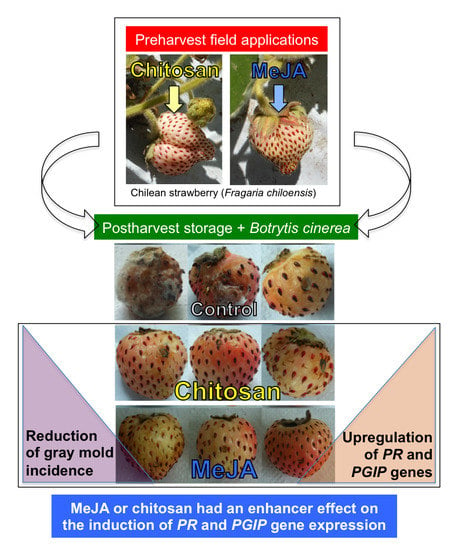
Independent Preharvest Applications of Methyl Jasmonate and Chitosan Elicit Differential Upregulation of Defense-Related Genes with Reduced Incidence of Gray Mold Decay during Postharvest Storage of Fragaria chiloensis Fruit
Abstract
:1. Introduction
2. Results and Discussion
2.1. Postharvest Decay Incidence of Gray Mold in F. chiloensis Fruit Is Lower in MeJA Than Chitosan-Treated Fruit
2.2. Priming Effect of MeJA and Chitosan on Chilean Strawberry Fruit
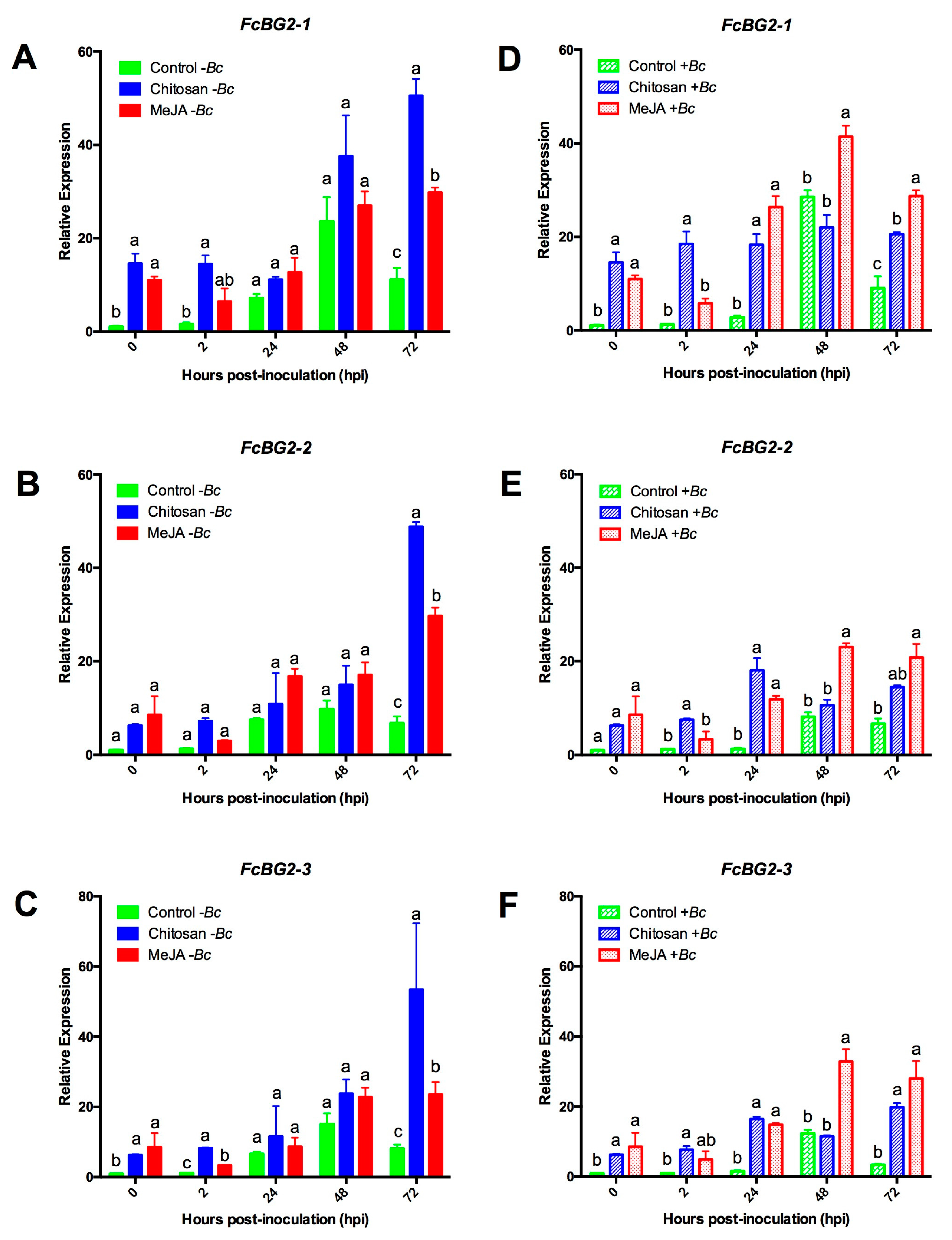
2.3. Differential Upregulation of Defense-Related Genes by MeJA or Chitosan during Postharvest Storage of F. chiloensis Fruit
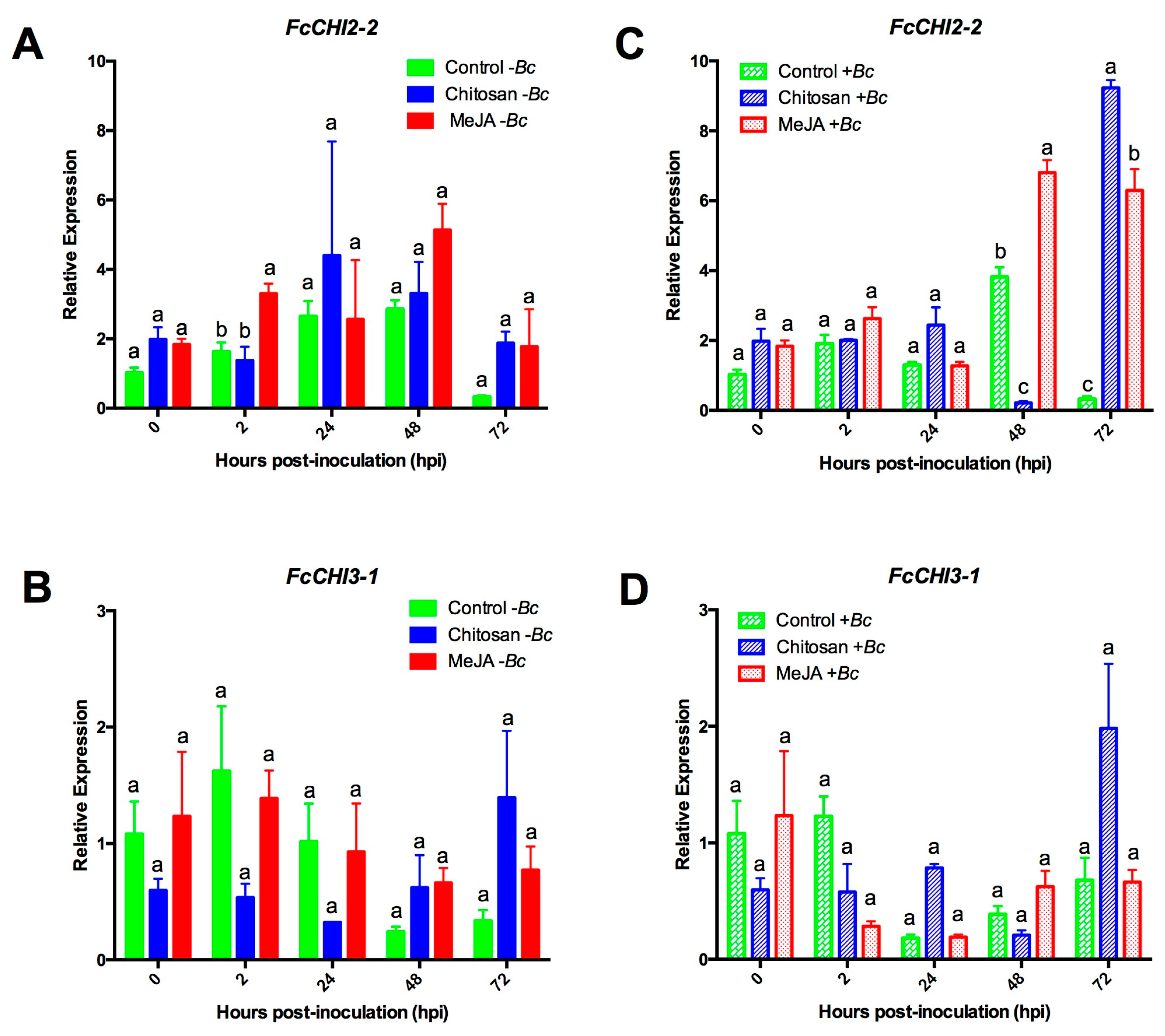
2.3.1. Expression Profiles for β-1,3-glucanases (BGs) and Chitinases (CHIs) Genes
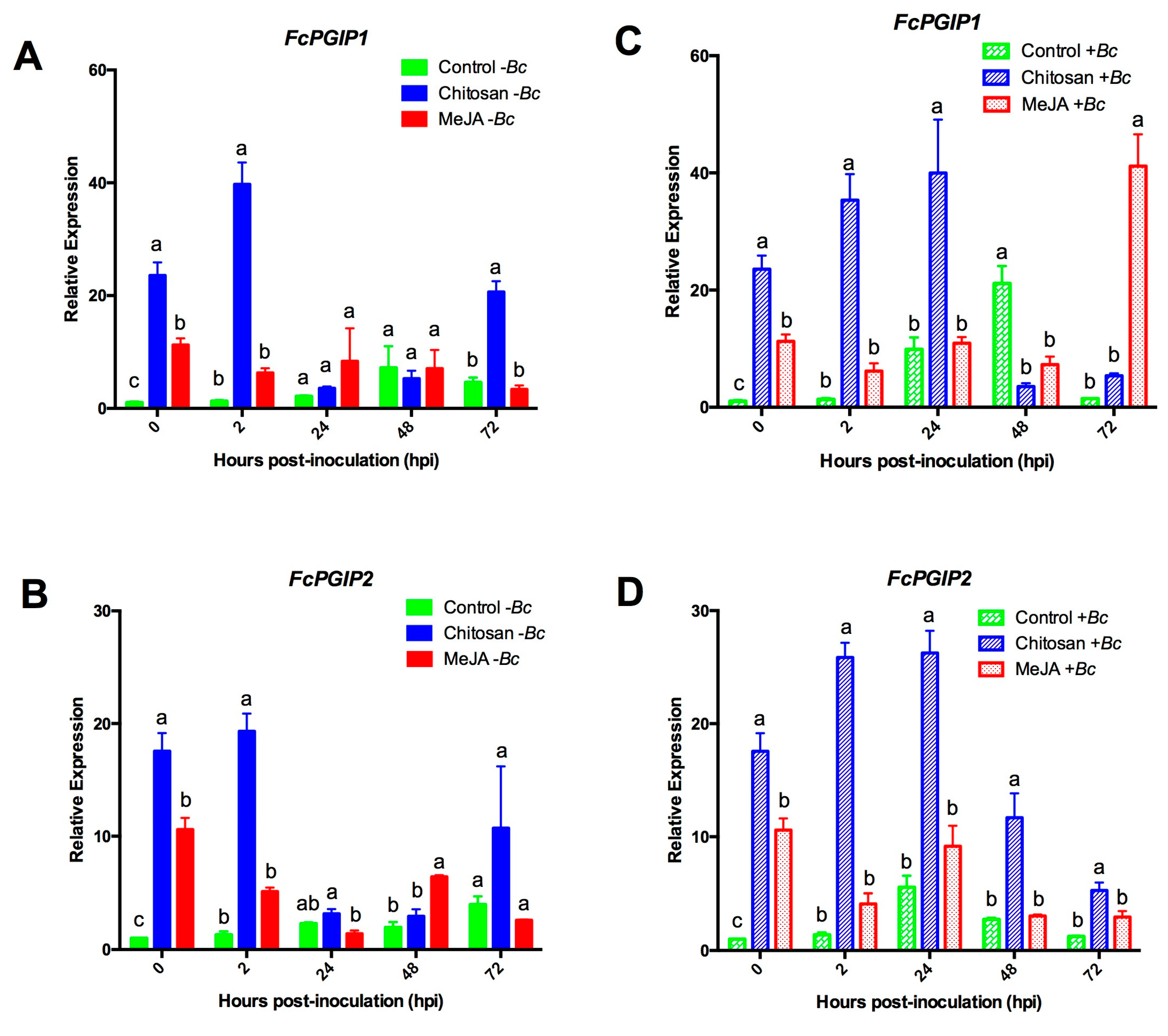
2.3.2. Expression Profiles for the Polygalacturonase Inhibiting Proteins (PGIPs) Genes
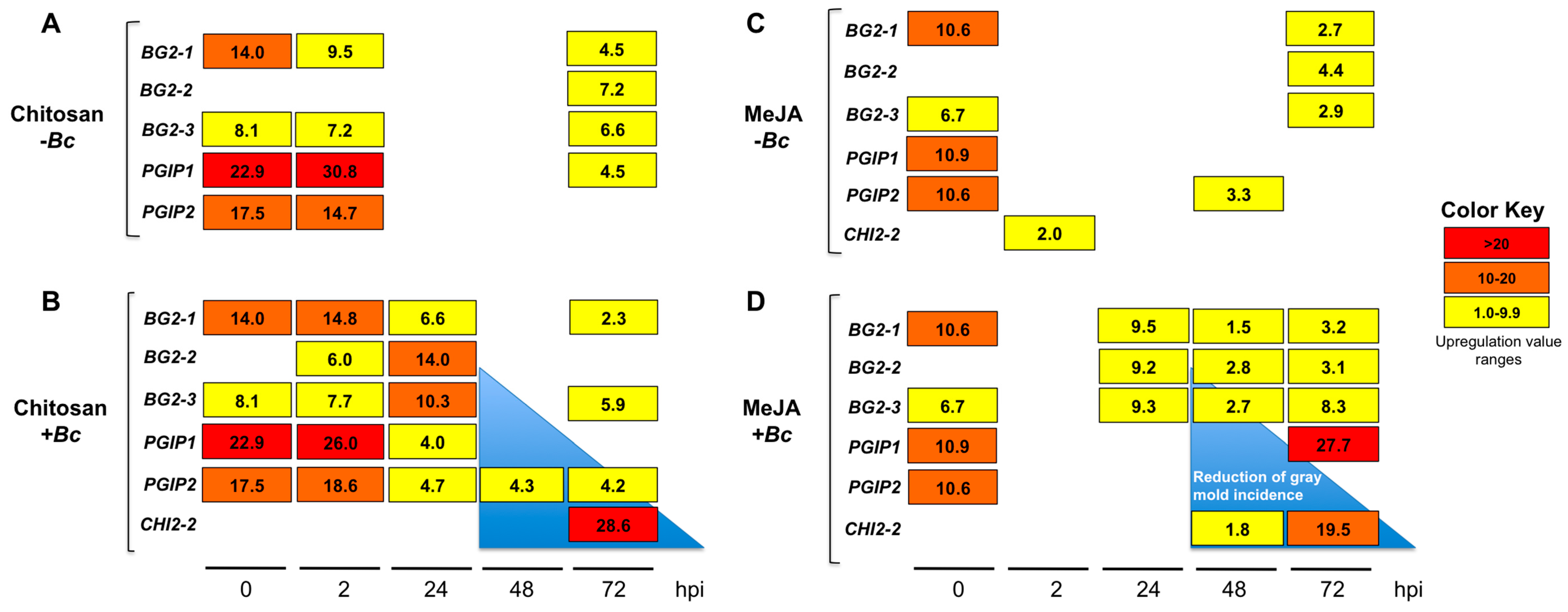
2.3.3. MeJA and Chitosan Upregulate FcBGs and FcPGIPs which Correlate with Reduction of B. cinerea Incidence in F. Chiloensis Fruit
3. Materials and Methods
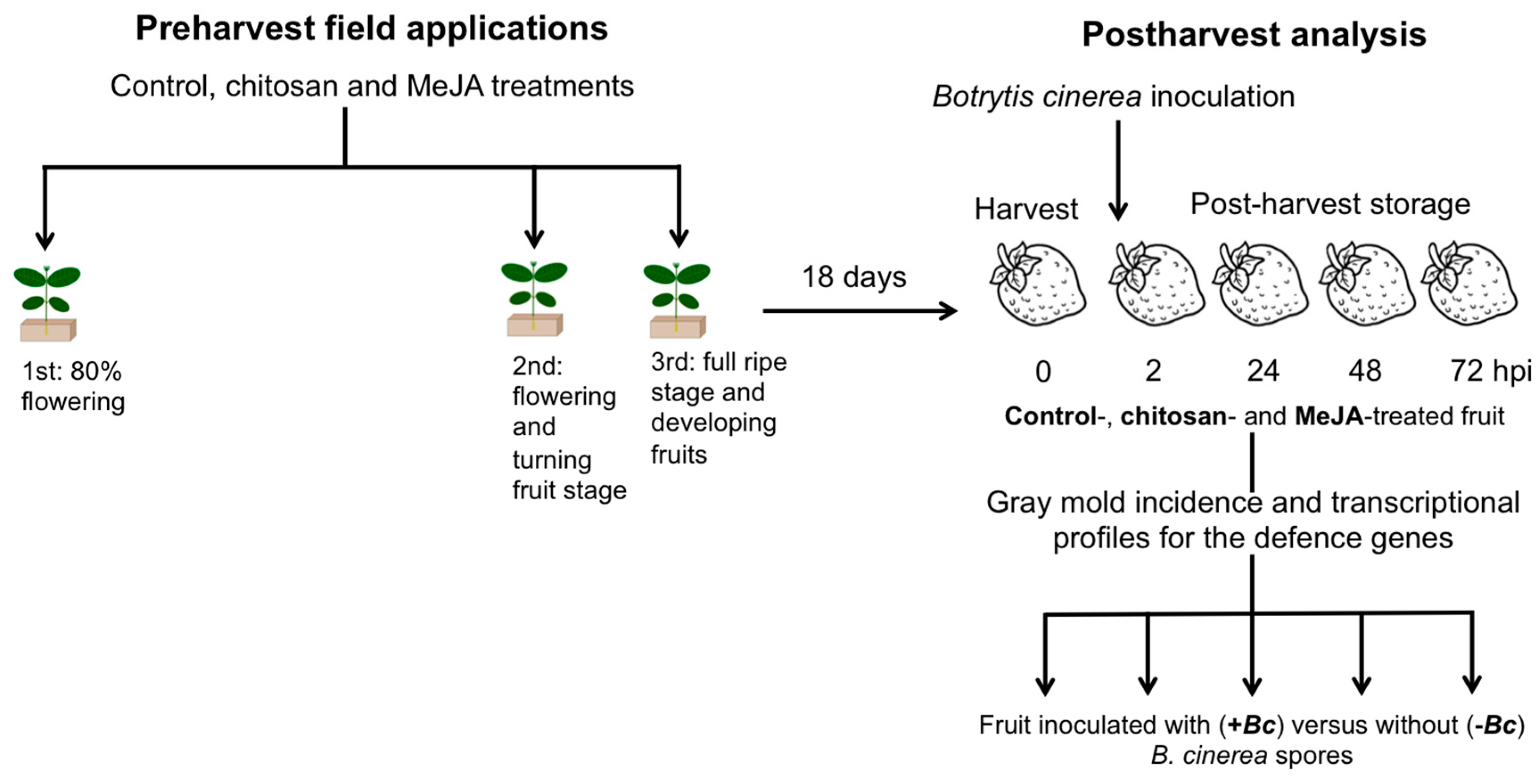
3.1. Plant Material, Treatments, and Fruit Quality Assessments
3.2. Botrytis cinerea Isolates and Aggressiveness Assay
3.3. Botrytis cinerea Inoculations in Postharvest Conditions
3.4. Analysis of Incidence and Gene Expression Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BG | β-1,3-glucanase |
| CHI | Chitinase |
