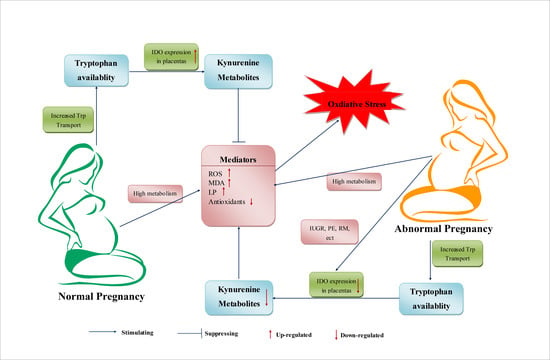
Redox Properties of Tryptophan Metabolism and the Concept of Tryptophan Use in Pregnancy
Abstract
:1. Introduction
2. OS during a Normal Pregnancy
3. The Defense Strategy against OS in Pregnancy
4. Trp Metabolism and Antioxidants
4.1. Tryptophan
4.2. Kynurenine
4.3. Kynurenic Acid
4.4. 3-HK
4.5. 3-Hydroxyanthranilic Acid
4.6. Xanthurenic Acid
4.7. Indoleamine 2,3-Dioxygenase
5. Trp Metabolism during Normal Pregnancy
5.1. Variation of Trp Metabolism in Different Stages of Pregnancy
5.2. Trp Metabolism in the Placenta
6. Trp Metabolism in Abnormal Pregnancies
6.1. Intrauterine Growth Restriction
6.2. Preeclampsia
6.3. Recurrent Miscarriage
6.4. Offspring Atopic Dermatitis (AD)
7. Factors Influencing Trp Metabolism and OS during Pregnancy
7.1. Inflammation
7.2. Social Stress
7.3. Heat Stress (HS)
7.4. Toxin Challenge
8. Potential Antioxidants of Trp Metabolism during Pregnancy
9. Summary and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Trp | Tryptophan |
| OS | Oxidative Stress |
| NAD | Nicotinamide Adenine Dinucleotide |
| NADP | Nicotinamide Adenine Dinucleotide Phosphate |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| ATP | Adenosine Triphosphate |
| MDA | Malonaldehyde |
| pO2 | Pressure of Oxygen |
| Hb | Hemoglobin |
| KYN | Kynurenine |
| KP | Kynurenine Pathway |
| TDO | Tryptophan 2,3-dioxygenase |
| IDO | Indoleamine 2,3-dioxygenase |
| KYNA | Kynurenic acid |
| 3HAA | 3-Hydroxyanthranilic acid |
| 3-HK | 3-Hydroxykynurenine |
| XA | Xanthurenic Acid |
| QUIN | Quinolinic Acid |
| SOD | Superoxide Dismutase |
| KATs | Kynurenine Aminotransferases |
| HOCl | Hypochlorous acid |
| GSH | Glutathione |
| GST | Glutathione S-transferase |
| NO | Nitric Oxide |
| LP | Lipid Peroxidation |
| IFN-γ | Interferon-γ |
| LPS | Lipopolysaccharides |
| iNOS | Inducible Nitric Oxide Synthase |
| HO-1, | Hemeoxygenase-1 |
| Nrf2 | NF-E2 Related Factor 2 |
| 5-HT | 5-Hydroxytryptamine |
| AA | Anthranilic Acid |
| Tph1 | Trp Hydroxylase-1 |
| IUGR | Intrauterine Growth Restriction |
| PE | Preeclampsia |
| RM | Recurrent Miscarriage |
| STAT3 | Signal transducer and activator of transcription 3 |
| MMP-9 | Matrix Metallopeptidase 9 |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-α |
| HS | Heat stress |
References
- Gupta, S.; Agarwal, A.; Sharma, R.K. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet. Gynecol. Surv. 2005, 60, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Raijmakers, M.T.; Dechend, R.; Poston, L. Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials. Hypertension 2004, 44, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.O.; Leskiw, M.; Chen, X.; Sims, M.; Stein, T.P. Oxidative stress, diet, and the etiology of preeclampsia. Am. J. Clin. Nutr. 2005, 81, 1390–1396. [Google Scholar] [PubMed]
- Peter Stein, T.; Scholl, T.O.; Schluter, M.D.; Leskiw, M.J.; Chen, X.; Spur, B.W.; Rodriguez, A. Oxidative stress early in pregnancy and pregnancy outcome. Free Radic. Res. 2008, 42, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Osawa, Y.; Kanamori, H.; Seki, E.; Hoshi, M.; Ohtaki, H.; Yasuda, Y.; Ito, H.; Suetsugu, A.; Nagaki, M.; Moriwaki, H.; et al. l-Tryptophan-mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J. Biol. Chem. 2011, 286, 34800–34808. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Diez-Ruiz, A.; Murr, C.; Theur, I.; Fuchs, D. Tryptophan metabolites as scavengers of reactive oxygen and chlorine species. Pteridines 2002, 13, 140. [Google Scholar] [CrossRef]
- Christen, S.; Peterhans, E.; Stocker, R. Antioxidant activities of some tryptophan metabolites: Possible implication for inflammatory diseases. Proc. Natl. Acad. Sci. USA 1990, 87, 2506–2510. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Cabrera, J.; D’Arpa, D. Melatonin and tryptophan derivatives as free radical scavengers and antioxidants. Adv. Exp. Med. Biol. 1999, 467, 379–387. [Google Scholar] [PubMed]
- Berchieri-Ronchi, C. Effects of oxidative stress during human and animal reproductions. Int. J. Nutrol. 2015, 8, 6. [Google Scholar]
- Idonije, O.; Festus, O.; Okhiai, O.; Akpamu, U. A comparative study of the status of oxidative stress in pregnant Nigerian women. Res. J. Obstet. Gynecol. 2011, 4, 28–36. [Google Scholar] [CrossRef]
- Kim, S.W.; Weaver, A.C.; Shen, Y.B.; Zhao, Y. Improving efficiency of sow productivity: Nutrition and health. J. Anim. Sci. Biotech. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, C.I.; Jordao, A.A.; Vannucchi, H. Antioxidant compounds and oxidative stress in female dogs during pregnancy. Res. Vet. Sci. 2007, 83, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Mutinati, M.; Piccinno, M.; Roncetti, M.; Campanile, D.; Rizzo, A.; Sciorsci, R.L. Oxidative Stress During Pregnancy In The Sheep. Reprod. Domest. Anim. 2013, 48, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Oxidative stress in development: Nature or nurture? Free Radic. Biol. Med. 2010, 49, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Prater, M.R.; Laudermilch, C.L.; Liang, C.; Holladay, S.D. Placental oxidative stress alters expression of murine osteogenic genes and impairs fetal skeletal formation. Placenta 2008, 29, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Flowers, W.L.; Saraiva, A.; Yeum, K.-J.; Kim, S.W. Effect of social ranks and gestation housing systems on oxidative stress status, reproductive performance, and immune status of sows. J. Anim. Sci. 2013, 91, 5848–5858. [Google Scholar] [CrossRef] [PubMed]
- Karowicz-Bilinska, A.; Kedziora-Kornatowska, K.; Bartosz, G. Indices of oxidative stress in pregnancy with fetal growth restriction. Free Radic. Res. 2007, 41, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Houghton, F.D.; Thompson, J.G.; Kennedy, C.J.; Leese, H.J. Oxygen consumption and energy metabolism of the early mouse embryo. Mol. Reprod. Dev. 1996, 44, 476–485. [Google Scholar] [CrossRef]
- Casanueva, E.; Viteri, F.E. Iron and oxidative stress in pregnancy. J. Nutr. 2003, 133, 1700S–1708S. [Google Scholar] [PubMed]
- Saikumar, P.; Jaya, D.; Devi, M.R. Oxidative stress in pregnancy. ISOR J. Dent. Med. Sci. 2013, 3, 12–13. [Google Scholar] [CrossRef]
