Oral Supplementation of Melatonin Protects against Fibromyalgia-Related Skeletal Muscle Alterations in Reserpine-Induced Myalgia Rats
Abstract
:1. Introduction
2. Results
2.1. Body Weight Time-Course
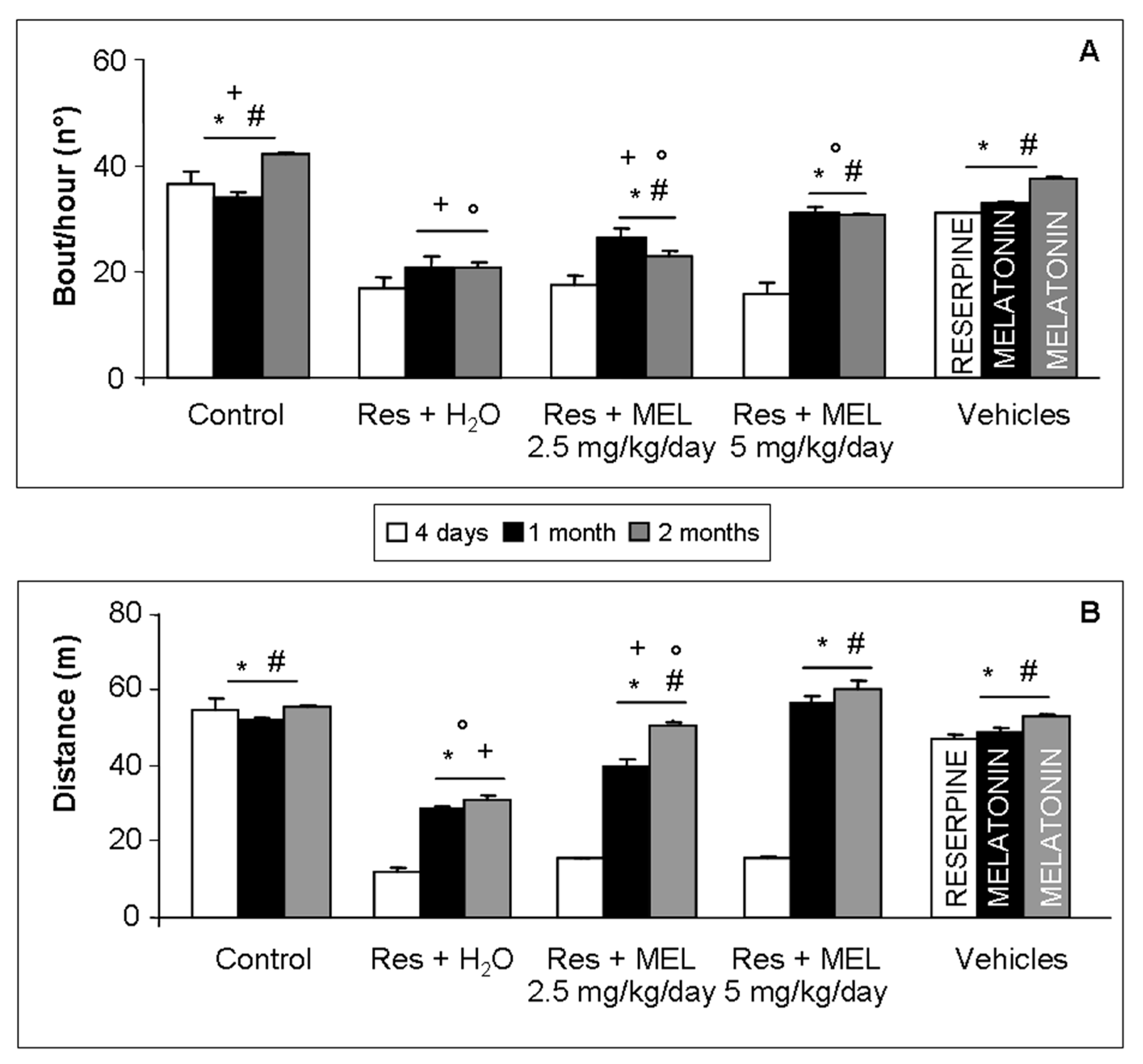
2.2. Voluntary Motor Activity
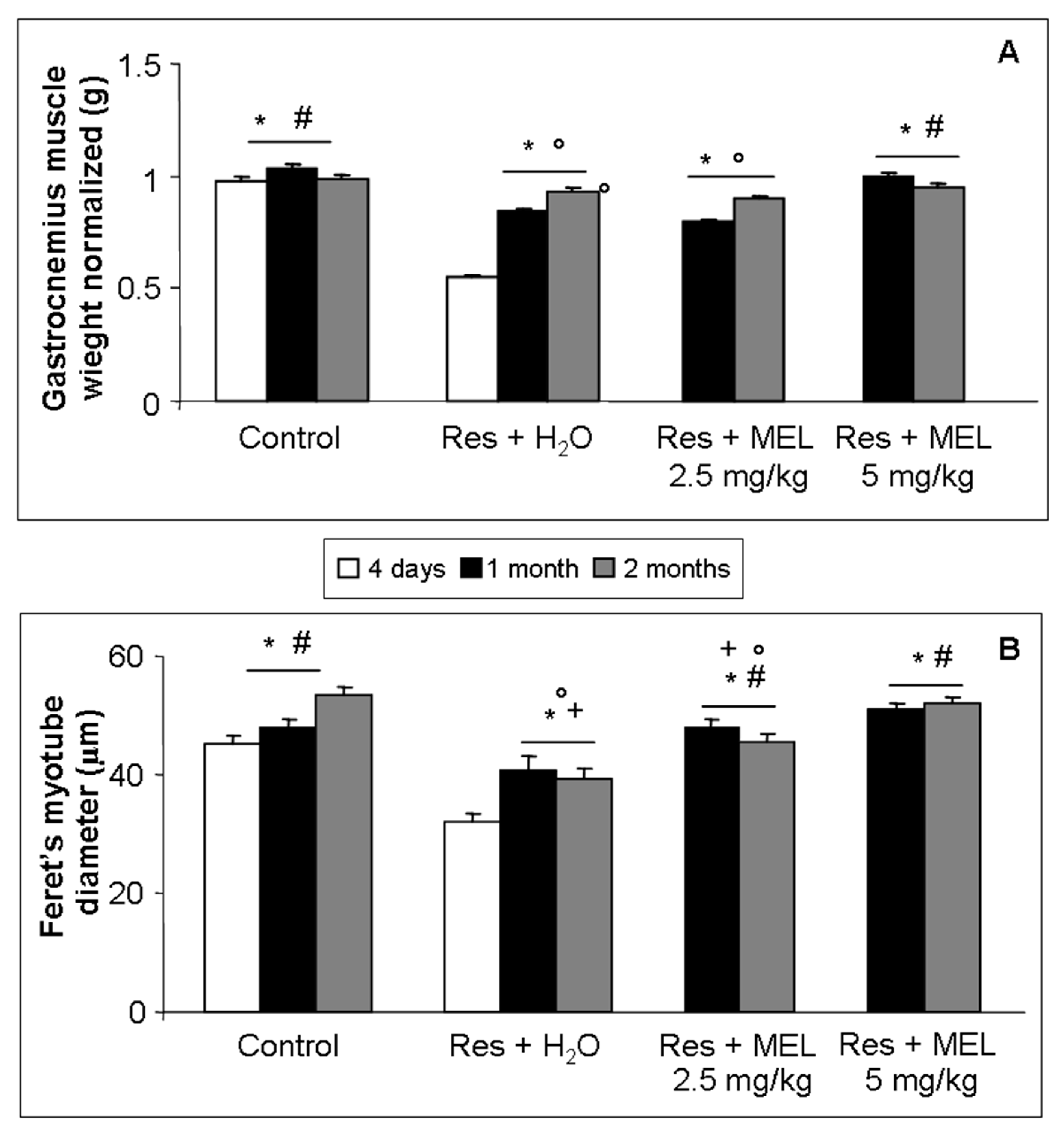
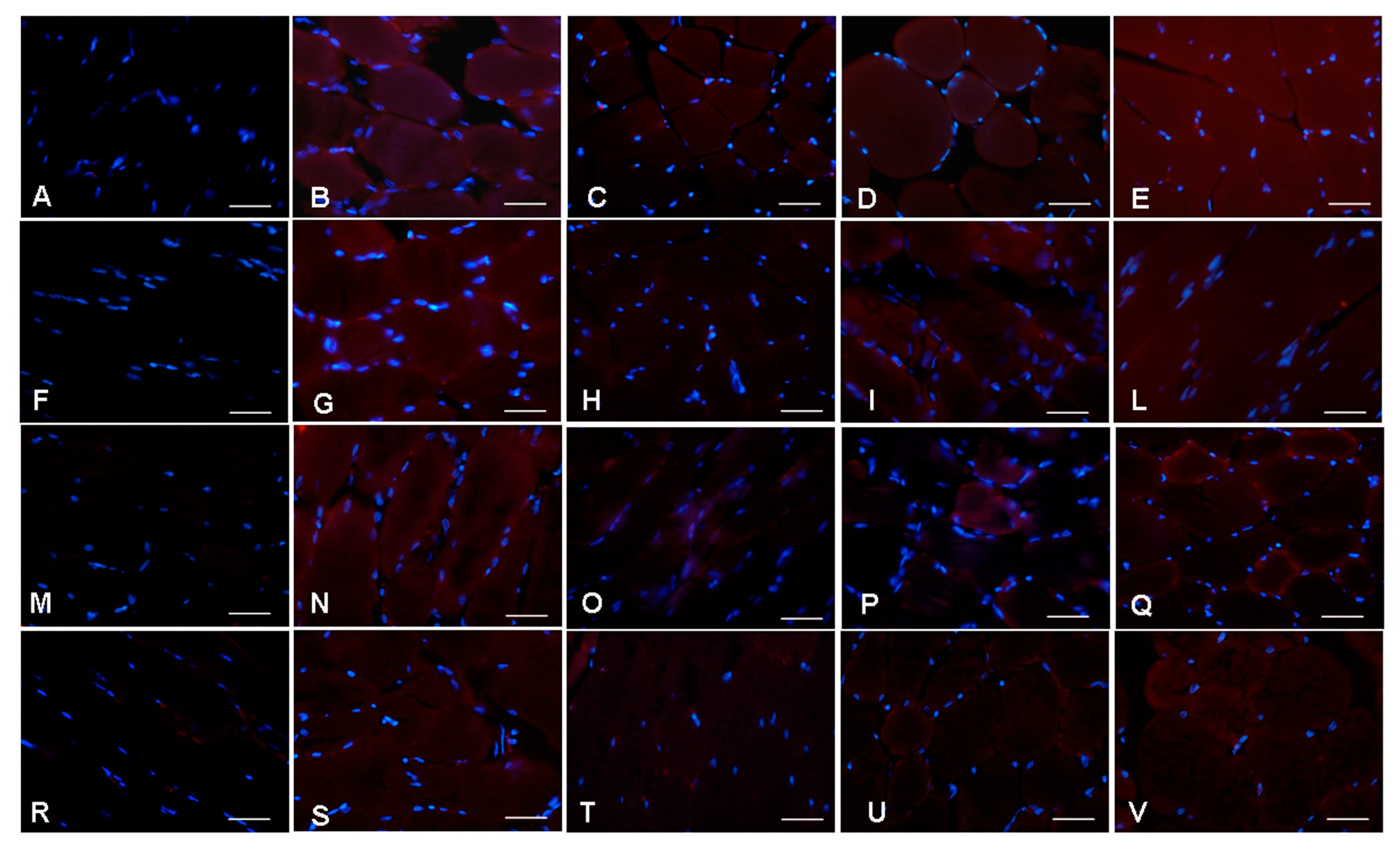
2.3. Morphological Evaluations on Gastrocnemius
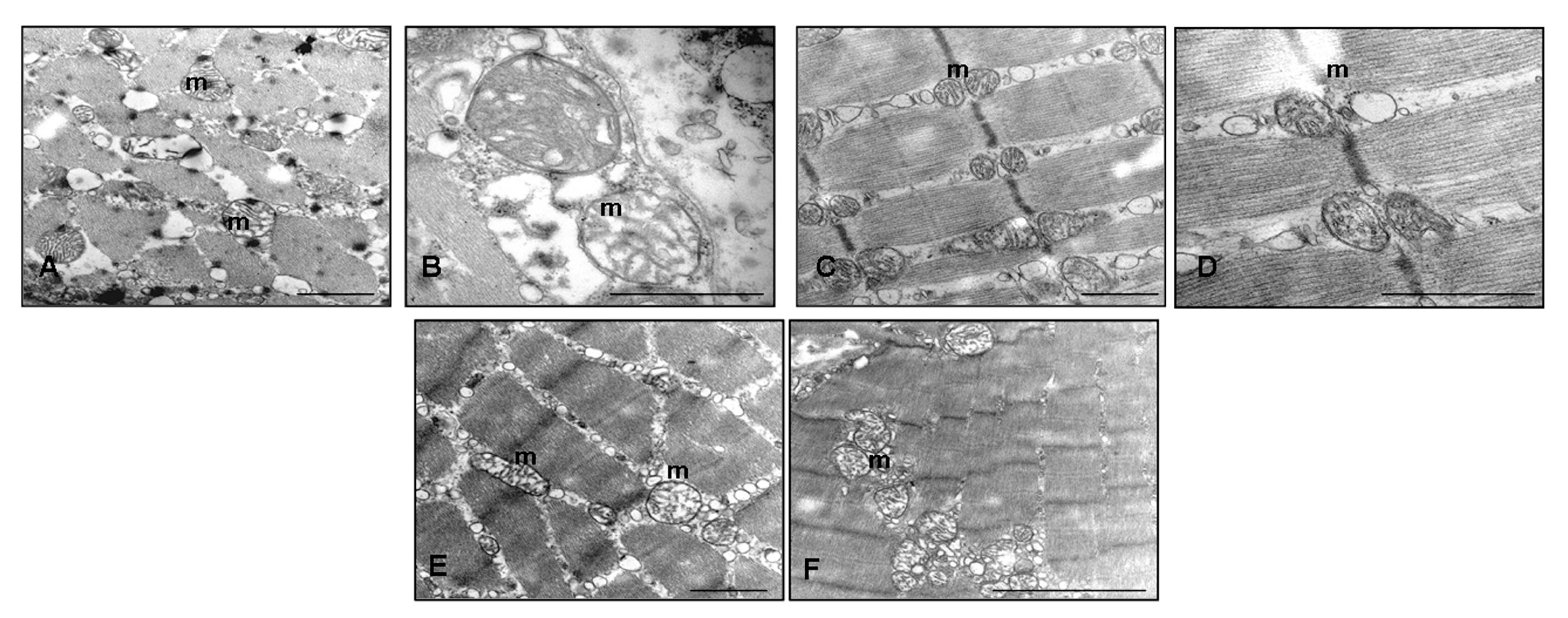
2.4. Ultrastructural Evaluation of Gastrocnemius Muscle
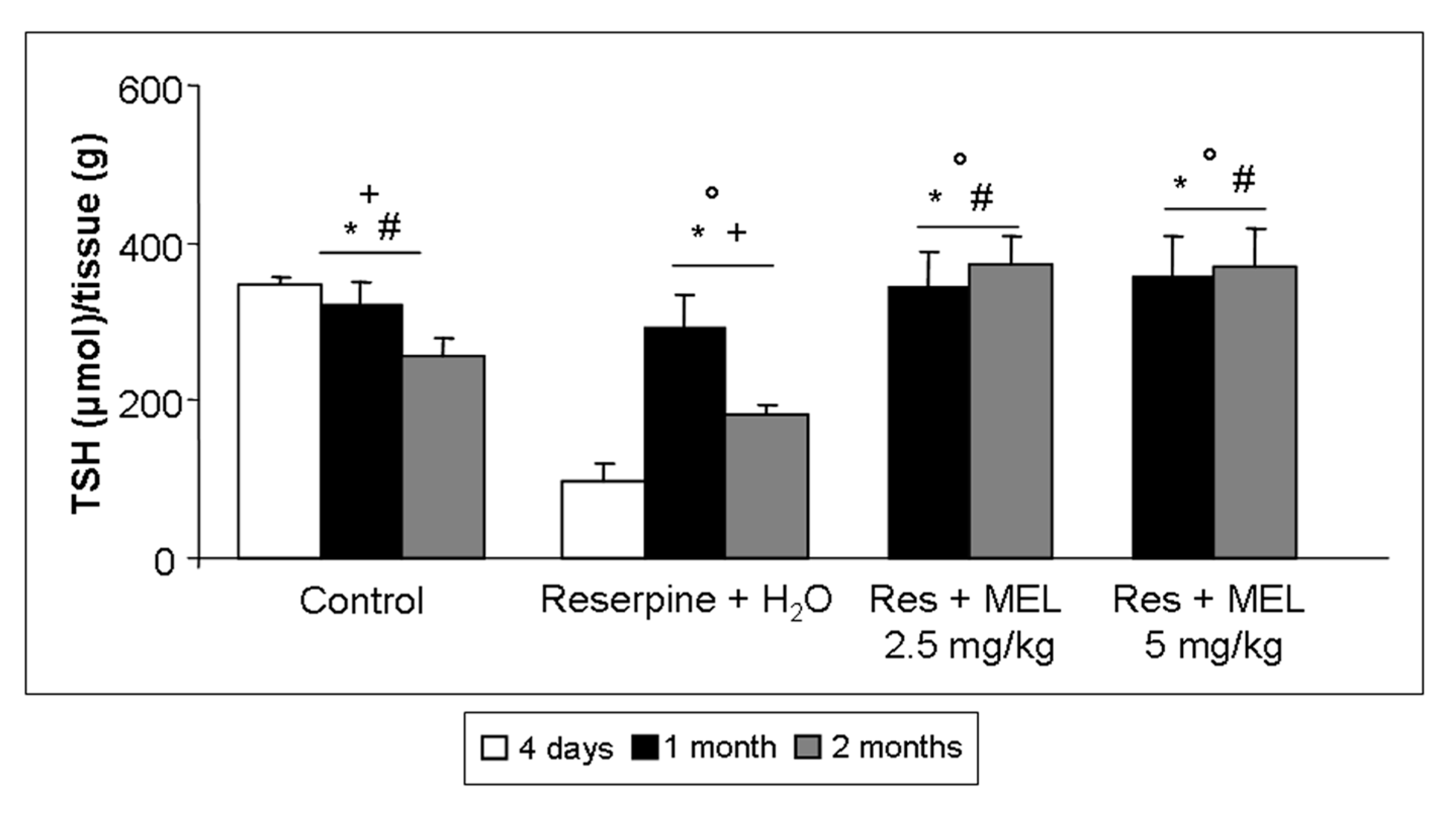
2.5. TSH Level Assessment
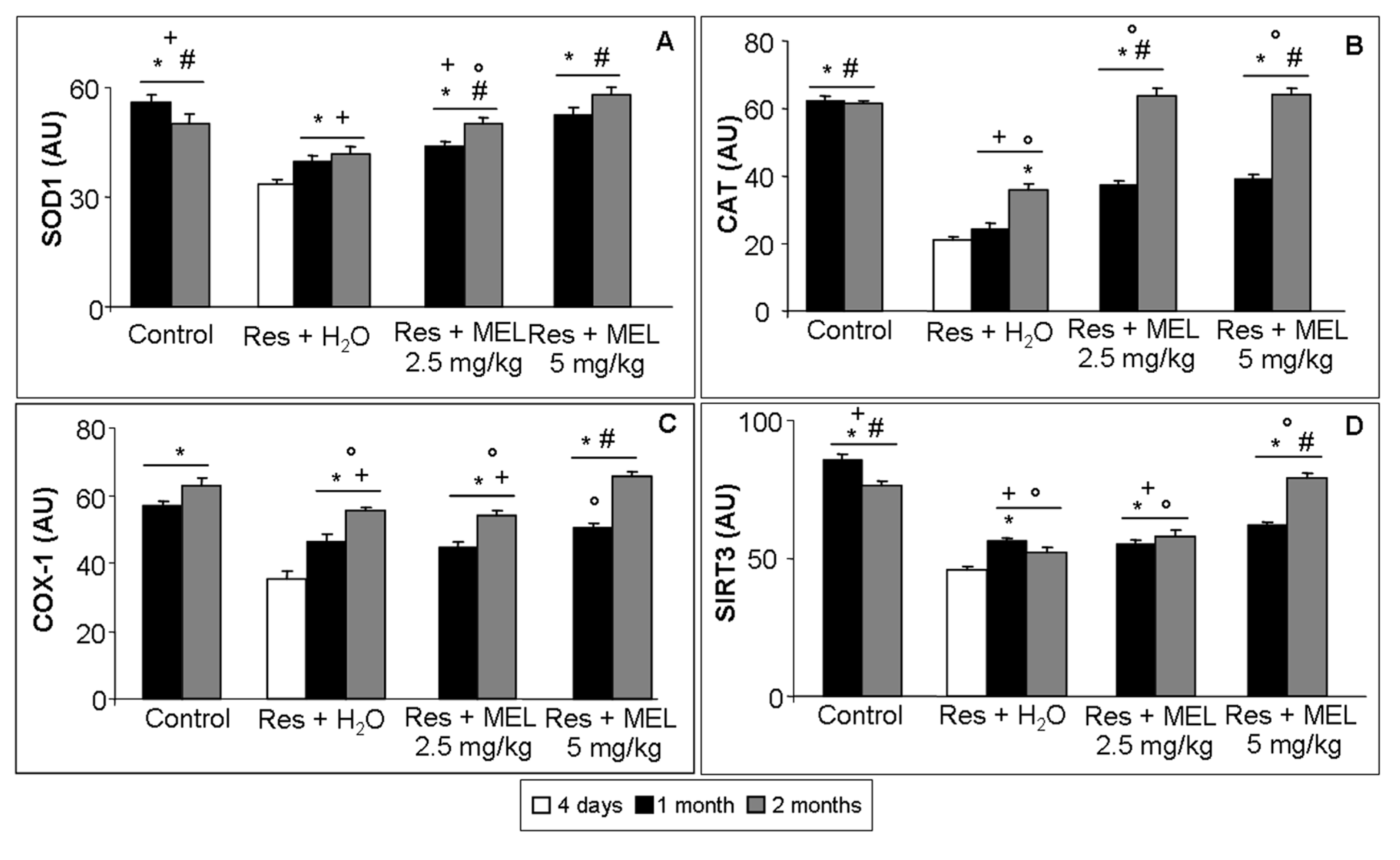
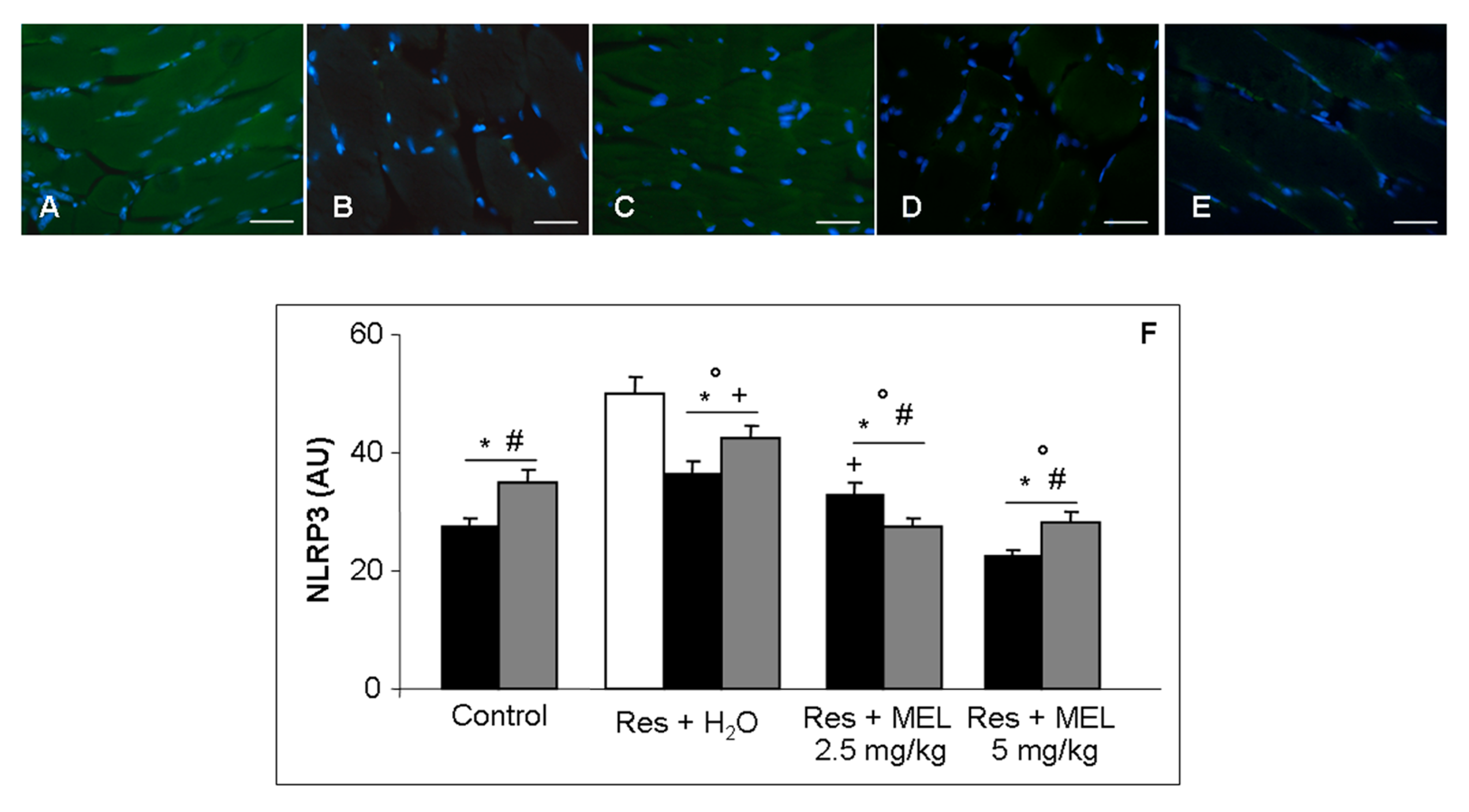
2.6. Inflammatory and Oxidative Stress Markers Analyses
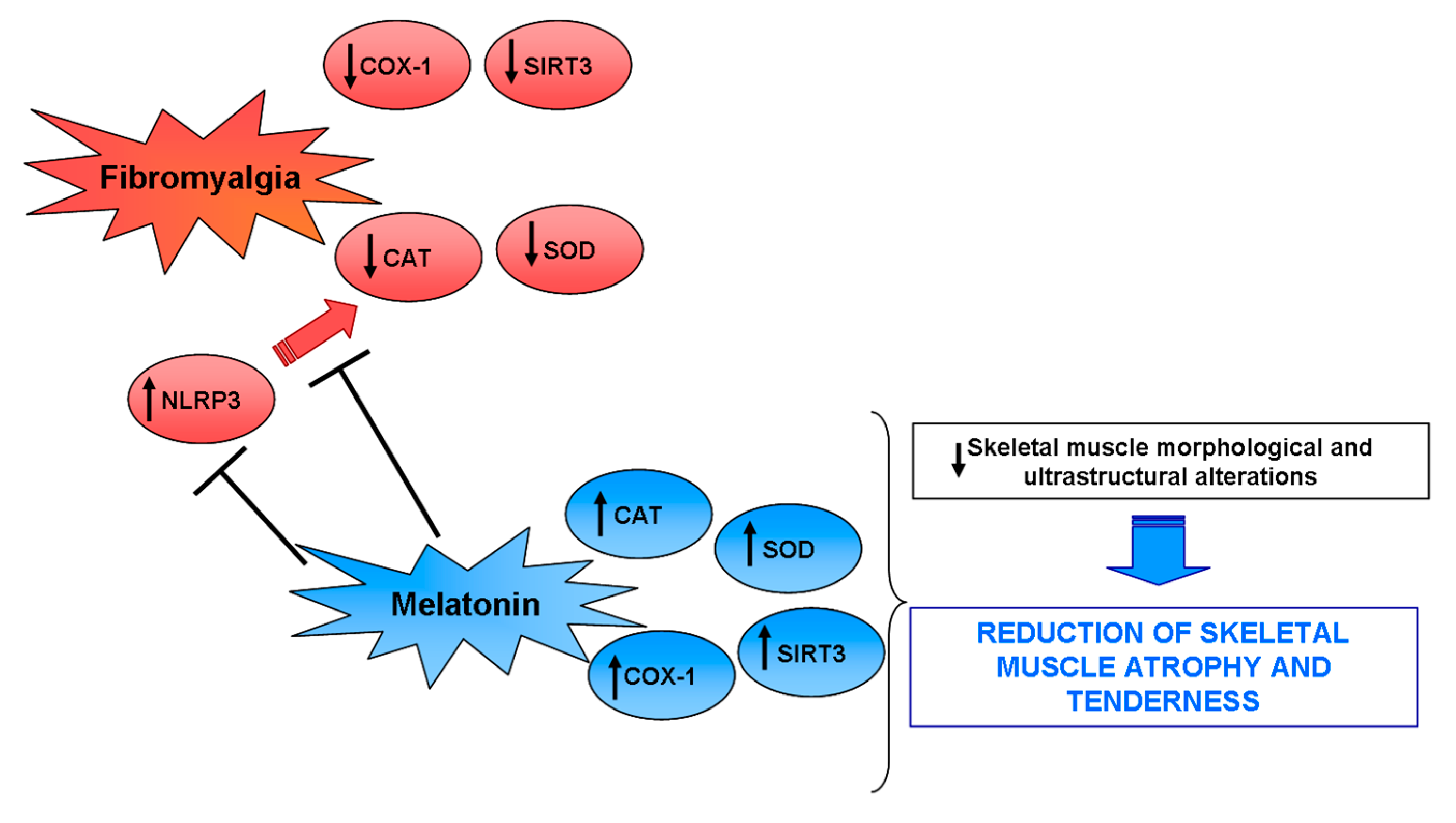
3. Discussion
4. Materials and Methods
4.1. Animal Treatment
4.2. Assessment of Volountary Locomotor Activity
4.3. Total Thiol Evaluation
4.4. Morphometrical Analysis
4.5. Ultrastructural Transmission Electron Microscopy Evaluation
4.6. Immunofluorescence Evaluations
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflict of Interest
References
- Bortolato, B.; Berk, M.; Maes, M.; McIntyre, R.S.; Carvalho, A.F. Fibromyalgia and bipolar disorder: Emerging epidemiological associations and shared pathophysiology. Curr. Mol. Med. 2016, 16, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- McBeth, J.; Jones, K. Epidemiology of chronic musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 2007, 21, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Acuna-Castroviejo, D.; Tan, D.X. Melatonin therapy in fibromyalgia. Curr. Pain Headache Rep. 2007, 11, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Gebke, K.B.; Choy, E.H. Fibromyalgia: Management strategies for primary care providers. Int. J. Clin. Pract. 2016, 70, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.L. Diagnosis and differential diagnosis of fibromyalgia. Am. J. Med. 2009, 122, S14–S21. [Google Scholar] [CrossRef] [PubMed]
- Bradley, L.A. Pathophysiologic mechanisms of fibromyalgia and its related disorders. J. Clin. Psychiatry 2008, 69, 6–13. [Google Scholar] [PubMed]
- Christidis, N.; Ghafouri, B.; Larsson, A.; Palstam, A.; Mannerkorpi, K.; Bileviciute-Ljungar, I.; Löfgren, M.; Bjersing, J.; Kosek, E.; Gerdle, B.; et al. Comparison of the levels of pro- inflammatory cytokines released in the vastus lateralis muscle of patients with fibromyalgia and healthy controls during contractions of the quadriceps Muscle—A microdialysis study. PLoS ONE 2015, 10, e0143856. [Google Scholar] [CrossRef] [PubMed]