| CTAB | Cetyltrimethylammonium bromide |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| JA | Jasmonic acid |
| JAs | Jasmonates |
| ISR | Induced systemic resistance |
| MAPK | Mitogen-activated protein kinase |
| MeJA | Methyl jasmonate |
| PDA | Potato dextrose agar |
| PGIP | Polygalacturonase-inhibiting protein |
| PR | Pathogenesis-related protein |
| SAR | Systemic acquired resistance |
| SSC | Soluble solids concentration |
| SDW | Sterile distilled water |
| RT-qPCR | Quantitative reverse transcription PCR |
| TA | Titratable acidity |
References
- Mora, F.; Concha, C.M.; Figueroa, C.R. Bayesian inference of genetic parameters for survival, flowering, fruit set, and ripening in a germplasm collection of Chilean strawberry using threshold models. J. Am. Soc. Hortic. Sci. 2016, 141, 285–291. [Google Scholar]
- Cherian, S.; Figueroa, C.R.; Nair, H. ′Movers and shakers′ in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.R.; Pimentel, P.; Gaete-Eastman, C.; Moya, M.; Herrera, R.; Caligari, P.D.; Moya-León, M.A. Softening rate of the Chilean strawberry (Fragaria chiloensis) fruit reflects the expression of polygalacturonase and pectate lyase genes. Postharvest Biol. Tec. 2008, 49, 210–220. [Google Scholar] [CrossRef]
- Saavedra, G.M.; Figueroa, N.E.; Poblete, L.A.; Cherian, S.; Figueroa, C.R. Effects of preharvest applications of methyl jasmonate and chitosan on postharvest decay, quality and chemical attributes of Fragaria chiloensis fruit. Food Chem. 2016, 190, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Essghaier, B.; Fardeau, M.