- Mueller, A.; Koebnick, C.; Binder, H.; Hoffmann, I.; Schild, R.L.; Beckmann, M.W.; Dittrich, R. Placental defence is considered sufficient to control lipid peroxidation in pregnancy. Med. Hypotheses 2005, 64, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–5160. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Murphy, M.P.; Oberley, T.D. Mitochondrial redox state regulates transcription of the nuclear-encoded mitochondrial protein manganese superoxide dismutase: A proposed adaptive response to mitochondrial redox imbalance. Free Radic. Biol. Med. 2005, 38, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of reactive oxygen species in biological processes. Klin. Wochenschr. 1991, 69, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Scholl, T.O. Oxidative stress: Changes in pregnancy and with gestational diabetes mellitus. Curr. Diab. Rep. 2005, 5, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Placental adaptive responses and fetal programming. J. Physiol. 2006, 572, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.I.; Fridovich, I. How does superoxide dismutase protect against tumor necrosis factor: A hypothesis informed by effect of superoxide on “free” iron. Free Radic. Biol. Med 1997, 23, 668–671. [Google Scholar] [CrossRef]
- Gür, S.; Türk, G.; Demirci, E.; Yüce, A.; Sönmez, M.; Özer, Ş.; Aksu, E.H. Effect of pregnancy and foetal number on diameter of corpus luteum, maternal progesterone concentration and oxidant/antioxidant balance in ewes. Reprod. Domest. Anim. 2011, 46, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Igosheva, N.; Mistry, H.D.; Seed, P.T.; Shennan, A.H.; Rana, S.; Karumanchi, S.A.; Chappell, L.C. Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am. J. Clin. Nutr. 2011, 94, 1980S–1985S. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R.; Kusanovic, J.P.; Hassan, S.S. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2011, 204, 503.e1–503.e12. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Briley, A.L.; Seed, P.T.; Kelly, F.J.; Shennan, A.H. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): Randomised placebo-controlled trial. Lancet 2006, 367, 1145–1154. [Google Scholar] [CrossRef]
- Roberts, J.M.; Myatt, L.; Spong, C.Y.; Thom, E.A.; Hauth, J.C.; Leveno, K.J.; Pearson, G.D.; Wapner, R.J.; Varner, M.W.; Thorp, J.M.J.; et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N. Engl. J. Med. 2010, 362, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.A. Biochemistry of tryptophan in health and disease. Mol. Asp. Med. 1983, 6, 101–197. [Google Scholar] [CrossRef]
- Badawy, A.A. Tryptophan: The key to boosting brain serotonin synthesis in depressive illness. J. Psychopharmacol. 2013, 27, 878–893. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379. [Google Scholar] [PubMed]
- Stone, T.W.; Darlington, L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002, 1, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Genestet, C.; Le Gouellec, A.; Chaker, H.; Polack, B.; Guery, B.; Toussaint, B.; Stasia, M.J. Scavenging of reactive oxygen species by tryptophan metabolites helps Pseudomonas aeruginosa escape neutrophil killing. Free Radic. Biol. Med. 2014, 73, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.L.A.; Dias, F.; Nunes, R.D.; Pereira, L.O.; Santos, T.S.R.; Chiarini, L.B.; Ramos, T.D.; Silva-Mendes, B.J.; Perales, J.; Valente, R.H.; et al. The antioxidant role of xanthurenic acid in the Aedes aegypti midgut during digestion of a blood meal. PLoS ONE 2012, 7, e38349. [Google Scholar] [CrossRef] [PubMed]
- Britan, A.; Maffre, V.; Tone, S.; Drevet, J.R. Quantitative and spatial differences in the expression of tryptophan-metabolizing enzymes in mouse epididymis. Cell Tissue Res. 2006, 324, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Egerton, M.; Christofides, J.; Stone, T.W.; Darlington, L.G. Tryptophan loading induces oxidative stress. Free Radic. Res. 2004, 38, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Reyes Ocampo, J.; Huitr, L.R.; Gonzalez-Esquivel, D.; Ugalde-Muniz, P.; Jimenez-Anguiano, A.; Pineda, B.; Pedraza-Chaverri, J.; Rios, C.; Perez de la Cruz, V. Kynurenines with neuroactive and redox properties: relevance to aging and brain diseases. Oxidative. Med. Cell. Longev. 2014, 2014, 22. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998, 70, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Kynurenines in the CNS: From endogenous obscurity to therapeutic importance. Prog. Neurobiol. 2001, 64, 185–218. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Free aromatic amino acids in egg yolk show antioxidant properties. Food Chem. 2011, 129, 155–161. [Google Scholar] [CrossRef]
- Yan, X.; Suzuki, M.; Ohnishi-Kameyama, M.; Sada, Y.; Nakanishi, T.; Nagata, T. Extraction and identification of antioxidants in the roots of yacon (Smallanthus sonchifolius). J. Agric. Food Chem. 1999, 47, 4711–4713. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, W.; Lu, Z.; Beta, T.; Hydamaka, A.W. Phenolic content, composition, antioxidant activity, and their changes during domestic cooking of potatoes. J. Agric. Food Chem. 2009, 57, 10231–10238. [Google Scholar] [CrossRef] [PubMed]
- Tsopmo, A.; Diehl-Jones, B.W.; Aluko, R.E.; Kitts, D.D.; Elisia, I.; Friel, J.K. Tryptophan released from mother’s milk has antioxidant properties. Pediatr. Res. 2009, 66, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Nayak Bichitra, N.; Buttar Harpal, S. Evaluation of the antioxidant properties of tryptophan and its metabolites in in vitro assay. J. Complement. Integr. Med. 2016, 13, 129–136. [Google Scholar]
- Bitzer-Quintero, O.K.; Dávalos-Marín, A.J.; Ortiz, G.G.; Meza, A.R.; Torres-Mendoza, B.M.; Robles, R.G.; Huerta, V.C.; Beas-Zárate, C. Antioxidant activity of tryptophan in rats under experimental endotoxic shock. Biomed. Pharmacother. 2010, 64, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Togashi, S.; Takahashi, N.; Fukui, T. l-tryptophan as an antioxidant in human placenta extract. J. Nutr. Sci. Vitaminol. 2002, 48, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S. Tryptophan Supplements Promote Pregnancy Success of Mice Challenged with Pseudorabies Virus (PRV) Via Regulating Systemic Cytokines, Immunoglobulins and PRV-Specific Protein Profiles and Toll-Like-Receptors Expression. Master’s Thesis, Sichuan Agricultural University, Yaan, China, 2009. [Google Scholar]