- Liptan, G.L. Fascia: A missing link in our understanding of the pathology of fibromyalgia. J. Bodyw. Mov. Ther. 2010, 14, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Titova, D.; Oeser, A.; Randels, M.; Avalos, I.; Milne, G.L.; Morrow, J.D.; Stein, C.M. Oxidative stress in fibromyalgia and its relationship to symptoms. Clin. Rheumatol. 2009, 28, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gómez, E.; Cano-García, F.J.; De Miguel, M.; Carrión, A.M.; Navas, P.; Sánchez Alcázar, J.A. Clinical symptoms in fibromyalgia are better associated to lipid peroxidation levels in blood mononuclear cells rather than in plasma. PLoS ONE 2011, 6, e26915. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, R.; Mughal, M.S.; Arshad, N.; Arshad, M. Pathophysiology and antioxidant status of patients with fibromyalgia. Rheumatol. Int. 2011, 31, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Boomershine, C.S. Fibromyalgia: The prototypical central sensitivity syndrome. Curr. Rheumatol. Rev. 2015, 11, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Sprott, H.; Salemi, S.; Gay, R.E.; Bradley, L.A.; Alarcón, G.S.; Oh, S.J.; Michel, B.A.; Gay, S. Increased DNA fragmentation and ultrastructural changes in fibromyalgic muscle fibres. Ann. Rheum. Dis. 2004, 63, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.C.; O’Connor, D.T.; Steinmeyer, R.; Del Mundo, J.D.; McMullan, D.R.; Whitt, J.A.; Ramos, J.E.; Gonzales, R.J. The influence of acute resistance exercise on cyclooxygenase-1 and -2 activity and protein levels in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R24–R30. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gómez, E.; Marín-Aguilar, F.; Rybkina, T.; Cotán, D.; Pérez-Pulido, A.; Alvarez-Suarez, J.M.; Battino, M.; Sánchez-Alcazar, J.A.; Carrión, A.M.; et al. Mutation in cytochrome b gene of mitochondrial DNA in a family with fibromyalgia is associated with NLRP3-inflammasome activation. J. Med. Genet. 2016, 53, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Meeus, M.; Nijs, J.; Hermans, L.; Goubert, D.; Calders, P. The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: Peripheral and central mechanisms as therapeutic targets? Expert. Opin. Ther. Targets 2013, 17, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Lukens, J.R.; Kanneganti, T.D. Mitochondria: Diversity in the regulation of the NLRP3 inflammasome. Trends Mol. Med. 2015, 21, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014, 5, 352. [Google Scholar] [CrossRef] [PubMed]