-L.; Cayol, J.-L.; Hajlaoui, M.; Boudabous, A.; Jijakli, H.; Sadfi-Zouaoui, N. Biological control of grey mould in strawberry fruit by halophilic bacteria. J. Appl. Microbiol. 2009, 106, 833–846. [Google Scholar] [CrossRef] [PubMed]
- González, G.; Moya, M.; Sandoval, C.; Herrera, R. Genetic diversity in Chilean strawberry (Fragaria chiloensis): Differential response to Botrytis cinerea infection. Span. J. Agric. Res. 2009, 7, 886–895. [Google Scholar] [CrossRef]
- Leroux, P.; Gredt, M.; Leroch, M.; Walker, A.-S. Exploring mechanisms of resistance to respiratory inhibitors in field strains of Botrytis cinerea, the causal agent of gray mold. Appl. Environ. Microbiol. 2010, 76, 6615–6630. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, W.J.; Korsten, L. Biological control of postharvest diseases of fruit. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Rajendran, S.R.C.K.; Gaur, M.; Sajeesh, P.; Kumar, A. Plant defense signaling and responses against necrotrophic fungal pathogens. J. Plant Growth Regul. 2016, 35, 1159–1174. [Google Scholar] [CrossRef]
- Van Loon, L.; Pierpoint, W.; Boller, T.; Conejero, V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol. Biol. Rep. 1994, 12, 245–264. [Google Scholar] [CrossRef]
- Odjakova, M.; Hadjiivanova, C. The complexity of pathogen defense in plants. Bulg. J. Plant Physiol. 2001, 27, 101–109. [Google Scholar]