- Ciji, A.; Sahu, N.P.; Pal, A.K.; Akhtar, M.S. Nitrite-induced alterations in sex steroids and thyroid hormones of Labeo rohita juveniles: Effects of dietary vitamin E and l-Tryptophan. Fish Physiol. Biochem. 2013, 39, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, C.; Fukuwatari, T.; Sano, M.; Saito, K.; Sasaki, S.; Shibata, K. Supplementing healthy women with up to 5.0 g/d of l-Tryptophan has no adverse effects. J. Nutr. 2013, 143, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Alvarez-Idaboy, J.R.; Galano, A. Free-radical scavenging by tryptophan and its metabolites through electron transfer based processes. J. Mol. Model. 2015, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Huitron, R.; Blanco-Ayala, T.; Ugalde-Muniz, P.; Carrillo-Mora, P.; Pedraza-Chaverri, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzon, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Park, H.; Kim, Y.S.; Kim, K.D.; Lee, H.K.; Cho, D.H.; Yang, J.W.; Hur, D.Y. l-Kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species. Int. Immunopharmacol. 2011, 11, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Zsizsik, B.K.; Poeggeler, B.; Fuhrberg, B.; Holst, S.; Coto-Montes, A. Indole-3-Pyruvic and -Propionic Acids, Kynurenic Acid, and Related Metabolites as Luminophores and Free-Radical Scavengers. In Tryptophan, Serotonin, and Melatonin: Basic Aspects and Applications; Huether, G., Kochen, W., Simat, T.J., Steinhart, H., Eds.; Springer: New York City, NY, USA, 1999; pp. 389–395. [Google Scholar]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Zsizsik, B.K.; Poeggeler, B.; Fuhrberg, B.; Holst, S.; Coto-Montes, A. Indole-3-pyruvic and -propionic acids, kynurenic acid, and related metabolites as luminophores and free-radical scavengers. Adv. Exp. Med. Biol. 1999, 467, 389–395. [Google Scholar] [PubMed]
- Hardeland, R. A Putative mechanism of kynurenic acid oxidation by free radicals: Scavenging of two hydroxyl radicals and a superoxide anion, release of NO an CO2. In Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites; Cuvillier: Göttingen, Germany, 2001; pp. 164–167. [Google Scholar]
- Colín-González, A.L.; Maya-López, M.; Pedraza-Chaverrí, J.; Ali, S.F.; Chavarría, A.; Santamaría, A. The Janus faces of 3-hydroxykynurenine: Dual redox modulatory activity and lack of neurotoxicity in the rat striatum. Brain Res. 2014, 1589, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goda, K.; Hamane, Y.; Kishimoto, R.; Ogishi, Y. Radical scavenging properties of tryptophan metabolites. Estimation of their radical reactivity. Adv. Exp. Med. Biol. 1999, 467, 397–402. [Google Scholar] [PubMed]
- Leipnitz, G.; Schumacher, C.; Dalcin, K.B.; Scussiato, K.; Solano, A.; Funchal, C.; Dutra-Filho, C.S.; Wyse, A.T.S.; Wannmacher, C.M.D.; Latini, A.; Wajner, M. In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochem. Int. 2007, 50, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Goshima, N.; Wadano, A.; Miura, K. 3-Hydroxykynurenine as O2−. scavenger in the blowfly, Aldrichina grahami. Biochem. Biophys Res. Commun. 1986, 139, 666–672. [Google Scholar] [CrossRef]
- Esaki, H.; Onozaki, H.; Kawakishi, S.; Osawa, T. New antioxidant isolated from tempeh. J. Agric. Food Chem. 1996, 44, 696–700. [Google Scholar] [CrossRef]
- Reyes-Ocampo, J.; Ramírez-Ortega, D.; Vázquez Cervantes, G.I.; Pineda, B.; Montes de Oca Balderas, P.; González-Esquivel, D.; Sánchez-Chapul, L.; Lugo-Huitrón, R.; Silva-Adaya, D.; Ríos, C.; et al. Mitochondrial dysfunction related to cell damage induced by 3-hydroxykynurenine and 3-hydroxyanthranilic acid: Non-dependent-effect of early reactive oxygen species production. Neurotoxicology 2015, 50, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.S.; Pae, H.O.; Choi, B.M.; Chae, S.C.; Lee, H.S.; Ryu, D.G.; Chung, H.T. 3-Hydroxyanthranilic acid, one of metabolites of tryptophan via indoleamine 2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochem. Biophys. Res. Commun. 2004, 320, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.; Suh, H.S.; Tarassishin, L.; Cui, Q.L.; Durafourt, B.A.; Choi, N.; Bauman, A.; Cosenza-Nashat, M.; Antel, J.P.; Zhao, M.L.; et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: Role of hemeoxygenase-1. Am. J. Pathol. 2011, 179, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Oh, G.S.; Lee, B.S.; Rim, J.S.; Kim, Y.M.; Chung, H.T. 3-Hydroxyanthranilic acid, one of l-tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1 expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis 2006, 187, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Waguri, S.; Sou, Y.S.; Kageyama, S.; Hasegawa, J.; Ishimura, R.; Saito, T.; Yang, Y.; Kouno, T.; Fukutomi, T.; et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell 2013, 51, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.A.; McCreanor, G.M. The preferred route of kynurenine metabolism in the rat. Biochim. Biophys. Acta (BBA) Gen. Subj. 1982, 717, 56–60. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Leopold, M.C.; Huang, X.; Atwood, C.S.; Saunders, A.J.; Hartshorn, M.; Lim, J.T.; Faget, K.Y.; Muffat, J.A.; Scarpa, R.C.; et al. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote α-crystallin cross-linking by metal ion reduction. Biochemistry 2000, 39, 7266–7275. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Saito, K.; Takemura, M.; Maekawa, N.; Fujigaki, S.; Fujii, H.; Wada, H.; Takeuchi, S.; Noma, A.; Seishima, M. 3-Hydroxyanthranilic acid, an l-tryptophan metabolite, induces apoptosis in monocyte-derived cells stimulated by interferon-γ. Ann. Clin. Biochem. 2001, 38, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Collins, C.A.; Stone, T.W.; Jacob, C. Electrochemical and in vitro evaluation of the redox-properties of kynurenine species. Biochem. Biophys. Res. Commun. 2003, 300, 719–724. [Google Scholar] [CrossRef]
- Murakami, K.; Ito, M.; Yoshino, M. Xanthurenic acid inhibits metal ion-induced lipid peroxidation and protects NADP-isocitrate dehydrogenase from oxidative inactivation. J. Nutr. Sci. Vitaminol. 2001, 47, 306–310. [Google Scholar] [CrossRef] [PubMed]
- López-Burillo, S.; Tan, D.-X.; Mayo, J.C.; Sainz, R.M.; Manchester, L.C.; Reiter, R.J. Melatonin, xanthurenic acid, resveratrol, EGCG, vitamin C and α-lipoic acid differentially reduce oxidative DNA damage induced by Fenton reagents: A study of their individual and synergistic actions. J. Pineal Res. 2003, 34, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Fukushima, K.; Kumamoto, K.; Iwahashi, H. Effects of some naturally occurring iron ion chelators on in vitro superoxide radical formation. Biol. Trace Elem. Res. 2005, 108, 77–85. [Google Scholar] [CrossRef]