- De Souza Nascimento, S.; Desantana, J.M.; Nampo, F.K.; Ribeiro, E.A.; da Silva, D.L.; Araújo-Júnior, J.X.; da Silva Almeida, J.R.; Bonjardim, L.R.; de Souza Araújo, A.A.; Quintans-Júnior, L.J. Efficacy and safety of medicinal plants or related natural products for fibromyalgia: A systematic review. Evid. Based Complement. Altern. Med. 2013, 2013, 149468. [Google Scholar] [CrossRef] [PubMed]
- Ablin, J.N.; Buskila, D. Fibromyalgia syndrome—Novel therapeutic targets. Maturitas 2013, 75, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D.E.; Malemud, C.J. Recent strategies for drug development in fibromyalgia syndrome. Expert. Rev. Neurother. 2016, 16, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Danilov, A.; Kurganova, J. Melatonin in chronic pain syndromes. Pain Ther. 2016, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pernambuco, A.P.; Schetino, L.P.; Viana, R.S.; Carvalho, L.S.; d’Ávila Reis, D. The involvement of melatonin in the clinical status of patients with fibromyalgia syndrome. Clin. Exp. Rheumatol. 2015, 33, S14–S19. [Google Scholar] [PubMed]
- Johnston, J.D.; Skene, D.J. 60 years of neuroendocrinology: Regulation of mammalian neuroendocrine physiology and rhythms by melatonin. J. Endocrinol. 2015, 226, T187–T198. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Tututi, M.; Granados-Soto, V. Oral and spinal melatonin reduces tactile allodynia in rats via activation of MT2 and opioid receptors. Pain 2007, 132, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P. The analgesic effects of exogenous melatonin in humans. Dan. Med. J. 2016, 63, B5289. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The safety of melatonin in humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, G.A.; Then, H.; Oezel, L.; Schwarzbach, H.; Ocker, M.; Thieme, K.; Di Fazio, P.; Kinscherf, R. Morphological alterations in gastrocnemius and soleus muscles in male and female mice in a fibromyalgia model. PLoS ONE 2016, 11, e0151116. [Google Scholar] [CrossRef] [PubMed]
