Effect of Cytomegalovirus (CMV) and Ageing on T-Bet and Eomes Expression on T-Cell Subsets
Abstract
:1. Introduction
2. Results
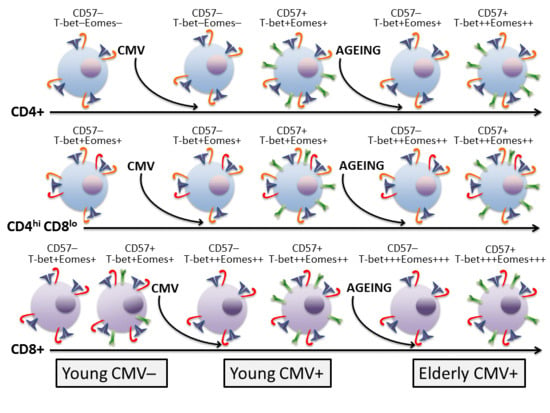
2.1. Molecular Signature of T-Cells in the Context of Ageing and CMV Infection
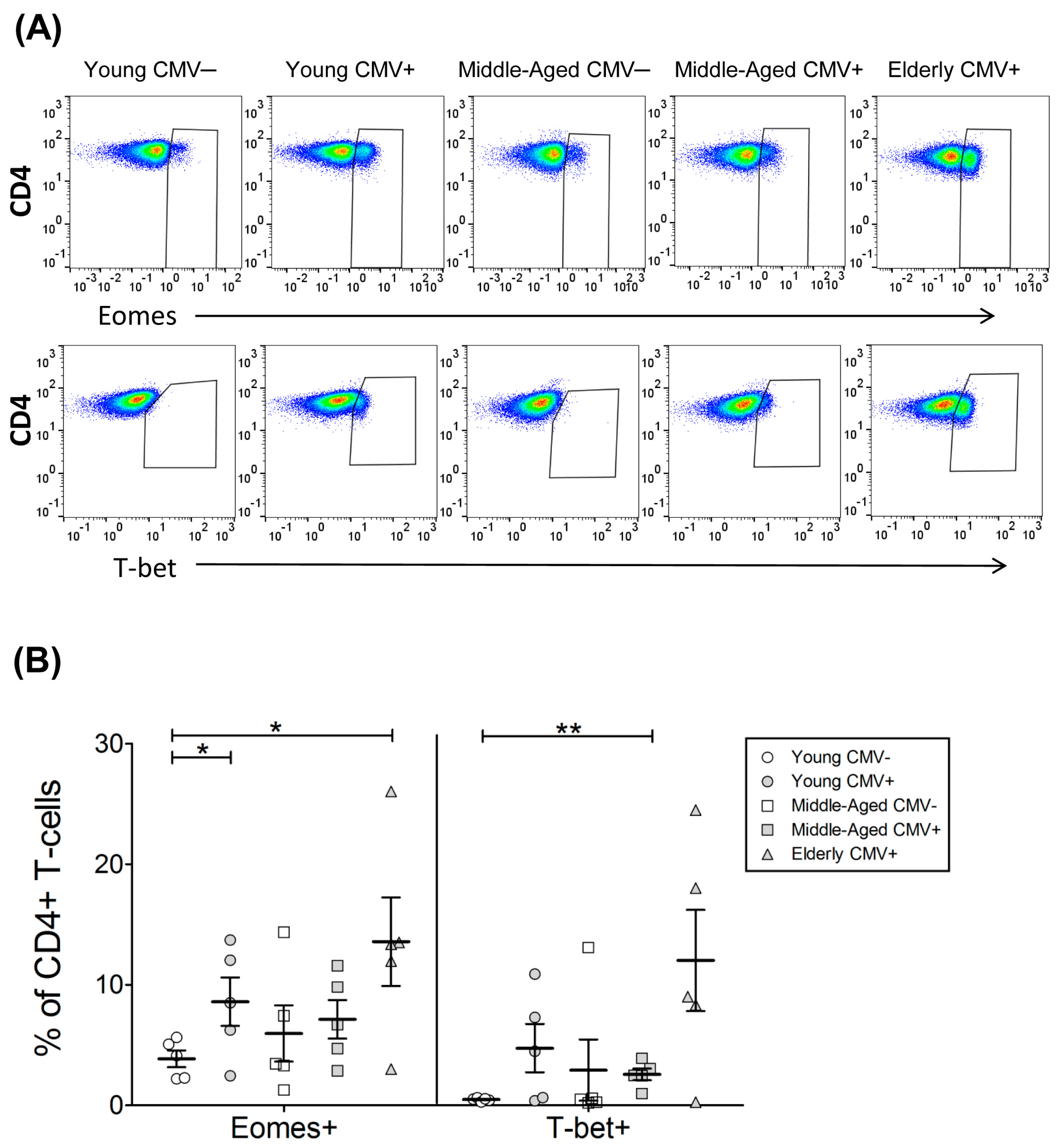
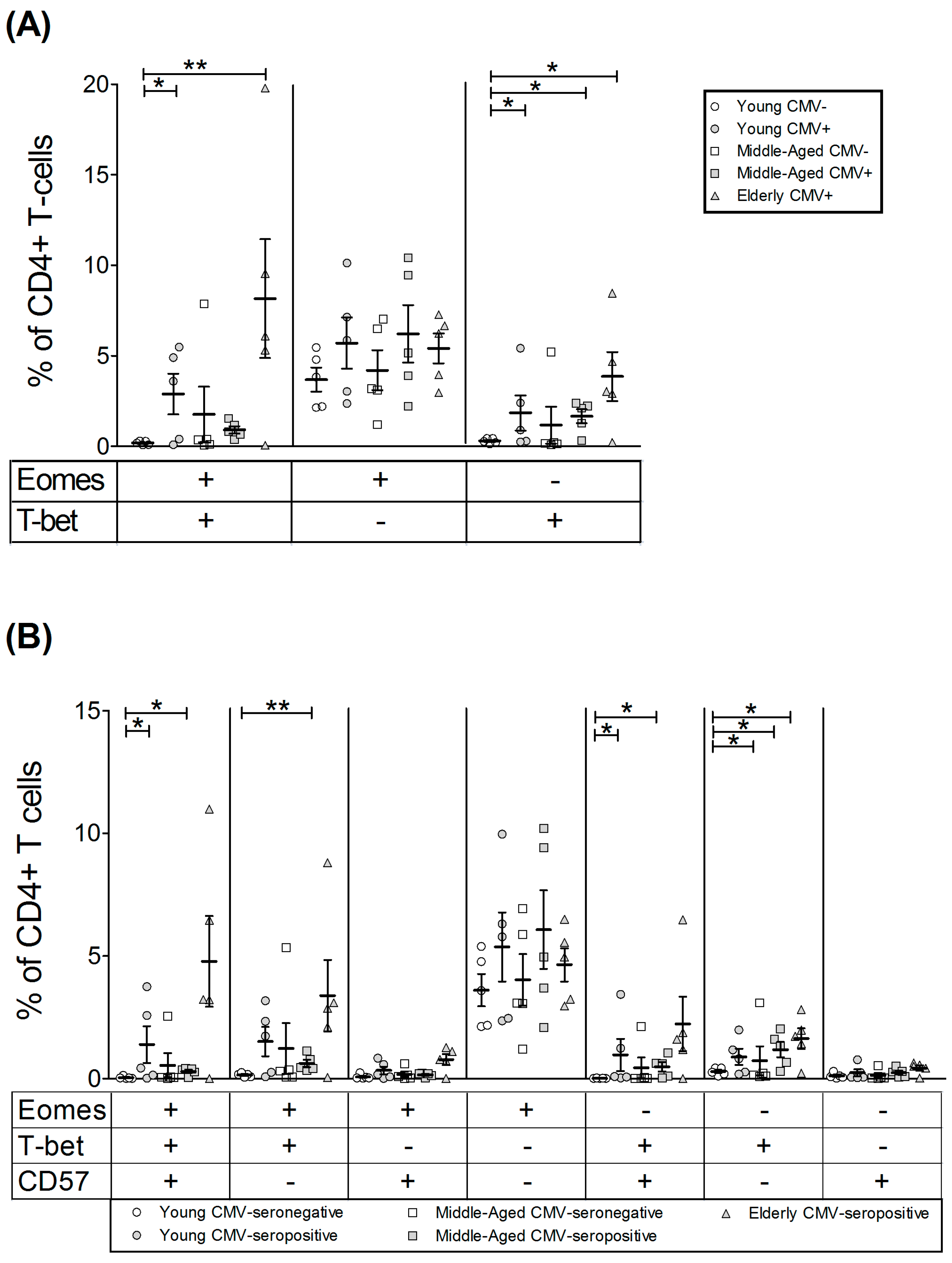
2.2. T-Bet and Eomes Expression in CD4+ T-Cells
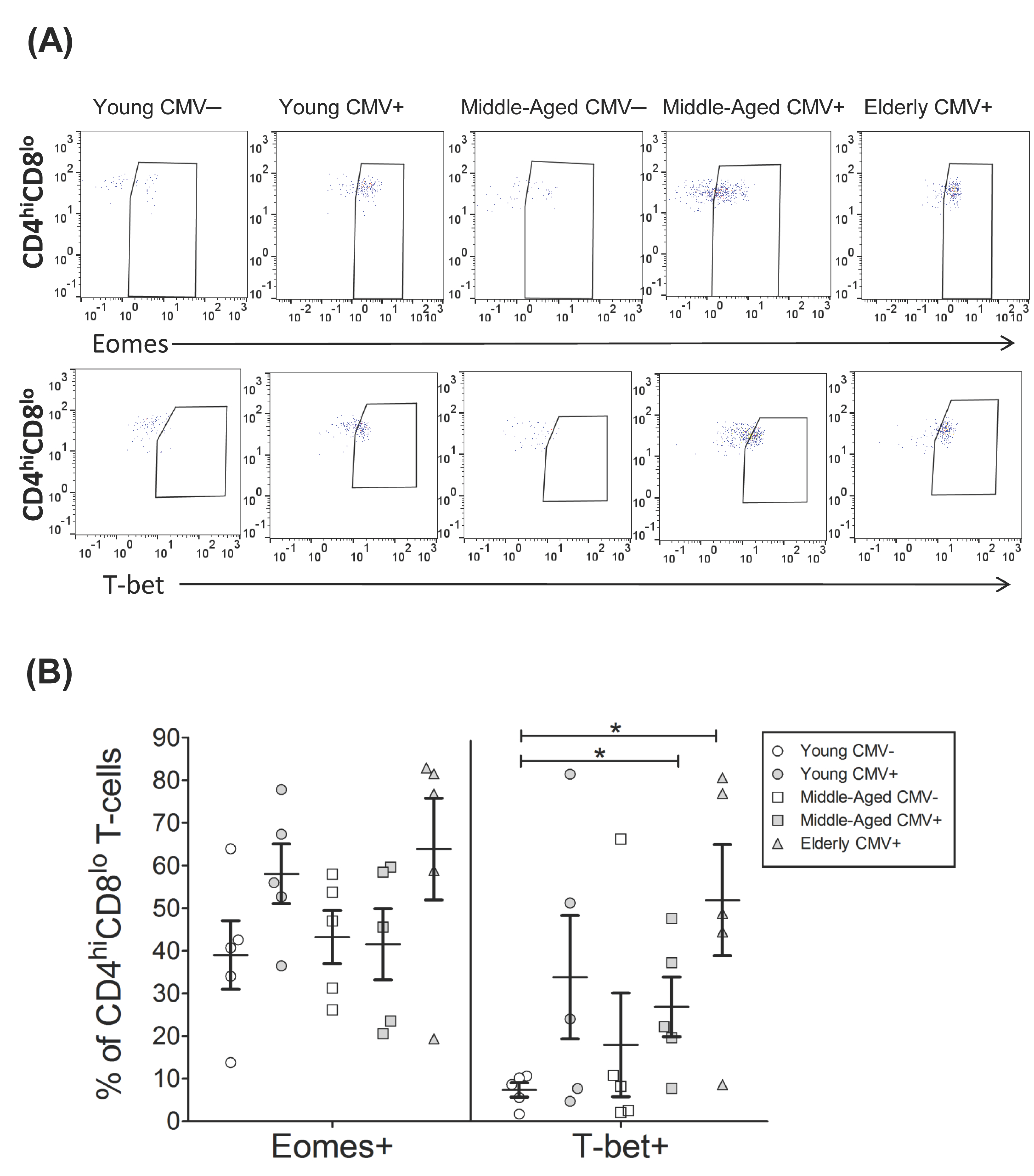
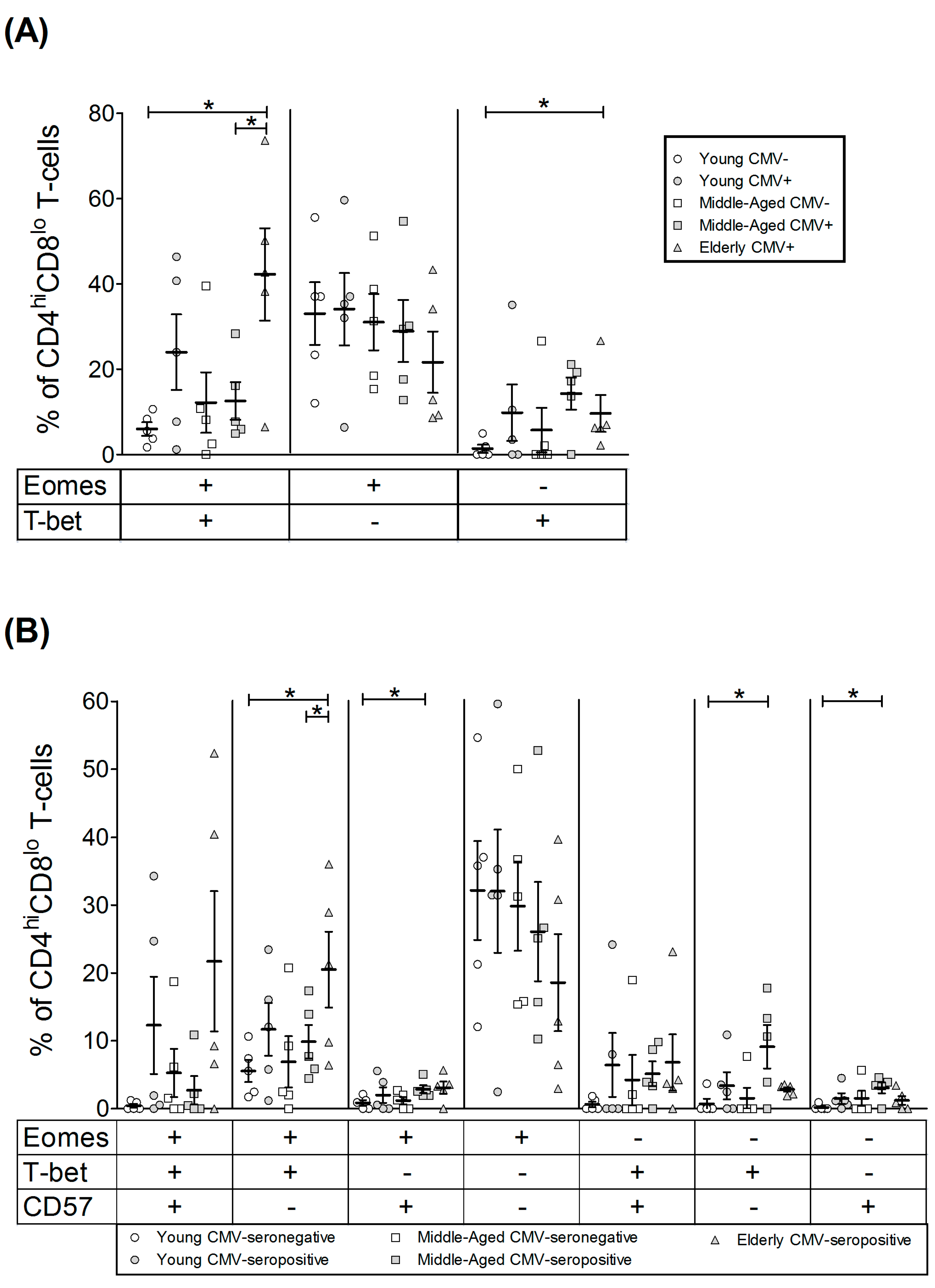
2.3. T-Bet and Eomes Expression on CD4hiCD8lo T-Cells
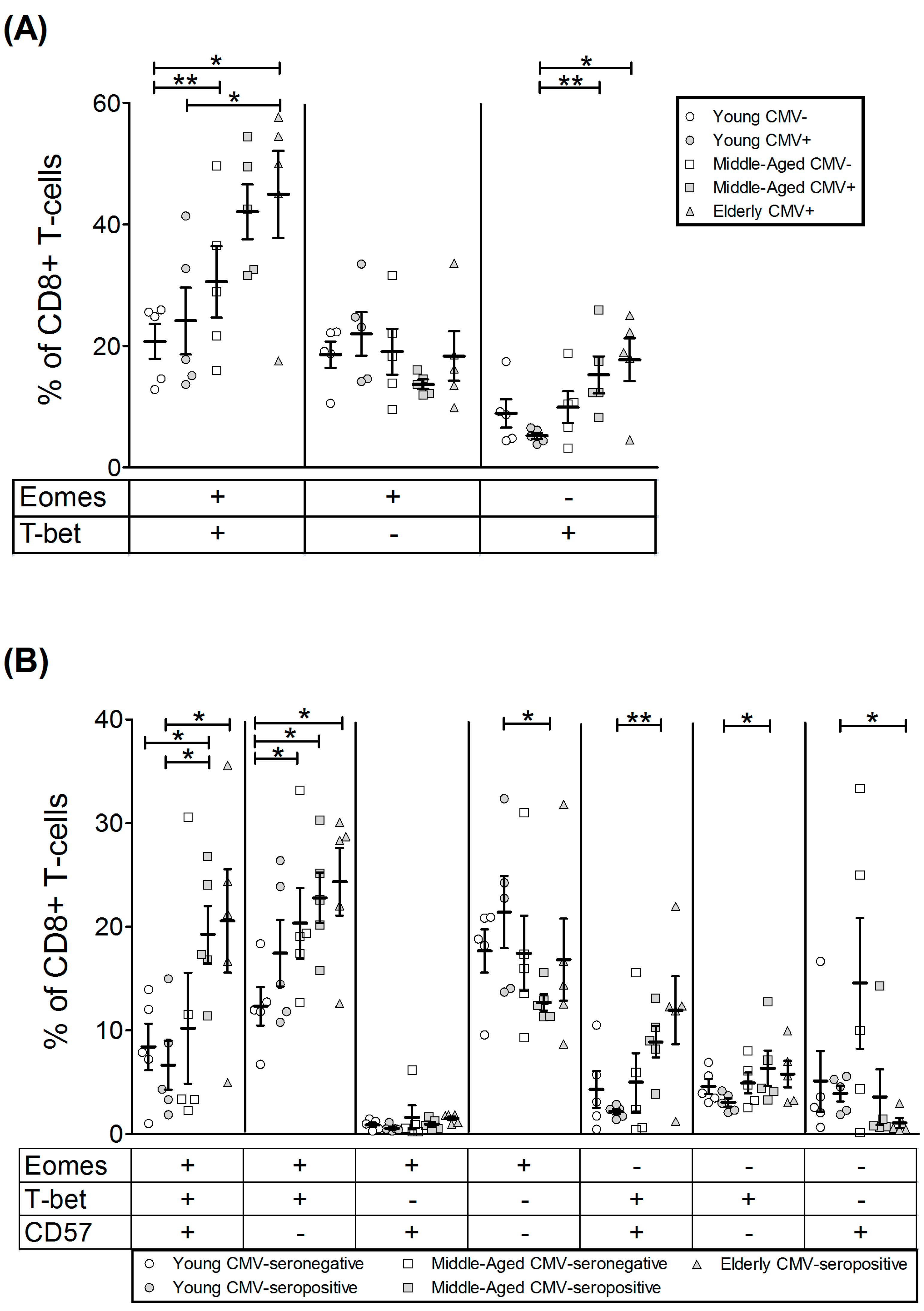
2.4. T-Bet and Eomes Expression in CD8+ T-Cells
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. CMV Serology
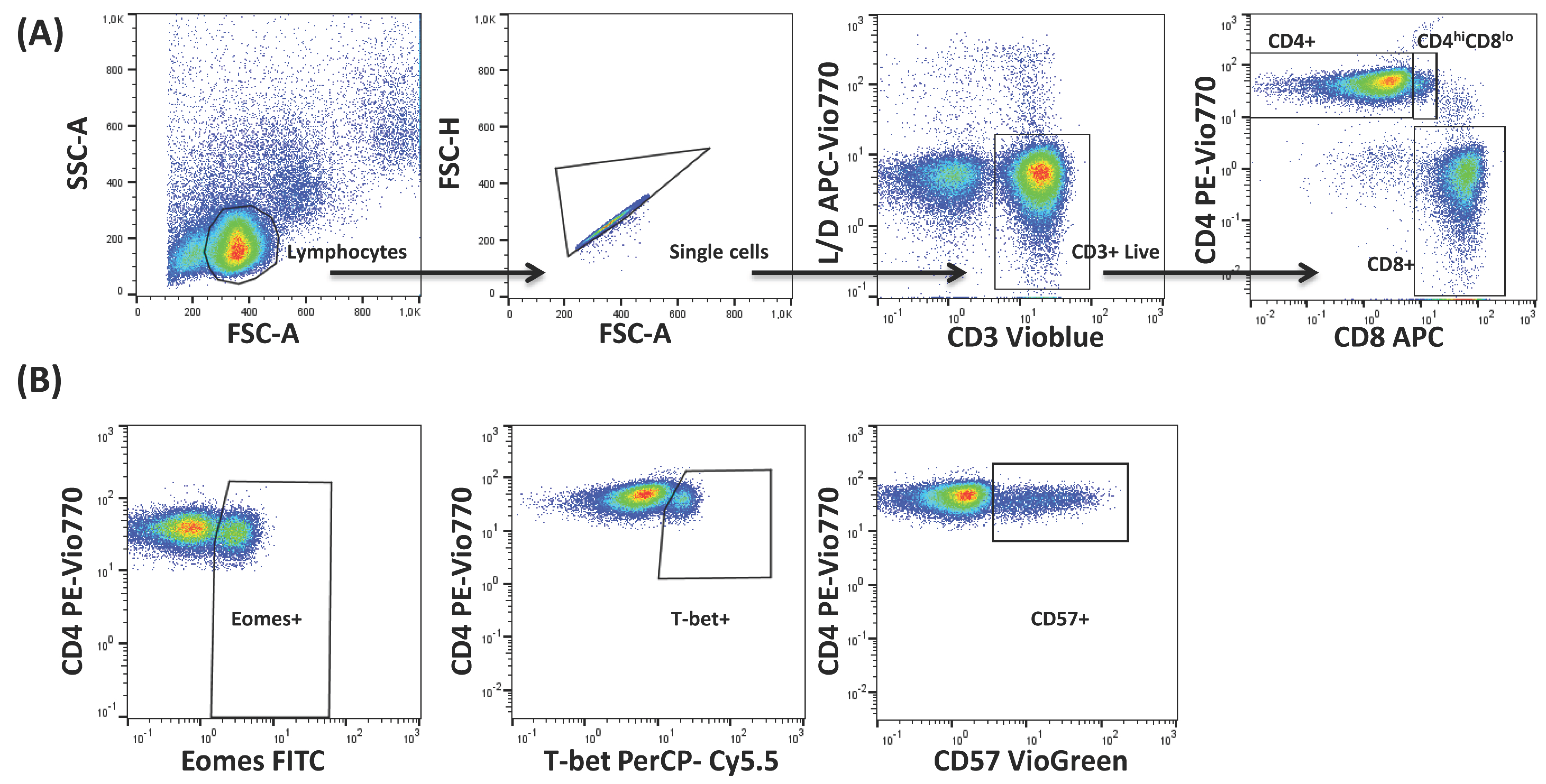
4.3. Flow Cytometry and Data Analysis
4.4. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muller, L.; Fulop, T.; Pawelec, G. Immunosenescence in vertebrates and invertebrates. Immun. Ageing 2013, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Cantisan, S.; Torre-Cisneros, J.; Lara, R.; Zarraga, S.; Montejo, M.; Solana, R. Impact of cytomegalovirus on early immunosenescence of CD8+ T lymphocytes after solid organ transplantation. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Witkowski, J.M.; Kotb, R.; Hirokawa, K.; Pawelec, G. Immunosenescence and cancer. Crit. Rev. Oncog. 2013, 18, 489–513. [Google Scholar] [CrossRef] [PubMed]
- Grubeck-Loebenstein, B.; Della, B.S.; Iorio, A.M.; Michel, J.P.; Pawelec, G.; Solana, R. Immunosenescence and vaccine failure in the elderly. Aging Clin. Exp. Res. 2009, 21, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, R.; Sanchez-Correa, B.; Casas-Aviles, I.; Campos, C.; Pera, A.; Morgado, S.; Lopez-Sejas, N.; Hassouneh, F.; Bergua, J.M.; Arcos, M.J.; et al. Immunosenescence: Limitations of natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2017, 66, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Di, B.S.; Derhovanessian, E.; Steinhagen-Thiessen, E.; Goldeck, D.; Muller, L.; Pawelec, G. Impact of age, sex and CMV-infection on peripheral T-cell phenotypes: Results from the Berlin BASE-II Study. Biogerontology 2015, 16, 631–643. [Google Scholar]
- Pawelec, G. Immunosenenescence: Role of cytomegalovirus. Exp. Gerontol. 2014, 54, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. T-cell immunity in the aging human. Haematologica 2014, 99, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Goldeck, D.; Derhovanessian, E. Inflammation, ageing and chronic disease. Curr. Opin. Immunol. 2014, 29, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef]
- Villarino, A.V.; Gallo, E.; Abbas, A.K. STAT1-activating cytokines limit Th17 responses through both T-bet-dependent and independent mechanisms1. J. Immunol. 2010, 185, 6461–6471. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, V.; Chen, X.; Shim, J.H.; Hwang, E.S.; Jang, E.; Bolm, A.N.; Oukka, M.; Kuchroo, V.K.; Glimcher, L.H. Transcription factor T-bet represses TH17 differentiation by preventing Runx1-mediated activation of the RORγt gene. Nat. Immunol. 2011, 12, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, V.; Glimcher, L.H. T-bet in disease. Nat. Immunol. 2011, 12, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, A.M.; Takemoto, N.; Wherry, E.J.; Longworth, S.A.; Northrup, J.T.; Palanivel, V.R.; Mullen, A.C.; Gasink, C.R.; Kaech, S.M.; Miller, J.D.; et al. Effector and memory CD8+ T-cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005, 6, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- McLane, L.M.; Banerjee, P.P.; Cosma, G.L.; Makedonas, G.; Wherry, E.J.; Orange, J.S.; Betts, M.R. Differential localization of T-bet and Eomes in CD8 T-cell memory populations. J. Immunol. 