- Cer-Kerčmar, K.; Tratar Pirc, E.; Modec, B.; Bukovec, P. Synthesis and characterization of two copper(II) compounds with xanthurenic acid. Monatshefte für Chem. Chem. Mon. 2012, 143, 413–420. [Google Scholar] [CrossRef]
- Murakami, K.; Haneda, M.; Yoshino, M. Prooxidant action of xanthurenic acid and quinoline compounds: Role of transition metals in the generation of reactive oxygen species and enhanced formation of 8-hydroxy-2′-deoxyguanosine in DNA. Biometals 2006, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.D.; Davis, T.Z.; Aust, S.D. Iron autoxidation and free radical generation: Effects of buffers, ligands, and chelators. Arch. Biochem. Biophys. 2002, 397, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Malina, H.Z.; Martin, X.D. Indoleamine 2,3-dioxygenase: Antioxidant enzyme in the human eye. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234, 457–462. [Google Scholar] [CrossRef]
- Murthi, P.; Wallace, E.M.; Walker, D.W. Altered placental tryptophan metabolic pathway in human fetal growth restriction. Placenta 2017, 52, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Suzuki, M.; Pryor-Koishi, K.; Sekiya, T.; Tada, S.; Kurahashi, H.; Udagawa, Y. Impact of indoleamine 2,3-dioxygenase on the antioxidant system in the placentas of severely pre-eclamptic patients. Syst. Biol. Reprod. Med. 2011, 57, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C.; Mattila, J.T. “Of mice and men”: Arginine metabolism in macrophages. Front. Immunol. 2014, 5, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneemann, M.; Schoeden, G. Macrophage biology and immunology: Man is not a mouse. J. Leukoc. Biol. 2007, 81, 579. [Google Scholar] [CrossRef] [PubMed]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide Biol. Chem. 2010, 23, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Samelson-Jones, B.J.; Yeh, S.R. Interactions between nitric oxide and indoleamine 2,3-dioxygenase. Biochemistry 2006, 45, 8527–8538. [Google Scholar] [CrossRef] [PubMed]
- Alberati-Giani, D.; Malherbe, P.; Ricciardi-Castagnoli, P.; Köhler, C.; Denis-Donini, S.; Cesura, A.M. Differential regulation of indoleamine 2,3-dioxygenase expression by nitric oxide and inflammatory mediators in IFN-γ-activated murine macrophages and microglial cells. J. Immunol. 1997, 159, 419–426. [Google Scholar] [PubMed]
- Hucke, C.; MacKenzie, C.R.; Adjogble, K.D.Z.; Takikawa, O.; Däubener, W. Nitric oxide-mediated regulation of gamma interferon-induced bacteriostasis: Inhibition and degradation of human indoleamine 2,3-dioxygenase. Infect. Immun. 2004, 72, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Sekkaı̈, D.; Guittet, O.; Lemaire, G.; Tenu, J.P.; Lepoivre, M. Inhibition of nitric oxide synthase expression and activity in macrophages by 3-hydroxyanthranilic acid, a tryptophan metabolite. Arch. Biochem. Biophys. 1997, 340, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Melillo, G.; Cox, G.W.; Biragyn, A.; Sheffler, L.A.; Varesio, L. Regulation of nitric-oxide synthase mRNA expression by interferon-γ and picolinic acid. J. Biol. Chem. 1994, 269, 8128–8133. [Google Scholar] [PubMed]
- Benz, D.; Cadet, P.; Mantione, K.; Zhu, W.; Stefano, G. Tonal nitric oxide and health-a free radical and a scavenger of free radicals. Med. Sci. Monit. 2002, 8, RA1-4. [Google Scholar] [PubMed]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Response to “species differences in macrophage NO production are important”. Nat. Immunol. 2002, 3, 102. [Google Scholar] [CrossRef]
- Thomas, S.R.; Salahifar, H.; Mashima, R.; Hunt, N.H.; Richardson, D.R.; Stocker, R. Antioxidants inhibit indoleamine 2,3-dioxygenase in IFN-γ-Activated human macrophages: Posttranslational regulation by pyrrolidine dithiocarbamate. J. Immunol. 2001, 166, 6332–6340. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, M.; Schoedon, G.; Hofer, S.; Blau, N.; Guerrero, L.; Schaffner, A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J. Infect. Dis. 1993, 167, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Pfister, H.; Remer, K.A.; Brcic, M.; Fatzer, R.; Christen, S.; Leib, S.; Jungi, T.W. Inducible nitric oxide synthase and nitrotyrosine in listeric encephalitis: A cross-species study in ruminants. Vet. Pathol. 2002, 39, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Werner-Felmayer, G.; Werner, E.R.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Wachter, H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1989, 1012, 140–147. [Google Scholar] [CrossRef]
- Schröcksnadel, K.; Widner, B.; Neurauter, G.; Fuchs, D.; Schröcksnadel, H.; Bergant, A. Tryptophan Degradation During And After Gestation. In Developments in Tryptophan and Serotonin Metabolism; Allegri, G., Costa, C.V.L., Ragazzi, E., Steinhart, H., Varesio, L., Eds.; Springer: New York City, NY, USA, 2003; pp. 77–83. [Google Scholar]
- Badawy, A.A. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology 2017, 112, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Schröcksnadel, H.; Baier-Bitterlich, G.; Dapunt, O.; Wachter, H.; Fuchs, D. Decreased plasma tryptophan in pregnancy. Obstet. Gynecol. 1996, 88, 47–50. [Google Scholar] [CrossRef]
- Badawy, A.A.B.; Namboodiri, A.M.A.; Moffett, J.R. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin. Sci. 2016, 130, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Bonney, E.A.; Matzinger, P. Much IDO about pregnancy. Nat. Med. 1998, 4, 1128–1129. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Braidman, I.P. Excretion of tryptophan metabolites as affected by pregnancy, contraceptive steroids, and steroid hormones. Am. J. Clin. Nutr. 1971, 24, 673–683. [Google Scholar] [PubMed]
- Stone, T.W. Tryptophan and kynurenines: Continuing to court controversy. Clin. Sci. (Lond. Engl. 1979) 2016, 130, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Toyofuku, Y.; Lynn, F.C.; Chak, E.; Uchida, T.; Mizukami, H.; Fujitani, Y.; Kawamori, R.; Miyatsuka, T.; Kosaka, Y.; et al. Serotonin regulates pancreatic β cell mass during pregnancy. Nat. Med. 2010, 16, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Fukuwatari, T.; Murakami, M.; Ohta, M.; Kimura, N.; Jin-No, Y.; Sasaki, R.; Shibata, K. Changes in the urinary excretion of the metabolites of the tryptopan-niacin pathway during pregnancy in japanese women and rats. J. Nutr. Sci. Vitaminol. 2004, 50, 392–398. [Google Scholar] [CrossRef]