- Cobos, E.J.; Ghasemlou, N.; Araldi, D.; Segal, D.; Duong, K.; Woolf, C.J. Inflammation- induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain 2012, 153, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y.; Oe, T.; Aoki, T.; Matsuoka, N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 2009, 146, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Serra, A.; Escrihuela-Vidal, F.; González-Soler, E.M.; Martínez-Expósito, F.; Blasco-Ausina, M.C.; Martínez-Bellver, S.; Cervera-Ferri, A.; Teruel-Martí, V.; Valverde-Navarro, A.A. Depressive-like symptoms in a reserpine-induced model of fibromyalgia in rats. Physiol. Behav. 2015, 151, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Chang, K.C.; Lin, R.F.; Wang, M.H.; Shih, R.L.; Tseng, H.C.; Soung, H.S.; Tsai, C.C. Nitric oxide pathway activity modulation alters the protective effects of (−) Epigallocatechin-3-gallate on reserpine-induced impairment in rats. Behav. Brain Res. 2016, 305, 198–211. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, P.; Lopes-Martins, R.Á.; Tomazoni, S.S.; Silva, J.A., Jr.; de Carvalho Pde, T.; Bjordal, J.M.; Leal Junior, E.C. Low-level laser therapy improves skeletal muscle performance, decreases skeletal muscle damage and modulates mRNA expression of COX-1 and COX-2 in a dose-dependent manner. Photochem. Photobiol. 2011, 87, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Patel, K.; Muldoon-Jacobs, K.; Bisht, K.S.; Aykin-Burns, N.; Pennington, J.D.; van der Meer, R.; Nguyen, P.; Savage, J.; Owens, K.M.; et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 2010, 17, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Coleman, M.C.; Pennington, J.D.; Ozden, O.; Park, S.H.; Jiang, H.; Kim, H.S.; Flynn, C.R.; Hill, S.; Hayes McDonald, W.; et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell. 2010, 40, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Park, H.H. Purification and analysis of the interactions of caspase-1 and ASC for assembly of the inflammasome. Appl. Biochem. Biotechnol. 2015, 175, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, B.; Wang, W.; Liu, X.; Liu, X.; Xia, Y.; Zhang, C.; Zhang, Y.; Zhang, M.; An, F. Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model. Cardiovasc. Drugs Ther. 2014, 28, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Zhang, N.; Chen, J. Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomed. Pharmacother. 2016, 83, 975–988. [Google Scholar] [CrossRef] [PubMed]