2013, 190, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.S.; Cui, W.; Chandele, A.; Lee, H.K.; Urso, D.R.; Hagman, J.; Gapin, L.; Kaech, S.M. Inflammation Directs Memory Precursor and Short-Lived Effector CD8(+) T Cell Fates via the Graded Expression of T-bet Transcription Factor. Immunity 2007, 27, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.E.; Jameson, S.C. CD8(+) T-cell differentiation: Choosing a path through T-bet. Immunity 2007, 27, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.J.; Cosma, G.L.; Betts, M.R.; McLane, L.M. Characterization of T-bet and eomes in peripheral human immune cells. Front. Immunol. 2014, 5, 217. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Oestreich, K.J.; Paley, M.A.; Crawford, A.; Angelosanto, J.M.; Ali, M.A.; Intlekofer, A.M.; Boss, J.M.; Reiner, S.L.; Weinmann, A.S.; et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T-cell responses during chronic infection. Nat. Immunol. 2011, 12, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Hersperger, A.R.; Martin, J.N.; Shin, L.Y.; Sheth, P.M.; Kovacs, C.M.; Cosma, G.L.; Makedonas, G.; Pereyra, F.; Walker, B.D.; Kaul, R.; et al. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 2011, 117, 3799–3808. [Google Scholar] [CrossRef] [PubMed]
- Paley, M.A.; Kroy, D.C.; Odorizzi, P.M.; Johnnidis, J.B.; Dolfi, D.V.; Barnett, B.E.; Bikoff, E.K.; Robertson, E.J.; Lauer, G.M.; Reiner, S.L.; et al. Progenitor and terminal subsets of CD8+ T-cells cooperate to contain chronic viral infection. Science 2012, 338, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Akbar, A.; Caruso, C.; Solana, R.; Grubeck-Loebenstein, B.; Wikby, A. Human immunosenescence: Is it infectious? Immunol. Rev. 2005, 205, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Solana, R.; DelaRosa, O.; Pawelec, G. Human cytomegalovirus infection and T-cell immunosenescence: A mini review. Mech. Ageing Dev. 2006, 127, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Solana, R.; Tarazona, R.; Aiello, A.E.; Akbar, A.N.; Appay, V.; Beswick, M.; Bosch, J.A.; Campos, C.; Cantisan, S.; Cicin-Sain, L.; et al. CMV and Immunosenescence: From basics to clinics. Immun. Ageing 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing 2012, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Simanek, A.M.; Dowd, J.B.; Pawelec, G.; Melzer, D.; Dutta, A.; Aiello, A.E. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS ONE 2011, 6, e16103. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Elhassen, D.; Gras, S.; Wynn, K.K.; Dasari, V.; Tellam, J.; Tey, S.K.; Rehan, S.; Liu, Y.C.; Rossjohn, J.; et al. Endogenous antigen presentation impacts on T-box transcription factor expression and functional maturation of CD8+ T-cells. Blood 2012, 120, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- Borrego, F.; Alonso, M.C.; Galiani, M.D.; Carracedo, J.; Ramirez, R.; Ostos, B.; Pena, J.; Solana, R. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp. Gerontol. 1999, 34, 253–265. [Google Scholar] [CrossRef]
- Pera, A.; Vasudev, A.; Tan, C.; Kared, H.; Solana, R.; Larbi, A. CMV induces expansion of highly polyfunctional CD4+ T-cell subset coexpressing CD57 and CD154. J. Leukoc. Biol. 2016, 101, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Silva, R.; Peruzzi, G.; Alvarez, Y.; Simhadri, V.R.; Debell, K.; Coligan, J.E.; Borrego, F. Human Th1 cells that express CD300a are polyfunctional and after stimulation up-regulate the T-box transcription factor eomesodermin. PLoS ONE 2010, 5, e10636. [Google Scholar] [CrossRef] [PubMed]