- Sano, M.; Ferchaud-Roucher, V.; Kaeffer, B.; Poupeau, G.; Castellano, B.; Darmaun, D. Maternal and fetal tryptophan metabolism in gestating rats: Effects of intrauterine growth restriction. Amino Acids 2016, 48, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Higuchi, T.; Fujiwara, H.; Nakayama, T.; Egawa, H.; Itoh, K.; Fujii, S.; Fujita, J. Induction of tryptophan 2,3-dioxygenase in the mouse endometrium during implantation. Biochem. Biophys. Res. Commun. 2000, 274, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Schröcksnadel, K.; Widner, B.; Bergant, A.; Neurauter, G.; Schennach, H.; Schröcksnadel, H.; Fuchs, D. Longitudinal study of tryptophan degradation during and after pregnancy. Life Sci. 2003, 72, 785–793. [Google Scholar] [CrossRef]
- Ligam, P.; Manuelpillai, U.; Wallace, E.M.; Walker, D. Localisation of Indoleamine 2,3-dioxygenase and kynurenine hydroxylase in the human placenta and decidua: implications for role of the kynurenine pathway in pregnancy. Placenta 2005, 26, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; Schröcksnadel, H.; Baier-Bitterlich, G.; Dapunt, O.; Wachter, H. Activated Cellular Immunity and Decreased Serum Tryptophan in Healthy Pregnancy. In Recent Advances in Tryptophan Research: Tryptophan and Serotonin Pathways; Filippini, G.A., Costa, C.V.L., Bertazzo, A., Eds.; Springer: New York City, NY, USA, 1996; pp. 149–153. [Google Scholar]
- Wang, M.; Liang, Q.; Li, H.; Xia, W.; Li, J.; Peng, Y.; Li, Y.; Ma, Z.; Xu, B.; Gao, Y.; et al. Normal pregnancy-induced amino acid metabolic stress in a longitudinal cohort of pregnant women: Novel insights generated from UPLC-QTOFMS-based urine metabolomic study. Metabolomics 2016, 12. [Google Scholar] [CrossRef]
- Luan, H.; Meng, N.; Liu, P.; Feng, Q.; Lin, S.; Fu, J.; Davidson, R.; Chen, X.; Rao, W.; Chen, F.; et al. Pregnancy-induced metabolic phenotype variations in maternal plasma. J. Proteome Res. 2014, 13, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Kamimura, S.; Yonezawa, M.; Mitsui, Y.; Mizutani, Y.; Kudo, T. Tryptophan and its metabolite concentrations in human plasma during the perinatal period. Nihon Sanka Fujinka Gakkai Zasshi 1992, 44, 663–668. [Google Scholar] [PubMed]
- Maes, M.; Verkerk, R.; Bonaccorso, S.; Ombelet, W.; Bosmans, E.; Scharpé, S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002, 71, 1837–1848. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Wallace, E.M.; Nicholls, T.; Guillemin, G.; Phillips, D.J.; Walker, D. Increased mRNA expression of kynurenine pathway enzymes in human placentae exposed to bacterial endotoxin. In Developments in Tryptophan and Serotonin Metabolism; Allegri, G., Costa, C.V.L., Ragazzi, E., Steinhart, H., Varesio, L., Eds.; Springer: New York City, NY, USA, 2003; pp. 85–89. [Google Scholar]
- Manuelpillai, U.; Ligam, P.; Smythe, G.; Wallace, E.M.; Hirst, J.; Walker, D.W. Identification of kynurenine pathway enzyme mRNAs and metabolites in human placenta: Up-regulation by inflammatory stimuli and with clinical infection. Am. J. Obstet. Gynecol. 2005, 192, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Sedlmayr, P.; Blaschitz, A.; Stocker, R. The role of placental tryptophan catabolism. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, A.; Levitt, P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 2011, 197, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.C.; de Vries, V.C.; Wasiuk, A.; Ahonen, C.; Bennett, K.A.; Le Mercier, I.; Ha, D.G.; Noelle, R.J. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J. Exp. Med. 2012, 209, 2127. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.W.; Powers, R.W.; Ness, R.B.; Cropcho, L.J.; Daftary, A.R.; Harger, G.F.; Vergona, R.; Finegold, D.N. Maternal and fetal amino acid concentrations and fetal outcomes during pre-eclampsia. Reproduction (Camb. Engl.) 2003, 125, 785–790. [Google Scholar] [CrossRef]
- Tsuji, A.; Nakata, C.; Sano, M.; Fukuwatari, T.; Shibata, K. L-Tryptophan metabolism in pregnant mice fed a high L-tryptophan diet and the effect on maternal, placental, and fetal growth. Int. J. Tryptophan Res. 2013, 6, 21–33. [Google Scholar] [PubMed]
- Sugino, N.; Takiguchi, S.; Umekawa, T.; Heazell, A.; Caniggia, I. Oxidative stress and pregnancy outcome: A workshop report. Placenta 2007, 28, S48–S50. [Google Scholar] [CrossRef] [PubMed]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–912. [Google Scholar] [CrossRef] [PubMed]
- Hracsko, Z.; Orvos, H.; Novak, Z.; Pal, A.; Varga, I.S. Evaluation of oxidative stress markers in neonates with intra-uterine growth retardation. Redox Rep. Commun. Free Radic. Res. 2008, 13, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Karowicz-Bilinska, A. Lipid peroxides concentration in women with intrauterine growth restriction. Ginekol. Pol. 2004, 75, 6–9. [Google Scholar] [PubMed]
- Karowicz-Bilinska, A.; Suzin, J.; Sieroszewski, P. Evaluation of oxidative stress indices during treatment in pregnant women with intrauterine growth retardation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2002, 8, CR211–CR216. [Google Scholar]
- Dang, Y.; Xia, C.; Brown, O.R. Effects of oxygen on 3-hydroxyanthranilate oxidase of the kynurenine pathway. Free Radic. Biol. Med. 1998, 25, 1033–1043. [Google Scholar] [CrossRef]
- Dang, Y.; Dale, W.E.; Brown, O.R. Comparative effects of oxygen on indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase of the kynurenine pathway. Free Radic. Biol. Med. 2000, 28, 615–624. [Google Scholar] [CrossRef]
- Dang, Y.; Dale, W.E.; Brown, O.R. Effects of oxygen on kynurenine-3-monooxygenase activity. Redox Rep. Commun. Free Radic. Res. 2000, 5, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, N.; Yamamoto, M.; Nanjo, S.; Toujima, S.; Minami, S.; Ino, K. Downregulation of indoleamine 2, 3-dioxygenase expression in the villous stromal endothelial cells of placentas with preeclampsia. J. Reprod. Immunol. 2017, 119, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Boyd, C.A.R.; Sargent, I.L.; Redman, C.W.G. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. Am. J. Obstet. Gynecol. 2003, 188, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, S.; Palmer, S.K.; Droma, T.; Stamm, E.; Coffin, C.; Moore, L.G. Effect of altitude on uterine artery blood flow during normal pregnancy. J. Appl. Physiol. 1995, 79, 7–14. [Google Scholar] [PubMed]