- González, G.; Fuentes, L.; Moya-León, M.A.; Sandoval, C.; Herrera, R. Characterization of two PR genes from Fragaria chiloensis in response to Botrytis cinerea infection: A comparison with Fragaria x ananassa. Physiol. Mol. Plant. Pathol. 2013, 82, 73–80. [Google Scholar] [CrossRef]
- Amil-Ruiz, F.; Blanco-Portales, R.; Muñoz-Blanco, J.; Caballero, J.L. The strawberry plant defense mechanism: A molecular review. Plant Cell Physiol. 2011, 52, 1873–1903. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, G.; Ferrari, S. Polygalacturonase-Inhibiting proteins in defense against phytopathogenic fungi. Curr. Opin. Plant Biol. 2002, 5, 295–299. [Google Scholar] [CrossRef]
- Schaart, J.G. Towards Consumer-Friendly Cisgenic Strawberries Which Are Less Susceptible to Botrytis cinerea. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2004. [Google Scholar]
- Yu, M.; Shen, L.; Fan, B.; Zhao, D.; Zheng, Y.; Sheng, J. The effect of MeJA on ethylene biosynthesis and induced disease resistance to Botrytis cinerea in tomato. Postharvest Biol. Tec. 2009, 54, 153–158. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Kan, J.; Han, L.; Zheng, Y. Response of direct or priming defense against Botrytis cinerea to methyl jasmonate treatment at different concentrations in grape berries. Int. J. Food Microbiol. 2015, 194, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Terry, L.A.; Joyce, D.C. Elicitors of induced disease resistance in postharvest horticultural crops: A brief review. Postharvest Biol. Tec. 2004, 32, 1–13. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Arul, J.; Grenier, J.; Asselin, A. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruit. Phytopathology 1992, 82, 398–402. [Google Scholar] [CrossRef]