- Hassouneh, F.; Lopez-Sejas, N.; Campos, C.; Tarazona, R.; Solana, R.; Pera, A. Effect of age and latent CMV infection on CD300a expression on T lymphocytes. Front. Immunol. 2017. under review. [Google Scholar]
- Edwards, E.S.; Smith, C.; Khanna, R. Phenotypic and transcriptional profile correlates with functional plasticity of antigen-specific CD4+ T-cells. Immunol. Cell Biol. 2014, 92, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Pipeling, M.R.; Mannem, H.; Shah, P.D.; Orens, J.B.; Connors, M.; Migueles, S.A.; McDyer, J.F. IL-12-Dependent Cytomegalovirus-Specific CD4+ T Cell Proliferation, T-bet Induction, and Effector Multifunction during Primary Infection Are Key Determinants for Early Immune Control. J. Immunol. 2016, 196, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Prato, G.; Stella, S.; Scielzo, C.; Geuna, M.; Caligaris-Cappio, F. Age-dependent accumulation of monoclonal CD4+CD8+ double positive T lymphocytes in the peripheral blood of the elderly. Br. J. Haematol. 2007, 139, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Parel, Y.; Chizzolini, C. CD4+ CD8+ double positive (DP) T-cells in health and disease. Autoimmun. Rev. 2004, 3, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Nascimbeni, M.; Shin, E.C.; Chiriboga, L.; Kleiner, D.E.; Rehermann, B. Peripheral CD4(+)CD8(+) T-cells are differentiated effector memory cells with antiviral functions. Blood 2004, 104, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Pita-Lopez, M.L.; Pera, A.; Solana, R. Adaptive Memory of Human NK-like CD8+ T-Cells to Aging, and Viral and Tumor Antigens. Front. Immunol. 2016, 7, 616. [Google Scholar] [CrossRef] [PubMed]