- Soothill, P.W.; Nicolaides, K.H.; Campbell, S. Prenatal asphyxia, hyperlacticaemia, hypoglycaemia, and erythroblastosis in growth retarded fetuses. Br. Med. J. (Clin. Res. Ed.) 1987, 294, 1051–1053. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A. A review of the mechanism for poor placentation in early-onset preeclampsia: The role of autophagy in trophoblast invasion and vascular remodeling. J. Reprod. Immunol. 2014, 101–102, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Hubel, C.A. Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 1999, 222, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin. Reprod. Endocrinol. 1998, 16, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nikaido, T.; Toki, T.; Kita, N.; Kanai, M.; Ashida, T.; Ohira, S.; Konishi, I. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004, 444, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Kossenjans, W.; Sahay, R.; Eis, A.; Brockman, D. Oxidative stress causes vascular dysfunction in the placenta. J. Matern.-Fetal Med. 2000, 9, 79–82. [Google Scholar] [CrossRef]
- Hung, T.H.; Skepper, J.N.; Burton, G.J. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am. J. Pathol. 2001, 159, 1031–1043. [Google Scholar] [CrossRef]
- Nishizawa, H.; Hasegawa, K.; Suzuki, M.; Kamoshida, S.; Kato, T.; Saito, K.; Tsutsumi, Y.; Kurahashi, H.; Udagawa, Y. The etiological role of allogeneic fetal rejection in pre-eclampsia. Am. J. Reprod. Immunol. 2007, 58, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Li, C.; Luo, C.; Zhao, X.; Liu, C.; Wang, K.; Jia, W.; Bai, M.; Yin, M.; Bao, S.; Guo, J.; Kang, J.; Duan, T.; Zhou, Q. Dysregulated expression of IDO may cause unexplained recurrent spontaneous abortion through suppression of trophoblast cell proliferation and migration. Sci. Rep. UK 2016, 6, 19916. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Chang, Y.; Dong, B.; Kong, B.; Qu, X. Indoleamine 2,3-dioxygenase levels at the normal and recurrent spontaneous abortion fetal-maternal interface. J. Int. Med. Res. 2013, 41, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Wilczynski, J.R. Immunological analogy between allograft rejection, recurrent abortion and pre-eclampsia - the same basic mechanism? Hum. Immunol. 2006, 67, 492–511. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Hasegawa, K.; Suzuki, M.; Achiwa, Y.; Kato, T.; Saito, K.; Kurahashi, H.; Udagawa, Y. Mouse model for allogeneic immune reaction against fetus recapitulates human pre-eclampsia. J. Obstet. Gynaecol. Res. 2008, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Santillan, M.K.; Pelham, C.J.; Ketsawatsomkron, P.; Santillan, D.A.; Davis, D.R.; Devor, E.J.; Gibson-Corley, K.N.; Scroggins, S.M.; Grobe, J.L.; Yang, B.; et al. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol. Rep. 2015, 3, e12257. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Hubel, C.A. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet 1999, 354, 788–789. [Google Scholar] [CrossRef]
- Thomas, S.R.; Stocker, R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999, 4, 199–220. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.R.; Thornton, M.J.; Price, J.M. The effect of vitamin supplementation on the urinary excretion of tryptophan metabolites by pregnant women. J. Clin. Investig. 1961, 40, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Banerjee, J.; Alvarez, J.G. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: A systematic review. Obstet. Gynecol. Surv. 2007, 62, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Simsek, M.; Naziroglu, M.; Simsek, H.; Cay, M.; Aksakal, M.; Kumru, S. Blood plasma levels of lipoperoxides, glutathione peroxidase, beta carotene, vitamin A and E in women with habitual abortion. Cell Biochem. Funct. 1998, 16, 227–231. [Google Scholar] [CrossRef]
- Vural, P.; Akgul, C.; Yildirim, A.; Canbaz, M. Antioxidant defence in recurrent abortion. Clin. Chim. Acta Int. J. Clin. Chem. 2000, 295, 169–177. [Google Scholar] [CrossRef]
- Safronova, V.G.; Matveeva, N.K.; Avkhacheva, N.V.; Sidel’nikova, V.M.; Van’ko, L.V.; Sukhikh, G.T. Changes in regulation of oxidase activity of peripheral blood granulocytes in women with habitual abortions. Bull. Exp. Biol. Med. 2003, 136, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, S.; Eguchi, K.; Yonezawa, M.; Sekiba, K. Localization and developmental change of indoleamine 2,3-dioxygenase activity in the human placenta. Acta Med. Okayama 1991, 45, 135–139. [Google Scholar] [PubMed]
- Miwa, N.; Hayakawa, S.; Miyazaki, S.; Myojo, S.; Sasaki, Y.; Sakai, M.; Takikawa, O.; Saito, S. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-gamma increase in normal pregnancy but decrease in spontaneous abortion. Mol. Hum. Reprod. 2005, 11, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Blois, S.; Kandil, J.; Handjiski, B.; Manuel, J.; Arck, P.C. Reduced uterine indoleamine 2,3-dioxygenase versus increased Th1/Th2 cytokine ratios as a basis for occult and clinical pregnancy failure in mice and humans. Am. J. Reprod. Immunol. 2005, 54, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, Y.; Ozaki, Y.; Goto, S.; Obayashi, S.; Suzumori, N.; Ohyama, F.; Tone, S.; Sugiura-Ogasawara, M. Role of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in patients with recurrent miscarriage. Am. J. Reprod. Immunol. 2016, 75, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef] [PubMed]
- Omata, N.; Tsukahara, H.; Ito, S.; Ohshima, Y.; Yasutomi, M.; Yamada, A.; Jiang, M.; Hiraoka, M.; Nambu, M.; Deguchi, Y.; Mayumi, M. Increased oxidative stress in childhood atopic dermatitis. Life Sci. 2001, 69, 223–228. [Google Scholar] [CrossRef]
- Tsukahara, H.; Shibata, R.; Ohshima, Y.; Todoroki, Y.; Sato, S.; Ohta, N.; Hiraoka, M.; Yoshida, A.; Nishima, S.; Mayumi, M. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. 2003, 72, 2509–2516. [Google Scholar] [CrossRef]
