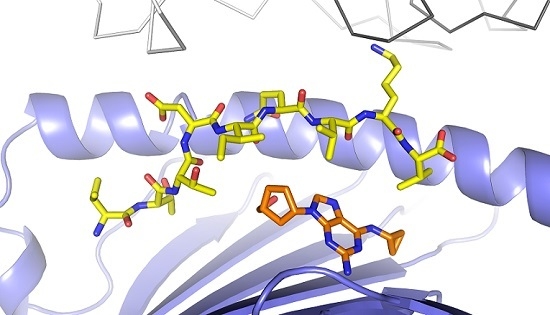
Structural Elements Recognized by Abacavir-Induced T Cells
Abstract
:1. Introduction
2. Methods
2.1. X-ray Crystallography and Structural Analysis
2.2. TCR-Transfectant Stimulation Assay
3. Results
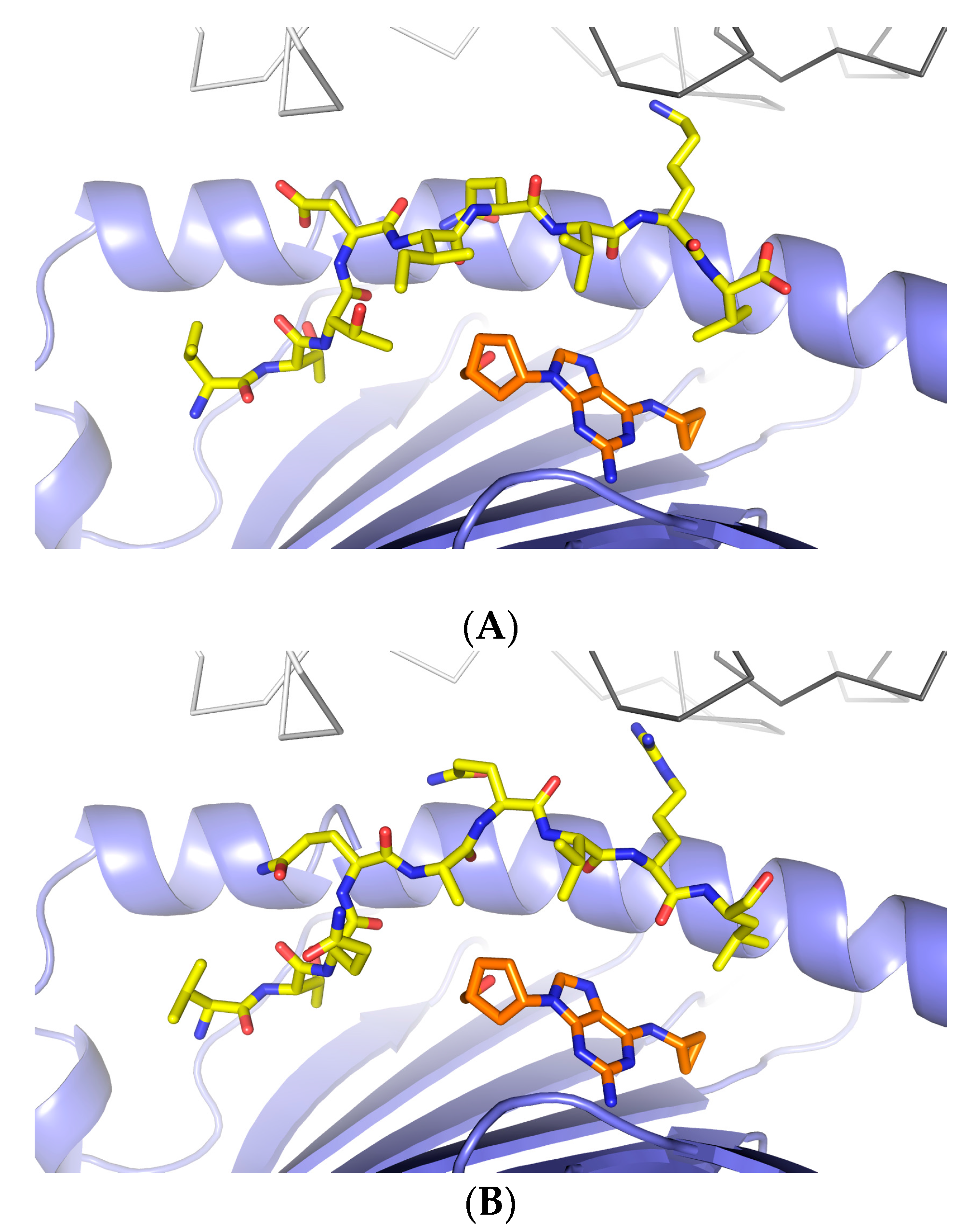
3.1. Defining TCR Contact Residues in a Self Peptide Complexed to Abacavir and HLA-B*57:01
3.2. Identification of Viral Peptides Similar to a Drug-Restricted Self Epitope
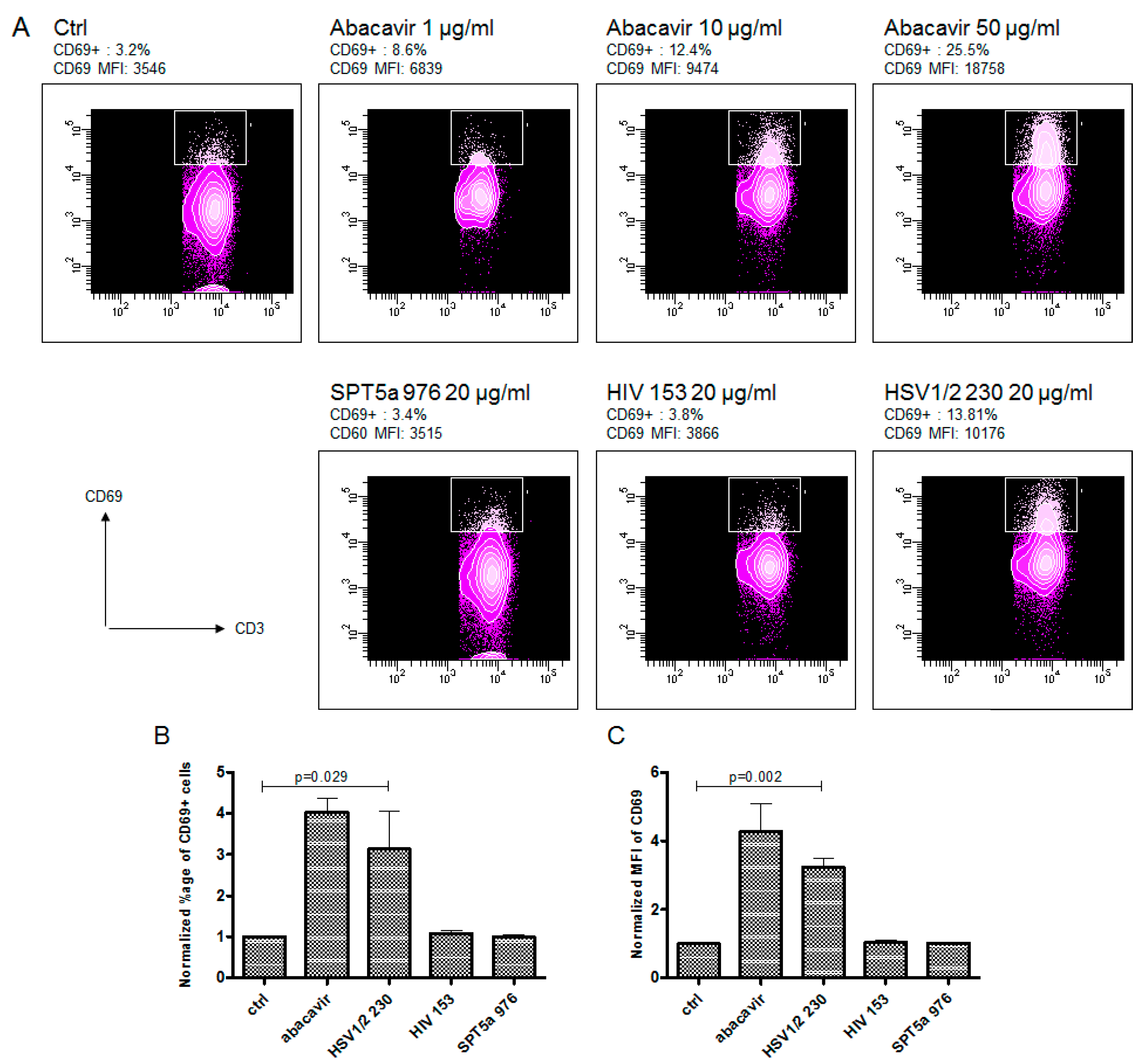
3.3. Abacavir Reactive T Cells Recognize a Herpes Simplex Peptide in the Context of Drug and HLA-B*57:01
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- White, K.D.; Chung, W.H.; Hung, S.I.; Mallal, S.; Phillips, E.J. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: The role of host, pathogens, and drug response. J. Allergy Clin. Immunol. 2015, 136, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V.; Mahe, E.; Houhou, N.; Abramowitz, L.; Rozenberg, F.; Ranger-Rogez, S.; Crickx, B. Drug-induced hypersensitivity syndrome associated with Epstein-Barr virus infection. Br. J. Dermatol. 2003, 148, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Janela, B.; Descamps, V.; D’Incan, M.; Courville, P.; Jacquot, S.; Rogez, S.; Mardivirin, L.; Moins-Teisserenc, H.; Toubert, A.; et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): A multiorgan antiviral T cell response. Sci. Transl. Med. 2010, 2, 46ra62. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.T.; Nguyen, T.T.; Daniels, K.A.; Welsh, R.M.; Stern, L.J. Disparate epitopes mediating protective heterologous immunity to unrelated viruses share peptide-MHC structural features recognized by cross-reactive T cells. J. Immunol. 2013, 191, 5139–5152. [Google Scholar] [CrossRef] [PubMed]
- Eliaszewicz, M.; Flahault, A.; Roujeau, J.C.; Fillet, A.M.; Challine, D.; Mansouri, S.; Wolkenstein, P.; Aractingi, S.; Penso-Assathiany, D.; Maslo, C.; et al. Prospective evaluation of risk factors of cutaneous drug reactions to sulfonamides in patients with AIDS. J. Am. Acad. Dermatol. 2002, 47, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, T.; Kano, Y. A complex interaction between drug allergy and viral infection. Clin. Rev. Allergy Immunol. 