- Derhovanessian, E.; Maier, A.B.; Hahnel, K.; Zelba, H.; de Craen, A.J.; Roelofs, H.; Slagboom, E.P.; Westendorp, R.G.; Pawelec, G. Lower proportion of naive peripheral CD8+ T-cells and an unopposed pro-inflammatory response to human Cytomegalovirus proteins in vitro are associated with longer survival in very elderly people. Age 2013, 35, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Pita-Lopez, M.L.; Gayoso, I.; DelaRosa, O.; Casado, J.G.; Alonso, C.; Munoz-Gomariz, E.; Tarazona, R.; Solana, R. Effect of ageing on CMV-specific CD8 T-cells from CMV seropositive healthy donors. Immun. Ageing 2009, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Larbi, A.; Derhovanessian, E.; Ozcelik, D.; Naumova, E.; Pawelec, G. Multiparameter flow cytometric analysis of CD4 and CD8 T-cell subsets in young and old people. Immun. Ageing 2008, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Larbi, A.; Ozcelik, D.; Solana, R.; Gouttefangeas, C.; Attig, S.; Wikby, A.; Strindhall, J.; Franceschi, C.; Pawelec, G. Cytomegalovirus infection: A driving force in human T-cell immunosenescence. Ann. N. Y. Acad. Sci. 2007, 1114, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Wagner, W.M.; Wikby, A.; Walter, S.; Aubert, G.; Dodi, A.I.; Travers, P.; Pawelec, G. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J. Clin. Immunol. 2003, 23, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Pera, A.; Campos, C.; Corona, A.; Sanchez-Correa, B.; Tarazona, R.; Larbi, A.; Solana, R. CMV latent infection improves CD8+ T response to SEB due to expansion of polyfunctional CD57+ cells in young individuals. PLoS ONE 2014, 9, e88538. [Google Scholar] [CrossRef] [PubMed]
- Barbarin, A.; Cayssials, E.; Jacomet, F.; Nunez, N.G.; Basbous, S.; Lefevre, L.; Abdallah, M.; Piccirilli, N.; Morin, B.; Lavoue, V.; et al. Phenotype of NK-Like CD8(+) T Cells with Innate Features in Humans and Their Relevance in Cancer Diseases. Front. Immunol. 