- De Zanette, S.A.; Vercelino, R.; Laste, G.; Rozisky, J.R.; Schwertner, A.; Machado, C.B.; Xavier, F.; de Souza, I.C.; Deitos, A.; Torres, I.L.; et al. Melatonin analgesia is associated with improvement of the descending endogenous pain-modulating system in fibromyalgia: A phase II, randomized, double-dummy, controlled trial. BMC Pharmacol. Toxicol. 2014, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Citera, G.; Arias, M.A.; Maldonado-Cocco, J.A.; Lázaro, M.A.; Rosemffet, M.G.; Brusco, L.I.; Scheines, E.J.; Cardinali, D.P. The effect of melatonin in patients with fibromyalgia: A pilot study. Clin. Rheumatol. 2000, 19, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Al-Khalifa, I.I.; Jasim, N.A.; Gorial, F.I. Adjuvant use of melatonin for treatment of fibromyalgia. J. Pineal Res. 2011, 50, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Stacchiotti, A.; Castrezzati, S.; Bonomini, F.; Albanese, M.; Rezzani, R.; Rodella, L.F. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr. Res. 2015, 35, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Aversa, S.; Arrigo, T.; Reiter, R.J.; Gitto, E. Analgesic, anxiolytic and anaesthetic effects of melatonin: New potential uses in pediatrics. Int. J. Mol. Sci. 2015, 16, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Chung, E.J.; Kwon, H.J.; Im, S.S.; Lim, J.G.; Song, D.K. Protective effect of melatonin on TNF-α-induced muscle atrophy in L6 myotubes. J. Pineal Res. 2013, 54, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective effects of melatonin and mitochondria-targeted antioxidants against oxidative stress: A review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen, M.; Amirian, I.; Reiter, R.J.; Rosenberg, J.; Gögenur, I. Analgesic effects of melatonin: A review of current evidence from experimental and clinical studies. J. Pineal Res. 2011, 51, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.K.; Ding, J.F.; Liu, J.; Yang, Y.Y. Analgesic effects of melatonin on post-herpetic neuralgia. Int. J. Clin. Exp. Med. 2015, 8, 5004–5009. [Google Scholar] [PubMed]