- El-Heis, S.; Crozier, S.R.; Robinson, S.M.; Harvey, N.C.; Cooper, C.; Inskip, H.M.; Godfrey, K.M. Higher maternal serum concentrations of nicotinamide and related metabolites in late pregnancy are associated with a lower risk of offspring atopic eczema at age 12 months. Clin. Exp. Allergy 2016, 46, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Ogawa, K.; Takeuchi, K.; Nakada, A.; Heishi, M.; Suto, H.; Mitsuishi, K.; Sugita, Y.; Ogawa, H.; Ra, C. Gene expression of enzymes for tryptophan degradation pathway is upregulated in the skin lesions of patients with atopic dermatitis or psoriasis. J. Dermatol. Sci. 2004, 36, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Von Bubnoff, D.; Matz, H.; Frahnert, C.; Rao, M.L.; Hanau, D.; de la Salle, H.; Bieber, T. Fc RI induces the tryptophan degradation pathway involved in regulating T cell responses. J. Immunol. 2002, 169, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Seve, B. Biological roles of tryptophan and its metabolism: Potential implications for pig feeding. Livest. Sci. 2007, 112, 23–32. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Melchior, D.; Sève, B. The importance of dietary tryptophan for preserving growth and controlling inflammatory response of weaned pigs submitted to immune stress. In Proceedings of the Animal production in Europe: The Way Foward in a Changing World, Saint-Malo, France, 11–13 October 2004; pp. 239–240. [Google Scholar]
- Le Floch, N.; Jondreville, C.; Matte, J.J.; Seve, B. Importance of sanitary environment for growth performance and plasma nutrient homeostasis during the post-weaning period in piglets. Arch. Anim. Nutr. 2006, 60, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Winberg, S.; Overli, O.; Lepage, O. Suppression of aggression in rainbow trout (Oncorhynchus mykiss) by dietary l-tryptophan. J. Exp. Biol. 2001, 204, 3867–7386. [Google Scholar] [PubMed]
- Mellor, A.L.; Munn, D.H. Tryptophan catabolism and T-cell tolerance: Immunosuppression by starvation? Immunol. Today 1999, 20, 469–473. [Google Scholar] [CrossRef]
- Romero, R.; Gotsch, F.; Pineles, B.; Kusanovic, J.P. Inflammation in Pregnancy: Its Roles in Reproductive Physiology, Obstetrical Complications, and Fetal Injury. Nutr. Rev. 2007, 65, 194–202. [Google Scholar] [CrossRef]
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F.; Petraglia, F. Inflammation and pregnancy. Reprod. Sci. 2009, 16, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G.P.; Studena, K.; Sargent, I.L.; Redman, C.W.G. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am. J. Obstet. Gynecol. 1998, 179, 80–86. [Google Scholar] [CrossRef]
- Kwak-Kim, J.; Yang, K.M.; Gilman-Sachs, A. Recurrent pregnancy loss: A disease of inflammation and coagulation. J. Obstet. Gynaecol. Res. 2009, 35, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Melchior, D.; Le Floc’h, N.; Seve, B. Effects of chronic lung inflammation on tryptophan metabolism in piglets. Adv. Exp. Med. Biol. 2003, 527, 359–362. [Google Scholar] [PubMed]
- Melchior, D.; Seve, B.; Le Floc’h, N. Chronic lung inflammation affects plasma amino acid concentrations in pigs. J. Anim. Sci. 2004, 82, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Melchior, D.; Meziere, N.; Seve, B.; Le Floc’h, N. Is tryptophan catabolism increased under indoleamine 2,3 dioxygenase activity during chronic lung inflammation in pigs? Reprod. Nutr. Dev. 2005, 45, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Melchior, D.; Seve, B. Dietary tryptophan helps to preserve tryptophan homeostasis in pigs suffering from lung inflammation. J. Anim. Sci. 2008, 86, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Asp, L.; Johansson, A.S.; Mann, A.; Owe-Larsson, B.; Urbanska, E.M.; Kocki, T.; Kegel, M.; Engberg, G.; Lundkvist, G.B.; Karlsson, H. Effects of pro-inflammatory cytokines on expression of kynurenine pathway enzymes in human dermal fibroblasts. J. Inflamm. 2011, 8, 1476–9255. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Schroecksnadel, S.; Féart, C.; Aubert, A.; Higueret, D.; Barberger-Gateau, P.; Layé, S.; Fuchs, D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: Role in neuropsychiatric symptoms. Biol. Psychiatry 2011, 70, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, H.; Wen, Q.; Zhang, Y. Indoleamine 2,3-dioxygenase expression in human inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2012, 24, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Moschen, A.R.; Kaser, A.; Obrist, P.; Fuchs, D.; Brandacher, G.; Winkler, C.; Geboes, K.; Rutgeerts, P.; Tilg, H. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin. Immunol. 2004, 113, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Zelante, T.; De Luca, A.; Fallarino, F.; Puccetti, P. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J. Immunol. 2008, 180, 5157–5162. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Stocker, R. Antioxidant activities and redox regulation of interferon-gamma-induced tryptophan metabolism in human monocytes and macrophages. In Tryptophan, Serotonin, and Melatonin; Huether, G., Kochen, W., Simat, T., Steinhart, H., Eds.; Springer: New York City, NY, USA, 1999; Volume 467, pp. 541–552. [Google Scholar]
- Lee, K.W.; Kim, J.B.; Seo, J.S.; Kim, T.K.; Im, J.Y.; Baek, I.S.; Kim, K.S.; Lee, J.K.; Han, P.L. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. J. Neurochem. 2009, 108, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Schwarcz, R. Restraint Stress during Pregnancy Rapidly Raises Kynurenic Acid Levels in Mouse Placenta and Fetal Brain. Dev. Neurosci. Basel 2016, 38, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Bjork, J.M.; Dougherty, D.M.; Moeller, F.G.; Swann, A.C. Differential behavioral effects of plasma tryptophan depletion and loading in aggressive and nonaggressive men. Neuropsychopharmacology 2000, 22, 357–369. [Google Scholar] [CrossRef]
- Shen, Y.B.; Voilqué, G.; Odle, J.; Kim, S.W. Dietary l-tryptophan supplementation with reduced large neutral amino acids enhances feed efficiency and decreases stress hormone secretion in nursery pigs under social-mixing stress. J. Nutr. 2012, 142, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Trejo, G.; Ortega-Cerrilla, M.; Rodarte-Covarrubias, L.; Herrera-Haro, J.; Figueroa-Velasco, J.; Galindo-Maldonado, F.; Sánchez-Martínez, O.; Lara-Bueno, A. Aggressiveness and productive performance of piglets supplemented with tryptophan. J. Anim. Vet. Adv. 2009, 8, 608–611. [Google Scholar]
- Walz, J.C.; Stertz, L.; Fijtman, A.; dos Santos, B.T.M.Q.; de Almeida, R.M.M. Tryptophan diet reduces aggressive behavior in male mice. Psychol. Neurosci. 2013, 6, 397–401. [Google Scholar] [CrossRef]