2017, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Seckert, C.K.; Griessl, M.; Buttner, J.K.; Scheller, S.; Simon, C.O.; Kropp, K.A.; Renzaho, A.; Kuhnapfel, B.; Grzimek, N.K.; Reddehase, M.J. Viral latency drives ‘memory inflation’: A unifying hypothesis linking two hallmarks of cytomegalovirus infection. Med. Microbiol. Immunol. 2012, 201, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Torti, N.; Walton, S.M.; Brocker, T.; Rulicke, T.; Oxenius, A. Non-hematopoietic cells in lymph nodes drive memory CD8 T-cell inflation during murine cytomegalovirus infection. PLoS Pathog. 2011, 7, e1002313. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Pipeling, M.R.; Shah, P.D.; Orens, J.B.; McDyer, J.F. T-bet: Eomes balance, effector function, and proliferation of cytomegalovirus-specific CD8+ T-cells during primary infection differentiates the capacity for durable immune control. J. Immunol. 2014, 193, 5709–5722. [Google Scholar] [CrossRef] [PubMed]
- De Ory, F.; Ramirez, R.; Garcia, C.L.; Leon, P.; Sagues, M.J.; Sanz, J.C. Is there a change in cytomegalovirus seroepidemiology in Spain? Eur. J. Epidemiol. 2004, 19, 85–89. [Google Scholar] [CrossRef] [PubMed]

| Group | Age Group | Mean Age (SD) | Sex (Male/Female) |
|---|---|---|---|
| Young CMV-Seronegative | Young (18–35) | 31 (6.82) | 5/0 |
| Young CMV-Seropositive | Young (18–35) | 26 (2.92) | 2/3 |
| Middle-aged CMV-Seronegative | Middle-aged (40–60) | 54.2 (8.79) | 3/2 |
| Middle-aged CMV-Seropositive | Middle-aged (40–60) | 52.6 (5.32) | 2/3 |
| Elderly CMV-Seropositive | Elderly (>65) | 74.6 (10.78) | 4/1 |
| Transcription Factors | Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young CMV− | Young CMV+ | Middle-Aged CMV− | Middle-Aged CMV+ | Elderly CMV+ | ||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| EOMES+ | 3.86 | 0.70 | 8.59 | 2.02 | 5.96 | 2.32 | 7.13 | 1.60 | 13.58 | 3.67 |