- Feliziani, E.; Santini, M.; Landi, L.; Romanazzi, G. Pre- and postharvest treatment with alternatives to synthetic fungicides to control postharvest decay of sweet cherry. Postharvest Biol. Tec. 2013, 78, 133–138. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Divenere, D.; Salerno, M. Effects of pre- and postharvest chitosan treatments to control storage grey mold of table grapes. J. Food Sci. 2002, 67, 1862–1867. [Google Scholar] [CrossRef]
- Gutiérrez-Martínez, P.; Chacón-López, A.; Xoca-Orozco, L.A.; Ramos-Guerrero, A.; Velázquez-Estrada, R.; Aguilera-Aguirre, S. Chitosan and changes in gene expression during fruit-pathogen interaction at postharvest stage. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Elsevier: London, UK, 2016; pp. 299–311. [Google Scholar]
- Herrera-Romero, I.; Ruales, C.; Caviedes, M.; Leon-Reyes, A. Postharvest evaluation of natural coatings and antifungal agents to control Botrytis cinerea in Rosa sp. Phytoparasitica 2017, 45, 9–20. [Google Scholar] [CrossRef]
- González, M.; Gaete-Eastman, C.; Valdenegro, M.; Figueroa, C.R.; Fuentes, L.; Herrera, R.; Moya-León, M.A. Aroma development during ripening of Fragaria chiloensis fruit and participation of an alcohol acyltransferase (FcAAT1) gene. J. Agric. Food Chem. 2009, 57, 9123–9132. [Google Scholar] [CrossRef] [PubMed]
- Porat, R.; McCollum, T.; Vinokur, V.; Droby, S. Effects of various elicitors on the transcription of a β-1,3-endoglucanase gene in citrus fruit. J. Phytopathol. 2002, 150, 70–75. [Google Scholar] [CrossRef]
