc. Pharmacol. 2014, 64, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Artas, G.; Ozercan, H.I. The expression of stat3, Bcl-Xl and Mmp-2 proteins in colon adenocarcinomas and their relationship with prognostic factors. Turk. Patoloji Dergisi 2014, 30, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Ricke-Hoch, M.; Stapel, B.; Gorst, I.; Hilfiker-Kleiner, D. Stat3, a key regulator of cell-to-cell communication in the heart. Cardiovasc. Res. 2014, 102, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, L.; Liu, X.; Zhou, H.; Fan, Q.; Luo, J.; Yao, L.; Wang, J.; Feng, J.; Ma, J. Janus kinase 2/signal transducers and activators of transcription signal inhibition regulates protective effects of probucol on mesangial cells treated with high glucose. Biol. Pharm. Bull. 2010, 33, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, W.; Lin, H.; Jiang, H.; Dai, H.; Zhang, Y. High glucose promotes the production of collagen types i and iii by cardiac fibroblasts through a pathway dependent on extracellular-signal-regulated kinase 1/2. Mol. Cell. Biochem. 2007, 301, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Gilde, A.J.; van der Lee, K.A.; Willemsen, P.H.; Chinetti, G.; van der Leij, F.R.; van der Vusse, G.J.; Staels, B.; van Bilsen, M. Peroxisome proliferator-activated receptor (PPAR) α and PPARβ/δ, but not PPARγ, modulate the expression of genes involved in cardiac lipid metabolism. Circ. Res. 2003, 92, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ding, G.; Qin, Q.; Huang, Y.; Lewis, W.; He, N.; Evans, R.M.; Schneider, M.D.; Brako, F.A.; Xiao, Y.; et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-δ deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat. Med. 2004, 10, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Planavila, A.; Rodriguez-Calvo, R.; Jove, M.; Michalik, L.; Wahli, W.; Laguna, J.C.; Vazquez-Carrera, M. Peroxisome proliferator-activated receptor β/δ activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc. Res. 2005, 65, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, B.E.; Smeets, P.J.; Willemsen, P.H.; De Windt, L.J.; Van der Vusse, G.J.; Van Bilsen, M. Activation of PPARδ inhibits cardiac fibroblast proliferation and the transdifferentiation into myofibroblasts. Cardiovasc. Res. 2007, 75, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Wagner, N. Peroxisome proliferator-activated