- Carpenter, J.S.; Abelmann, A.C.; Hatton, S.N.; Robillard, R.; Hermens, D.F.; Bennett, M.R.; Lagopoulos, J.; Hickie, I.B. Pineal volume and evening melatonin in young people with affective disorders. Brain Imaging Behav. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Wang, M.; Lian, B.; Sun, H.; Wang, G.; Li, Q.; Sun, L. Melatonin produces a rapid onset and prolonged efficacy in reducing depression-like behaviors in adult rats exposed to chronic unpredictable mild stress. Neurosci. Lett. 2017, 642, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Lauterbach, E.C.; Ho, K.Y.; Acuña-Castroviejo, D.; Zakaria, R.; Brzezinski, A. Melatonin in antinociception: Its therapeutic applications. Curr. Neuropharmacol. 2012, 10, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.R.; Laste, G.; Deitos, A.; Stefani, L.C.; Cambraia-Canto, G.; Torres, I.L.; Brunoni, A.R.; Fregni, F.; Caumo, W. Combined neuromodulatory interventions in acute experimental pain: Assessment of melatonin and non-invasive brain stimulation. Front. Behav. Neurosci. 2015, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K. Potential drug therapies for the treatment of fibromyalgia. Expert Opin. Investig. Drugs 2016, 25, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Canul, M.; Palazzo, E.; Dominguez-Lopez, S.; Luongo, L.; Lacoste, B.; Comai, S.; Angeloni, D.; Fraschini, F.; Boccella, S.; Spadoni, G.; et al. Selective melatonin MT2 receptor ligands relieve neuropathic pain through modulation of brainstem descending antinociceptive pathways. Pain 2015, 156, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Posa, L.; De Gregorio, D.; Gobbi, G.; Comai, S. Targeting melatonin MT2 receptors: A novel pharmacological avenue for inflammatory and neuropathic pain. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, J.; Hong, Y.; Lee, M.; Kim, K.; Lee, S.R.; Chang, K.T.; Hong, Y. Beneficial effects of melatonin on stroke-induced muscle atrophy in focal cerebral ischemic rats. Lab. Anim. Res. 2012, 28, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Rodella, L.F.; Nardo, L.; Giugno, L.; Cocchi, M.A.; Borsani, E.; Reiter, R.J.; Rezzani, R. A comparison of melatonin and á-lipoic acid in the induction of antioxidant defences in L6 rat skeletal muscle cells. Age 2015, 37, 9824. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y.; Takahashi, M.; Noto, T.; Sekizawa, T.; Oe, T.; Yoshimi, E.; Tamaki, K.; Shimizu, Y. Different pathophysiology underlying animal models of fibromyalgia and neuropathic pain: Comparison of reserpine-induced myalgia and chronic constriction injury rats. Behav. Brain Res. 2012, 226, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Nagakura, Y.; Tsukamoto, M.; Watabiki, T.; Ozawa, T.; Oe, T.; Shimizu, Y.; Ito, H. Systemic administration of 5-HT(2C) receptor agonists attenuates muscular hyperalgesia in reserpine-induced myalgia model. Pharmacol. Biochem. Behav. 