- Wang, K.; Jin, P.; Cao, S.; Shang, H.; Yang, Z.; Zheng, Y. Methyl jasmonate reduces decay and enhances antioxidant capacity in Chinese bayberries. J. Agric. Food Chem. 2009, 57, 5809–5815. [Google Scholar] [CrossRef] [PubMed]
- Concha, C.M.; Figueroa, N.E.; Poblete, L.A.; Oñate, F.A.; Schwab, W.; Figueroa, C.R. Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol. Biochem. 2013, 70, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, P.; Han, L.; Shang, H.; Tang, S.; Rui, H.; Duan, Y.; Kong, F.; Kai, X.; Zheng, Y. Methyl jasmonate induces resistance against Penicillium citrinum in Chinese bayberry by priming of defense responses. Postharvest Biol. Tec. 2014, 98, 90–97. [Google Scholar] [CrossRef]
- Landi, L.; Feliziani, E.; Romanazzi, G. Expression of defense genes in strawberry fruit treated with different resistance inducers. J. Agric. Food Chem. 2014, 62, 3047–3056. [Google Scholar] [CrossRef] [PubMed]
- Burketova, L.; Trda, L.; Ott, P.G.; Valentova, O. Bio-Based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in systemic plant immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.; Poinssot, B.; Pozo, M.J. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Pombo, M.A.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria × ananassa Duch.). Postharvest Biol. Tec. 2011, 59, 94–102. [Google Scholar] [CrossRef]
- GenBank. Available online: http://www.ncbi.nlm.nih.gov/genbank/ (accessed on 6 January 2014).