2007, 33, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa-Mineoka, R.; Katoh, N.; Nara, T.; Nishimura, Y.; Yamamoto, S.; Kishimoto, S. DRESS syndrome caused by teicoplanin and vancomycin, associated with reactivation of human herpesvirus-6. Int. J. Dermatol. 2007, 46, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Takigawa, M. Drug-induced hypersensitivity syndrome associated with cytomegalovirus reactivation: Immunological characterization of pathogenic T cells. Acta Derm. Venereol. 2005, 85, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Mallal, S. Drug hypersensitivity in HIV. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Leisner, C.; Loeth, N.; Lamberth, K.; Justesen, S.; Sylvester-Hvid, C.; Schmidt, E.G.; Claesson, M.; Buus, S.; Stryhn, A. One-pot, mix-and-read peptide-MHC tetramers. PLoS ONE 2008, 3, e1678. [Google Scholar] [CrossRef] [PubMed]
- Sidney, J.; Southwood, S.; Moore, C.; Oseroff, C.; Pinilla, C.; Grey, H.M.; Sette, A. Measurement of MHC/peptide interactions by gel filtration or monoclonal antibody capture. In Current Protocols in Immunology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; Chapter 18, Unit 18.3. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkoczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Ostrov, D.A.; Grant, B.J.; Pompeu, Y.A.; Sidney, J.; Harndahl, M.; Southwood, S.; Oseroff, C.; Lu, S.; Jakoncicf, J.; de Oliveira, C.A.F.; et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc. Natl. Acad. Sci. USA 2012, 109, 9959–9964. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Yamaguchi, Y.; Wada, T.; Handa, H.; Sharp, P.A. Tat-SF1 protein associates with RAP30 and human SPT5 proteins. Mol. Cell. Biol. 1999, 19, 5960–5968. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Peachman, K.K.; Kim, J.; Gao, G.; Alving, C.R.; Michael, N.L.; Venigalla, B.R. HIV-1 variable loop 2 and its importance in HIV-1 infection and vaccine development. Curr. HIV Res. 2013, 11, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Sidney, J.; Assarsson, E.; Moore, C.; Ngo, S.; Pinilla, C.; Sette, A.; Peters, B. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. 2008, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.; Wuillemin, N.; Watkins, S.; Jamin, H.; Eriksson, K.K.; Villiger, P.; Fontana, S.; Pichler, W.J.; Yerly, D. Abacavir induced T cell reactivity from drug naive individuals shares features of allo-immune responses. PLoS ONE 2014, 9, e95339. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.; Eriksson, K.K.; Schnyder, B.; Fontana, S.; Pichler, W.J.; Yerly, D. Avidity determines T-cell reactivity in abacavir hypersensitivity. Eur. J. Immunol. 2012, 42, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, M.; Illing, P.; Theodossis, A.; Purcell, A.W.; Rossjohn, J.; McCluskey, J. Drug Hypersensitivity and Human Leukocyte Antigens of the Major Histocompatibility Complex. Annu. Rev. Pharmacol. Toxicol. 2011, 52, 401–431. [Google Scholar] [CrossRef] [PubMed]
- Yip, V.L.; Alfirevic, A.; Pirmohamed, M. Genetics of immune-mediated adverse drug reactions: A comprehensive and clinical review. Clin. Rev. Allergy Immunol. 2015, 48, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J.; Beeler, A.; Keller, M.; Lerch, M.; Posadas, S.; Schmid, D.; Spanou, Z.; Zawodniak, A.; Gerber, B. Pharmacological interaction of drugs with immune receptors: The p-i concept. Allergol. Int. 2006, 55, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, L.; Meng, X.; Park, B.K.; Naisbitt, D.J. The importance of hapten-protein complex formation in the development of drug allergy. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Dao, R.L.; Chung, W.H. Immunopathogenesis and risk factors for allopurinol severe cutaneous adverse reactions. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Mallal, S.; Nolan, D.; Witt, C.; Masel, G.; Martin, A.M.; Moore, C.; Sayer, D.; Castley, A; Mamotte, C; Maxwell, D.; et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 2002, 359, 727–732. [Google Scholar] [CrossRef]
- Hetherington, S.; Hughes, A.R.; Mosteller, M.; Shortino, D.; Baker, K.L.; Spreen, W.; Lai, E.; Davies, K.; Handley, A.; Dow, D.J.; et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet 2002, 359, 1121–1122. [Google Scholar] [CrossRef]
- Phillips, E.J.; Wong, G.A.; Kaul, R.; Shahabi, K.; Nolan, D.A.; Knowles, S.R.; Martin, A.M.; Mallal, S.A; Shear, N.H. Clinical and immunogenetic correlates of abacavir hypersensitivity. AIDS 2005, 19, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Lichtenfels, M.; Farrell, J.; Ogese, M.O.; Bell, C.C.; Eckle, S.; McCluskey, J.; Park, B.K.; Alfirevic, A.; Naisbitt, D.J.; Pirmohamed, M. HLA restriction of carbamazepine-specific T-Cell clones from an HLA-A*31:01-positive hypersensitive patient. Chem. Res. Toxicol. 