receptor β/δ (PPARβ/δ) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol. Ther. 2010, 125, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Lee, K.S.; Chen, L.J.; Wang, L.Y.; Niu, H.S.; Cheng, J.T. Cardiac peroxisome proliferator-activated receptor δ (PPARδ) as a new target for increased contractility without altering heart rate. PLoS ONE 2013, 8, e64229. [Google Scholar] [CrossRef] [PubMed]
- Cernecka, H.; Doka, G.; Srankova, J.; Pivackova, L.; Malikova, E.; Galkova, K.; Kyselovic, J.; Krenek, P.; Klimas, J. Ramipril restores PPARβ/δ and PPARγ expressions and reduces cardiac nadph oxidase but fails to restore cardiac function and accompanied myosin heavy chain ratio shift in severe anthracycline-induced cardiomyopathy in rat. Eur. J. Pharmacol. 2016, 791, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ting, S.; Lau, H.; Pulinilkunnil, T.; An, D.; Qi, D.; Abrahani, M.A.; Rodrigues, B. Increased efflux of glutathione conjugate in acutely diabetic cardiomyocytes. Can. J. Physiol. Pharmacol. 2004, 82, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Ignarro, L.J. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch. Pharm. Res. 2009, 32, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.; Awad, E.M.; Nagy, M.A. Effects of panax quinquefolium on streptozotocin-induced diabetic rats: Role of c-peptide, nitric oxide and oxidative stress. Int. J. Clin. Exp. Med. 2011, 4, 136–147. [Google Scholar] [PubMed]
- Lee, T.I.; Kao, Y.H.; Chen, Y.C.; Pan, N.H.; Chen, Y.J. Oxidative stress and inflammation modulate peroxisome proliferator-activated receptors with regional discrepancy in diabetic heart. Eur. J. Clin. Investig. 2010, 40, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; Bernardo, B.C.; McMullen, J.R.; Ritchie, R.H. Diabetic cardiomyopathy: Mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol. Ther. 2014, 142, 375–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, R.; Yan, H.; Tian, L.; You, Q.; Li, S.; Huang, R.; Wu, K. Naringin inhibits Ros-activated mapk pathway in high glucose-induced injuries in H9c2 cardiac cells. Basic Clin. Pharmacol. Toxicol. 