- Khan, A.A.; Shi, Y.; Shih, D.S. Cloning and partial characterization of a β-1,3-glucanase gene from strawberry. DNA Seq. 2003, 14, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Y.; Shih, D.S. Cloning and expression analysis of two β-1,3-glucanase genes from strawberry. J. Plant Physiol. 2006, 163, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Curry, K.J.; Abril, M.; Avant, J.B.; Smith, B.J. Strawberry anthracnose: Hispathology of Colletotrichum acutatum and C. fragariae. Phytopathology 2002, 92, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Shih, D.S. Molecular cloning, characterization, and expression analysis of two class II chitinase genes from the strawberry plant. Plant Sci. 2004, 166, 753–762. [Google Scholar] [CrossRef]
- Mehli, L.; Kjellsen, T.D.; Dewey, F.M.; Hietala, A.M. A case study from the interaction of strawberry and Botrytis cinerea highlights the benefits of comonitoring both partners at genomic and mRNA level. New Phytol. 2005, 168, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.B.; Junior, M.L.; Grossi-de-Sá, M.F.; Petrofeza, S. Exogenous application of methyl jasmonate induces a defense response and resistance against Sclerotinia sclerotiorum in dry bean plants. J. Plant Physiol. 2015, 182, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Nishizawa, Y.; Tabei, Y.; Hibi, T.; Nakajima, M.; Akutsu, K. Detailed analysis of rice chitinase gene expression in transgenic cucumber plants showing different levels of disease resistance to gray mold (Botrytis cinerea). Plant Sci. 2002, 162, 655–662. [Google Scholar] [CrossRef]
- Rickauer, M.; Brodschelm, W.; Bottin, A.; Veronesi, C.; Grimal, H.; Esquerre-Tugaye, M. The jasmonate pathway is involved differentially in the regulation of different defense responses in tobacco cells. Planta 1997, 202, 155–162. [Google Scholar] [CrossRef]
- Khan, A.; Wu, J.; Shih, D. Cloning and sequence analysis of a class III chitinase gene (accession no. AF134347) from Fragaria ananassa Duch. (PGR99–069). Plant Physiol. 1999, 120, 340. [Google Scholar]
- Kalunke, R.M.; Tundo, S.; Benedetti, M.; Cervone, F.; de Lorenzo, G.; D'Ovidio, R. An update on polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein that protects crop plants against pathogens. Front. Plant Sci. 2015, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Mehli, L.; Schaart, J.G.; Kjellsen, T.D.; Tran, D.H.; Salentijn, E.M.; Schouten, H.J.; Iversen, T.H. A gene encoding a polygalacturonase-inhibiting protein (PGIP) shows developmental regulation and pathogen-induced expression in strawberry. New Phytol. 2004, 163, 99–110. [Google Scholar] [CrossRef]
- Schaart, J.G.; Mehli, L.; Schouten, H.J. Quantification of allele-specific expression of a gene encoding strawberry polygalacturonase-inhibiting protein (PGIP) using Pyrosequencing. Plant J. 2005, 41, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Molina Mercader, G.; Zaldúa Flores, S.; González Vargas, G.; Sanfuentes Von, E. Selección de hongos antagonistas para el control biológico de Botrytis cinerea en viveros forestales en Chile. Bosque (Valdivia) 2006, 27, 126–134. [Google Scholar] [CrossRef]