2013, 108, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Ghelardini, C.; Thurmond, R.L.; Masini, E.; Galeotti, N. Behavioural phenotype of histamine H(4) receptor knockout mice: Focus on central neuronal functions. Neuropharmacology 2017, 114, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Murai, N.; Fushiki, H.; Honda, S.; Murakami, Y.; Iwashita, A.; Irie, M.; Tamura, S.; Nagakura, Y.; Aoki, T. Relationship between serotonin transporter occupancies and analgesic effects of AS1069562, the (+)-isomer of indeloxazine, and duloxetine in reserpine-induced myalgia rats. Neuroscience 2015, 289, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Naidu, P.S.; Kulkarni, S.K. Reversal of reserpine-induced vacuous chewing movements in rats by melatonin: Involvement of peripheral benzodiazepine receptors. Brain Res. 2001, 904, 149–152. [Google Scholar] [CrossRef]
- Abílio, V.C.; Vera, J.A., Jr.; Ferreira, L.S.; Duarte, C.R.; Carvalho, R.C.; Grassl, C.; Martins, C.R.; Torres-Leite, D.; Bignotto, M.; Tufik, S.; et al. Effects of melatonin on orofacial movements in rats. Psychopharmacology 2002, 161, 340–347. [Google Scholar] [PubMed]
- Luna-Sánchez, M.; Díaz-Casado, E.; Barca, E.; Tejada, M.Á.; Montilla-García, Á.; Cobos, E.J.; Escames, G.; Acuña-Castroviejo, D.; Quinzii, C.M.; López, L.C. The clinical heterogeneity of coenzyme Q10 deficiency results from genotypic differences in the Coq9 gene. EMBO Mol. Med. 2015, 7, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhang, W.; Wang, J.; Luo, F.; Chang, J.; Xu, R. Impaired voluntary wheel running behavior in the unilateral 6-hydroxydopamine rat model of Parkinson’s disease. J. Korean Neurosurg. Soc. 2015, 57, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.A.; Oliveira, C.S.; Ineu, R.P.; Moraes-Silva, L.; de Siqueira, L.F.; Pereira, M.E. Lactating and non-lactating rats differ in sensitivity to HgCl2: Protective effect of ZnCl2. J. Trace Elem. Med. Biol. 2014, 28, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.A.; Favero, G.; Stacchiotti, A.; Giugno, L.; Buffoli, B.; de Oliveira, C.S.; Lavazza, A.; Albanese, M.; Rodella, L.F.; Pereira, M.E.; et al. Acute mercuri exposition of virgin, pregnant, and lactating rats: Histopathological kidney and liver evaluations. Environ. Toxicol. 