| T-bet+ | 0.48 | 0.06 | 4.74 | 2.00 | 2.92 | 2.54 | 2.57 | 0.48 | 12.02 | 4.19 |
| Transcription Factors | Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young CMV− | Young CMV+ | Middle-Aged CMV− | Middle-Aged CMV+ | Elderly CMV+ | ||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| EOMES+ | 39.01 | 8.05 | 58.05 | 6.98 | 43.22 | 6.24 | 41.53 | 8.35 | 63.87 | 11.93 |
| T-bet+ | 7.35 | 1.66 | 33.81 | 14.48 | 17.93 | 12.17 | 26.85 | 7.00 | 51.87 | 13.01 |
| Transcription Factors | Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young CMV− | Young CMV+ | Middle-Aged CMV− | Middle-Aged CMV+ | Elderly CMV+ | ||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| EOMES+ | 39.28 | 4.57 | 46.09 | 3.54 | 49.58 | 6.57 | 55.73 | 4.42 | 63.24 | 3.31 |
| T-bet+ | 29.60 | 3.18 | 29.28 | 5.91 | 40.43 | 8.46 | 57.30 | 6.17 | 62.64 | 10.51 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassouneh, F.; Lopez-Sejas, N.; Campos, C.; Sanchez-Correa, B.; Tarazona, R.; Pera, A.; Solana, R. Effect of Cytomegalovirus (CMV) and Ageing on T-Bet and Eomes Expression on T-Cell Subsets. Int. J. Mol. Sci. 2017, 18, 1391. https://doi.org/10.3390/ijms18071391
Hassouneh F, Lopez-Sejas N, Campos C, Sanchez-Correa B, Tarazona R, Pera A, Solana R. Effect of Cytomegalovirus (CMV) and Ageing on T-Bet and Eomes Expression on T-Cell Subsets. International Journal of Molecular Sciences. 2017; 18(7):1391. https://doi.org/10.3390/ijms18071391
Chicago/Turabian StyleHassouneh, Fakhri, Nelson Lopez-Sejas, Carmen Campos, Beatriz Sanchez-Correa, Raquel Tarazona, Alejandra Pera, and Rafael Solana. 2017. "Effect of Cytomegalovirus (CMV) and Ageing on T-Bet and Eomes Expression on T-Cell Subsets" International Journal of Molecular Sciences 18, no. 7: 1391. https://doi.org/10.3390/ijms18071391