2014, 27, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.A.; Depta, J.P.; Luthi, M.; Pichler, W.J. Transfection of drug-specific T-cell receptors into hybridoma cells: Tools to monitor drug interaction with T-cell receptors and evaluate cross-reactivity to related compounds. Mol. Pharmacol. 2006, 70, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Depta, J.P.; Altznauer, F.; Gamerdinger, K.; Burkhart, C.; Weltzien, H.U.; Pichler, W.J. Drug interaction with T-cell receptors: T-cell receptor density determines degree of cross-reactivity. J. Allergy Clin. Immunol. 2004, 113, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Illing, P.T.; Vivian, J.P.; Dudek, N.L.; Kostenko, L.; Chen, Z.; Bharadwaj, M.; Miles, J.J.; Kjer-Nielsen, L.; Gras, S.; Williamson, N.A.; et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 2012, 486, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.C.; Teyton, L.; Wilson, I.A. Structural basis of T cell recognition. Annu. Rev. Immunol. 1999, 17, 369–397. [Google Scholar] [CrossRef] [PubMed]
- Mallal, S.; Phillips, E.; Carosi, G.; Molina, J.M.; Workman, C.; Tomazic, J.; Jägel-Guedes, E.; Rugina, S.; Kozyrev, O.; Cid, J.F.; et al. HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl. J. Med. 2008, 358, 568–579. [Google Scholar] [CrossRef] [PubMed]

| Sequence | Protein | Sequence Number | Identity to SPT5a |
|---|---|---|---|
| VTTDIQVKV | Human SPT5A | 976–984 | 100 % |
| VTTNIQTKV | HIV-1 | 153–161 | 77.8% |
| VTQQAQVRL | HSV1/2 | 230–238 | 44.4% |
| VTTDSVRAL | HSV1 | 12–20 | 44.4% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerly, D.; Pompeu, Y.A.; Schutte, R.J.; Eriksson, K.K.; Strhyn, A.; Bracey, A.W.; Buus, S.; Ostrov, D.A. Structural Elements Recognized by Abacavir-Induced T Cells. Int. J. Mol. Sci. 2017, 18, 1464. https://doi.org/10.3390/ijms18071464
Yerly D, Pompeu YA, Schutte RJ, Eriksson KK, Strhyn A, Bracey AW, Buus S, Ostrov DA. Structural Elements Recognized by Abacavir-Induced T Cells. International Journal of Molecular Sciences. 2017; 18(7):1464. https://doi.org/10.3390/ijms18071464
Chicago/Turabian StyleYerly, Daniel, Yuri Andreiw Pompeu, Ryan J. Schutte, Klara. K. Eriksson, Anette Strhyn, Austin. W. Bracey, Soren Buus, and David A. Ostrov. 2017. "Structural Elements Recognized by Abacavir-Induced T Cells" International Journal of Molecular Sciences 18, no. 7: 1464. https://doi.org/10.3390/ijms18071464