2016, 32, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Agabiti-Rosei, C.; Favero, G.; De Ciuceis, C.; Rossini, C.; Porteri, E.; Rodella, L.F.; Franceschetti, L.; Maria Sarkar, A.; Agabiti-Rosei, E.; Rizzoni, D.; et al. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens. Res. 2017, 40, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Favero, G.; Lavazza, A.; Golic, I.; Aleksic, M.; Korac, A.; Rodella, L.F.; Rezzani, R. Hepatic macrosteatosis is partially converted to microsteatosis by melatonin supplementation in ob/ob mice non-alcoholic fatty liver disease. PLoS ONE 2016, 11, e0148115. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Favero, G.; Stacchiotti, A.; Rodella, L.F. Endothelial and vascular smooth muscle cell dysfunction mediated by cyclophylin A and the atheroprotective effects of melatonin. Life Sci. 2013, 92, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Rossini, C.; Favero, G.; Foglio, E.; Loreto, C.; Rezzani, R. Nicotine-induced morphological changes in rat aorta: The protective role of melatonin. Cells Tissues Organs 2012, 195, 252–259. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favero, G.; Trapletti, V.; Bonomini, F.; Stacchiotti, A.; Lavazza, A.; Rodella, L.F.; Rezzani, R. Oral Supplementation of Melatonin Protects against Fibromyalgia-Related Skeletal Muscle Alterations in Reserpine-Induced Myalgia Rats. Int. J. Mol. Sci. 2017, 18, 1389. https://doi.org/10.3390/ijms18071389
Favero G, Trapletti V, Bonomini F, Stacchiotti A, Lavazza A, Rodella LF, Rezzani R. Oral Supplementation of Melatonin Protects against Fibromyalgia-Related Skeletal Muscle Alterations in Reserpine-Induced Myalgia Rats. International Journal of Molecular Sciences. 2017; 18(7):1389. https://doi.org/10.3390/ijms18071389
Chicago/Turabian StyleFavero, Gaia, Valentina Trapletti, Francesca Bonomini, Alessandra Stacchiotti, Antonio Lavazza, Luigi Fabrizio Rodella, and Rita Rezzani. 2017. "Oral Supplementation of Melatonin Protects against Fibromyalgia-Related Skeletal Muscle Alterations in Reserpine-Induced Myalgia Rats" International Journal of Molecular Sciences 18, no. 7: 1389. https://doi.org/10.3390/ijms18071389