2014, 114, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Collino, M.; Castiglia, S.; Fantozzi, R.; Thiemermann, C. Activation of peroxisome proliferator-activated receptor-β/δ attenuates myocardial ischemia/reperfusion injury in the rat. Shock 2010, 34, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Pesant, M.; Sueur, S.; Dutartre, P.; Tallandier, M.; Grimaldi, P.A.; Rochette, L.; Connat, J.L. Peroxisome proliferator-activated receptor δ (PPARδ) activation protects H9c2 cardiomyoblasts from oxidative stress-induced apoptosis. Cardiovasc. Res. 2006, 69, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Kocalis, H.E.; Turney, M.K.; Printz, R.L.; Laryea, G.N.; Muglia, L.J.; Davies, S.S.; Stanwood, G.D.; McGuinness, O.P.; Niswender, K.D. Neuron-specific deletion of peroxisome proliferator-activated receptor δ (PPARδ) in mice leads to increased susceptibility to diet-induced obesity. PLoS ONE 2012, 7, e42981. [Google Scholar] [CrossRef] [PubMed]
- Musende, A.G.; Eberding, A.; Wood, C.; Adomat, H.; Fazli, L.; Hurtado-Coll, A.; Jia, W.; Bally, M.B.; Guns, E.T. Pre-clinical evaluation of Rh2 in PC-3 human xenograft model for prostate cancer in vivo: Formulation, pharmacokinetics, biodistribution and efficacy. Cancer Chemother Pharmacol. 2009, 64, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, G.J.; Sun, J.G.; Jia, Y.W.; Wang, W.; Xu, M.J.; Lv, T.; Zheng, Y.T.; Sai, Y. Pharmacokinetic characterization of ginsenoside Rh2, an anticancer nutrient from ginseng, in rats and dogs. Food Chem. Toxicol. 2009, 47, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Seldinger, S.I. Catheter replacement of the needle in percutaneous arteriography. A new technique. Acta Radiol. Suppl. 2008, 434, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Peng, D.F.; Hu, Y.J.; Chen, J. Protective effects of valsartan and benazepril combined with atorvastatin on cardiorenal syndrome in rats. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 759–766. [Google Scholar] [PubMed]
- Li, C.J.; Lv, L.; Li, H.; Yu, D.M. Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by α-lipoic acid. Cardiovasc. Diabetol. 2012, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Hwang, S.M.; Cui, Z.; Gilda, J.E.; Gomes, A.V. Different effects of the nonsteroidal anti-inflammatory drugs meclofenamate sodium and naproxen sodium on proteasome activity in cardiac cells. J. Mol. Cell. Cardiol. 2016, 94, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.C.; Chen, L.J.; Niu, H.S.; Yang, T.T.; Lin, K.C.; Cheng, J.T. Signals for increase of mu-opioid receptor expression in muscle by hyperglycemia. Neurosci. Lett. 2014, 582, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xia, Z.; Han, Y.; Rong, J. Plant natural product formononetin protects rat cardiomyocyte H9c2 cells against oxygen glucose deprivation and reoxygenation via inhibiting ROS formation and promoting GSK-3β phosphorylation. Oxidative Med. Cell. Longev. 2016, 2016, 2060874. [Google Scholar] [CrossRef] [PubMed]