- Shes-moore, M.M.; Thomas, O.P.; Mench, J.A. Decreases in aggression in tryptophan-supplemented broiler breeder males are not due to increases in blood niacin levels. Poult. Sci. 1996, 75, 370–374. [Google Scholar] [CrossRef]
- Koopmans, S.J.; Ruis, M.; Dekker, R.; van Diepen, H.; Korte, M.; Mroz, Z. Surplus dietary tryptophan reduces plasma cortisol and noradrenaline concentrations and enhances recovery after social stress in pigs. Physiol. Behav. 2005, 85, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Baidoo, S.K.; Johnston, L.J.; Anderson, J.E. Effects of tryptophan supplementation on aggression among group-housed gestating sows. J. Anim. Sci. 2011, 89, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Behera, K.; Lone, S.; Behera, D. Influence of stocking density on growth performance of vencobb broiler. Asian J. Anim. Sci. 2015, 10, 187–192. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, I.H. Effect of stocking density on pig production. Afr. J. Biotechnol. 2013, 10, 13688–13692. [Google Scholar]
- Zhang, Z.F.; Li, J.; Park, J.C.; Kim, I.H. Effect of Vitamin Levels and Different Stocking Densities on Performance, Nutrient Digestibility, and Blood Characteristics of Growing Pigs. Asian Australas. J. Anim. Sci. 2013, 26, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef] [PubMed]
- El-Gogary, M.; Azzam, M. Effects of dietary tryptophan levels and stocking density during the growing-finishing phase on broiler performance and immunity. Asian J. Anim. Vet. Adv. 2014, 9, 568–577. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, J.M.; Zhang, L.S.; Zhang, Y.R.; Cai, S.M.; Yu, J.H.; Xia, Z.F. Effects of tryptophan supplementation on growth performance, antioxidative activity, and meat quality of ducks under high stocking density. Poult. Sci. 2015, 94, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Coffey, M.T.; Kim, S.W. Effects of short term supplementation of l-tryptophan and reducing large neutral amino acid along with l-tryptophan supplementation on growth and stress response in pigs. Anim. Feed Sci. Technol. 2015, 207, 245–252. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Sato, K.; Akiba, Y.; Toyomizu, M. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult. Sci. 2006, 85, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Rosado Montilla, S.I. The Effects of Heat Stress in Redox Balance and Inflammatory Signaling in Porcine Skeletal Muscle. Master’s Thesis, Iowa State University, Ames, IA, USA, 2013. [Google Scholar]
- Montilla, S.I. R.; Johnson, T.P.; Pearce, S.C.; Gardan-Salmon, D.; Gabler, N.K.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Lonergan, S.M.; Selsby, J.T. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature 2014, 1, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Hirabayashi, M.; Kanai, Y. Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or in vitro. Reproduction (Camb. Engl.) 2002, 124, 683–689. [Google Scholar] [CrossRef]
- Matsuzuka, T.; Ozawa, M.; Nakamura, A.; Ushitani, A.; Hirabayashi, M.; Kanai, Y. Effects of heat stress on the redox status in the oviduct and early embryonic development in mice. J. Reprod. Dev. 2005, 51, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Flowers, W.L.; Saraiva, A.; Yeum, K.-J.; Kim, S.W. Effect of heat stress on oxidative stress status and reproductive performance of sows. J. Anim. Sci. 2011, 89, 683–689. [Google Scholar]
- Manjunath, R.; Ramasarma, T. Stimulation of liver tryptophan pyrrolase during heat exposure. Biochem. J. 1985, 226, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Tabiri, H.Y.; Sato, K.; Takahashi, K.; Toyomizu, M.; Akiba, Y. Effect of acute heat stress on plasma amino acids concentration of broiler chickens. Jpn. Poult. Sci. 2000, 37, 86–94. [Google Scholar] [CrossRef]
- Tabiri, H.Y.; Sato, K.; Takahashi, K.; Toyomizu, M.; Akiba, Y. Effects of heat stress and dietary tryptophan on performance and plasma amino acid concentrations of broiler chickens. Asian Australas. J. Anim. Sci. 2002, 15, 247–253. [Google Scholar] [CrossRef]
- Murr, C.; Widner, B.; Gerlach, D.; Werner-Felmayer, G.; Dierich, M.; Wachter, H.; Fuchs, D. Streptococcal erythrogenic toxins induce tryptophan degradation in human peripheral blood mononuclear cells. Int. Arch. Allergy Immunol. 1997, 114, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Hoeflich, A. Endotoxin-induced tryptophan degradation along the kynurenine pathway: The role of indolamine 2,3-dioxygenase and aryl hydrocarbon receptor-mediated immunosuppressive effects in endotoxin tolerance and cancer and its implications for immunoparalysis. J. Amino Acids 2015, 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef]
- Chaytor, A.C.; See, M.T.; Hansen, J.A.; de Souza, A.L.; Middleton, T.F.; Kim, S.W. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J. Anim. Sci. 2011, 89, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Yu, B.; Mao, X.B.; Zheng, P.; He, J.; Chen, D.W. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal 2012, 6, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Lv, M.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Wang, Q.; Chen, D. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets. J. Anim. Sci. Biotechnol. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Liu, H.; Xu, K.; Oso, A.O.; Wu, X.; Liu, G.; Tossou, M.C.B.; Al-Dhabi, N.A.; Duraipandiyan, V.; Xi, Q.; Yin, Y. A review of the immunomodulatory role of dietary tryptophan in livestock and poultry. Amino Acids 2017, 49, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Liu, H.; Bai, M.; Gao, J.; Wu, X.; Yin, Y. Redox Properties of Tryptophan Metabolism and the Concept of Tryptophan Use in Pregnancy. Int. J. Mol. Sci. 2017, 18, 1595. https://doi.org/10.3390/ijms18071595
Xu K, Liu H, Bai M, Gao J, Wu X, Yin Y. Redox Properties of Tryptophan Metabolism and the Concept of Tryptophan Use in Pregnancy. International Journal of Molecular Sciences. 2017; 18(7):1595. https://doi.org/10.3390/ijms18071595
Chicago/Turabian StyleXu, Kang, Hongnan Liu, Miaomiao Bai, Jing Gao, Xin Wu, and Yulong Yin. 2017. "Redox Properties of Tryptophan Metabolism and the Concept of Tryptophan Use in Pregnancy" International Journal of Molecular Sciences 18, no. 7: 1595. https://doi.org/10.3390/ijms18071595