- Osorio, S.; Castillejo, C.; Quesada, M.A.; Medina-Escobar, N.; Brownsey, G.J.; Suau, R.; Heredia, A.; Botella, M.A.; Valpuesta, V. Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defense responses in wild strawberry (Fragaria vesca). Plant J. 2008, 54, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
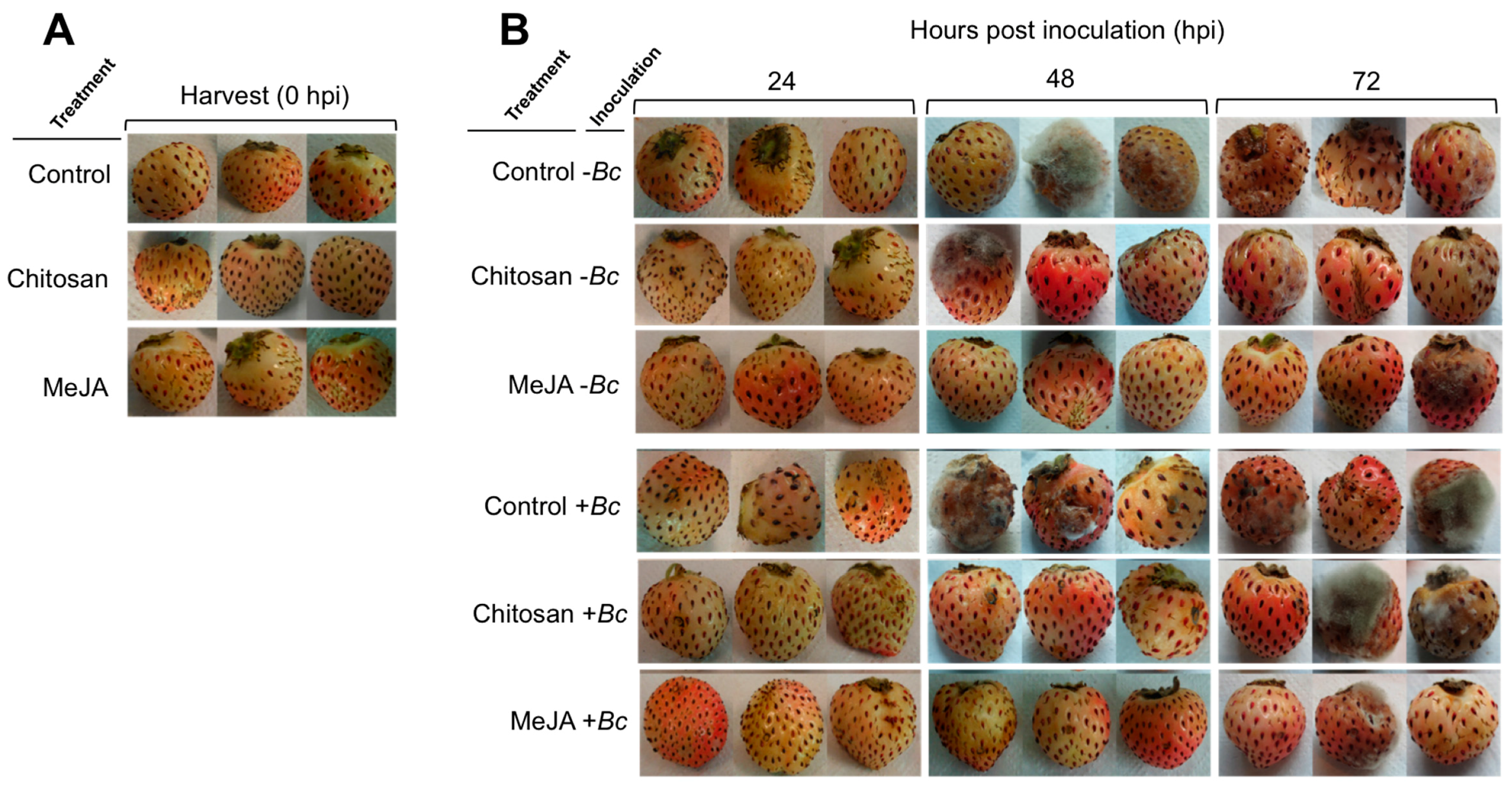
| Treatment 2 | Inoculation 2 | Hours Post Inoculation (hpi) 3 | ||||
|---|---|---|---|---|---|---|
| 0 | 2 | 24 | 48 | 72 | ||
| Control | –Bc | 0 | 0 | 0 | 67 | 67 |
| Chitosan | –Bc | 0 | 0 | 33 | 67 | 67 |
| MeJA | –Bc | 0 | 0 | 0 | 0 | 33 |
| Control | +Bc | 0 | 0 | 33 | 100 | 100 |
| Chitosan | +Bc | 0 | 0 | 0 | 67 | 67 |
| MeJA | +Bc | 0 | 0 | 33 | 0 | 33 |
| Gene 2 | Accession No. 1 | Protein Family 3 | Strawberry Gene ID or Standardized Gene Name 3 | Forward Primer | Reverse Primer | Amplicon Size (pb) | Efficiency (%) |
|---|---|---|---|---|---|---|---|
| BG2-1 | AY170375 | PR2 | FaBG2-1 | CTAAATATCTTCTTCCTGCCATA | AATGTTGTATCTATTGCTGTT | 101 | 103 |
| BG2-2 | AY989818 | PR2 | FaBG2-2 | ACCGGGACTCCCAAGAGACCAAATG | TGTGAGCCTGCACTAGCCAAAGGTG | 162 | 86 |
| BG2-3 | AY989819 | PR2 | FaBG2-3 | TCCGAGAGTGGTTGGCCATCTGAAG | TCCATTTGGTCTCTTCGGAGTCCCG | 117 | 101 |
| CHI2-2 | AF320111 | PR3 | FaCHI2-2 | GCACAACAGGTGATGTTGC | GTAATGACGTCGTGGCTTGA | 183 | 100 |
| CHI3-1 | AF134347 | PR8 | FaCHI3-1 | AGGTCTTCTTAGGACTCCCTG | CTTGGACCAAAGCATGACACCGCC | 134 | 81 |
| PGIP1 | EU117215 | PGIP | FrveA6 PGIP | TGCTAGAATTCGATCTGTCCAAGG | ATTATCCAATTGGGTCAACTGCTC | 113 | 103 |
| PGIP2 | EU117213 | PGIP | FaPGIP-1.8 | TCCTCATGGAAATCCGACGCCGAC | ACCTGTGAGATTGGGGAGCTTGCG | 189 | 101 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saavedra, G.M.; Sanfuentes, E.; Figueroa, P.M.; Figueroa, C.R. Independent Preharvest Applications of Methyl Jasmonate and Chitosan Elicit Differential Upregulation of Defense-Related Genes with Reduced Incidence of Gray Mold Decay during Postharvest Storage of Fragaria chiloensis Fruit. Int. J. Mol. Sci. 2017, 18, 1420. https://doi.org/10.3390/ijms18071420
Saavedra GM, Sanfuentes E, Figueroa PM, Figueroa CR. Independent Preharvest Applications of Methyl Jasmonate and Chitosan Elicit Differential Upregulation of Defense-Related Genes with Reduced Incidence of Gray Mold Decay during Postharvest Storage of Fragaria chiloensis Fruit. International Journal of Molecular Sciences. 2017; 18(7):1420. https://doi.org/10.3390/ijms18071420
Chicago/Turabian StyleSaavedra, Gabriela M., Eugenio Sanfuentes, Pablo M. Figueroa, and Carlos R. Figueroa. 2017. "Independent Preharvest Applications of Methyl Jasmonate and Chitosan Elicit Differential Upregulation of Defense-Related Genes with Reduced Incidence of Gray Mold Decay during Postharvest Storage of Fragaria chiloensis Fruit" International Journal of Molecular Sciences 18, no. 7: 1420. https://doi.org/10.3390/ijms18071420