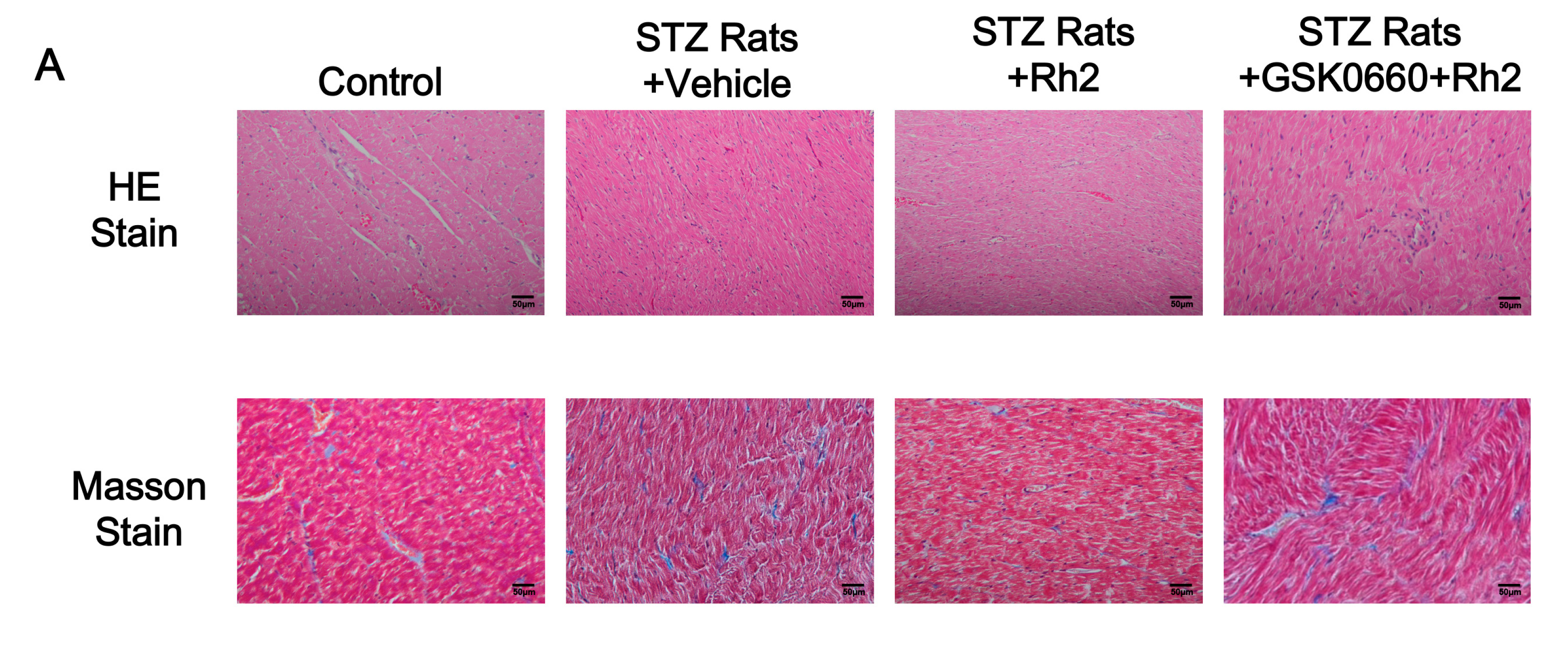
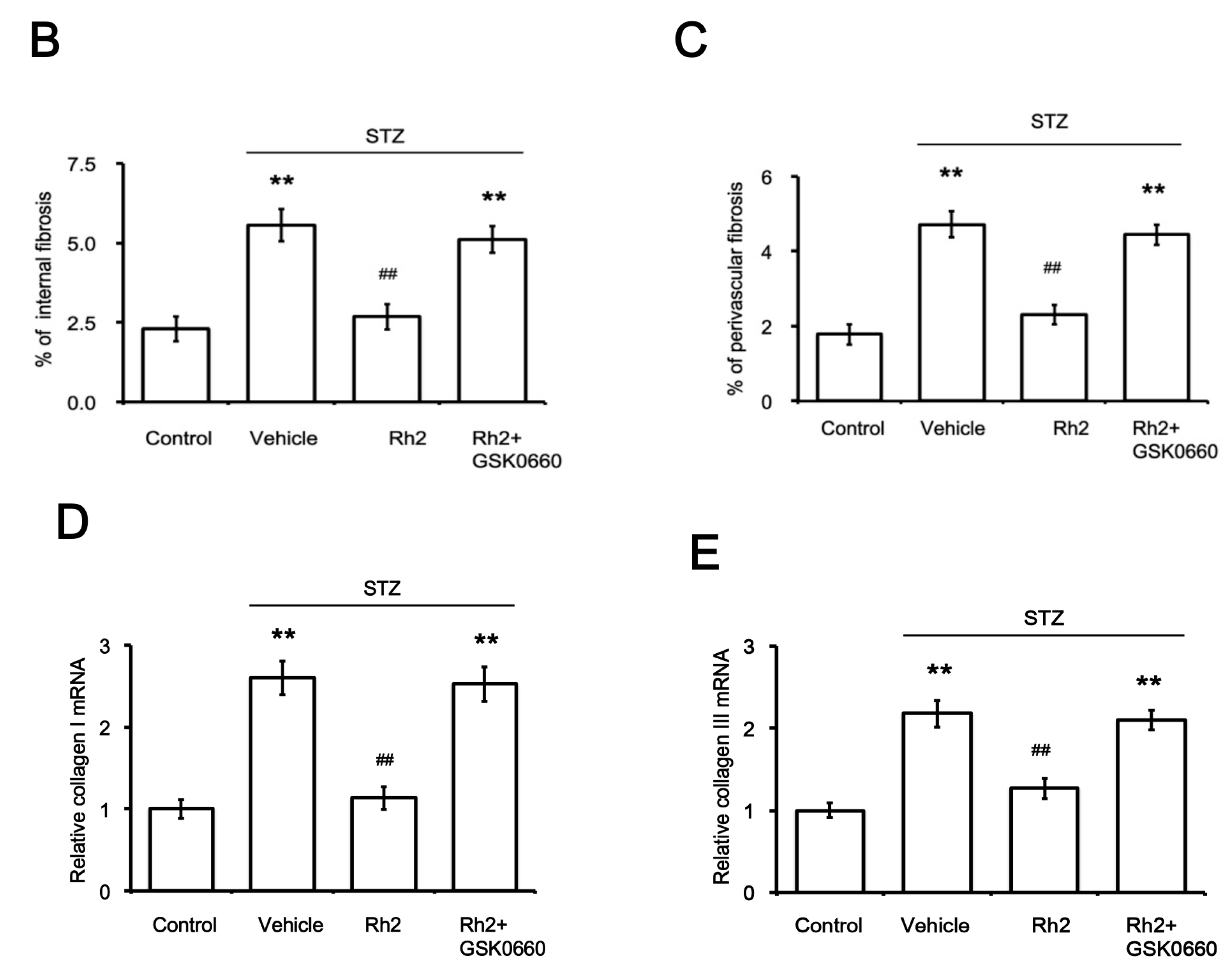

| Group | Control | STZ Rats | |||
|---|---|---|---|---|---|
| +Vehicle | +Rh2 | +Vehicle | +Rh2 | +Rh2 +GSK0660 | |
| Blood glucose (mg/dL) | 101.4 ± 4.0 | 99.3 ± 5.3 | 328.1 ± 13.0 ** | 237.4 ± 15.9 **,# | 315.3 ± 14.8 ** |
| Body weight (g) | 363.4 ± 6.5 | 348.9 ± 7.3 | 271.1 ± 6.6 ** | 304.0 ± 6.5 *,# | 275.8 ± 8.2 ** |
| Heart weight (g) | 1.28 ± 0.04 | 1.17 ± 0.07 | 1.05 ± 0.04 * | 0.86 ± 0.04 **,# | 1.08 ± 0.06 ** |
| Cardiac weight index (mg/g) | 3.52 ± 0.14 | 3.35 ± 0.16 | 3.89 ± 0.18 * | 2.85 ± 0.16 *,# | 3.91 ± 0.22 * |
| Heart rate (beats /min) | 379.50 ± 21.46 | 384.25 ± 26.87 | 289.25 ± 31.95 * | 330.38 ± 24.73 *,# | 294.50 ± 22.49 * |
| LVSP (mmHg) | 144.4 ± 4.6 | 145.7 ± 5.2 | 113.6 ± 3.0 * | 139.5 ± 3.5 *,# | 112.1 ± 4.0 * |
| LVEDP (mmHg) | 4.77 ± 0.46 | 4.95.1 ± 0.31 | 8.94 ± 0.32 * | 5.11 ± 0.45 *,# | 8.82 ± 0.56 * |
| Cardiac Performance | |||||
| +dp/dtmax (mmHg/S) | 2525.9 ± 92.4 | 2602.6 ± 61.2 | 2089.4 ± 58.4 ** | 2301.1 ± 53.1 *,# | 2106.4 ± 46.6 ** |
| −dp/dtmax (mmHg/S) | 1501.1 ± 61.5 | 1458.8 ± 46.3 | 931.3 ± 51.3 ** | 1246.7 ± 35.5 *,# | 967.3 ± 69.5 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, S.-H.; Hsu, C.-T.; Niu, H.-S.; Niu, C.-S.; Cheng, J.-T.; Chen, Z.-C. Ginsenoside Rh2 Improves Cardiac Fibrosis via PPARδ–STAT3 Signaling in Type 1-Like Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 1364. https://doi.org/10.3390/ijms18071364
Lo S-H, Hsu C-T, Niu H-S, Niu C-S, Cheng J-T, Chen Z-C. Ginsenoside Rh2 Improves Cardiac Fibrosis via PPARδ–STAT3 Signaling in Type 1-Like Diabetic Rats. International Journal of Molecular Sciences. 2017; 18(7):1364. https://doi.org/10.3390/ijms18071364
Chicago/Turabian StyleLo, Shih-Hsiang, Chao-Tien Hsu, Ho-Shan Niu, Chiang-Shan Niu, Juei-Tang Cheng, and Zhih-Cherng Chen. 2017. "Ginsenoside Rh2 Improves Cardiac Fibrosis via PPARδ–STAT3 Signaling in Type 1-Like Diabetic Rats" International Journal of Molecular Sciences 18, no. 7: 1364. https://doi.org/10.3390/ijms18071364