Porcine Interferon Stimulated Gene 12a Restricts Porcine Reproductive and Respiratory Syndrome Virus Replication in MARC-145 Cells
Abstract
:1. Introduction
2. Results
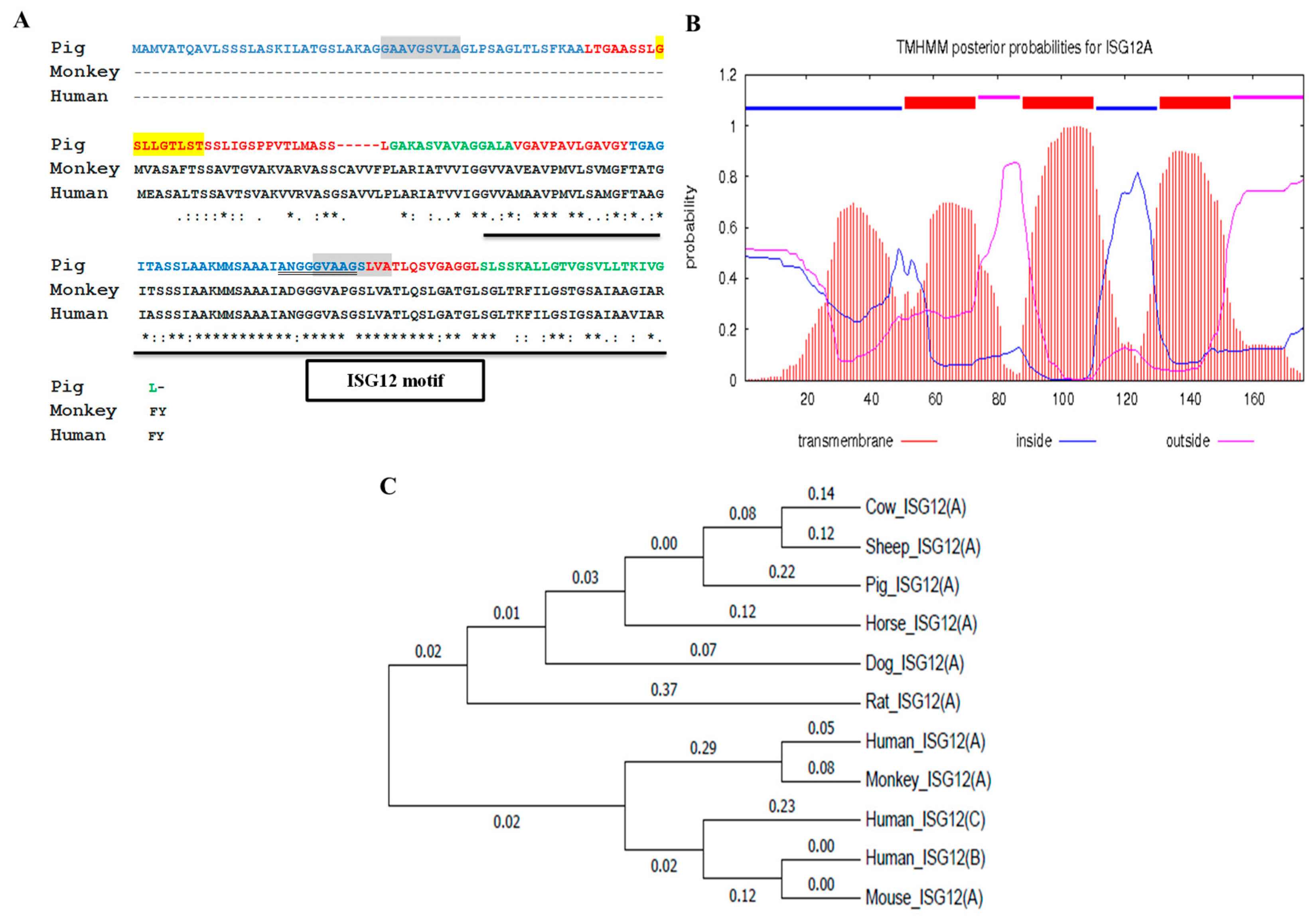
2.1. Porcine ISG12A Sequence and Phylogenetic Analysis
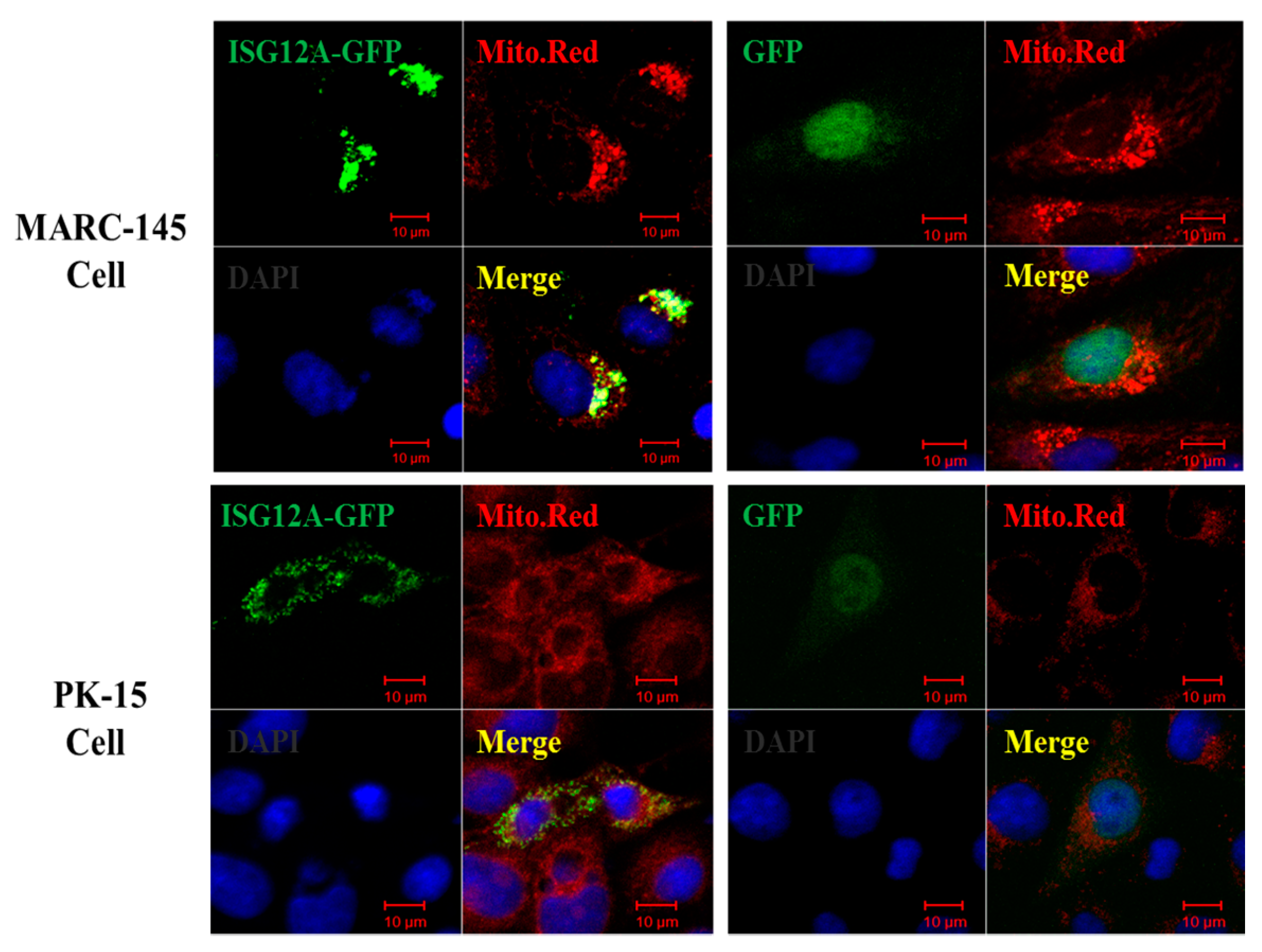
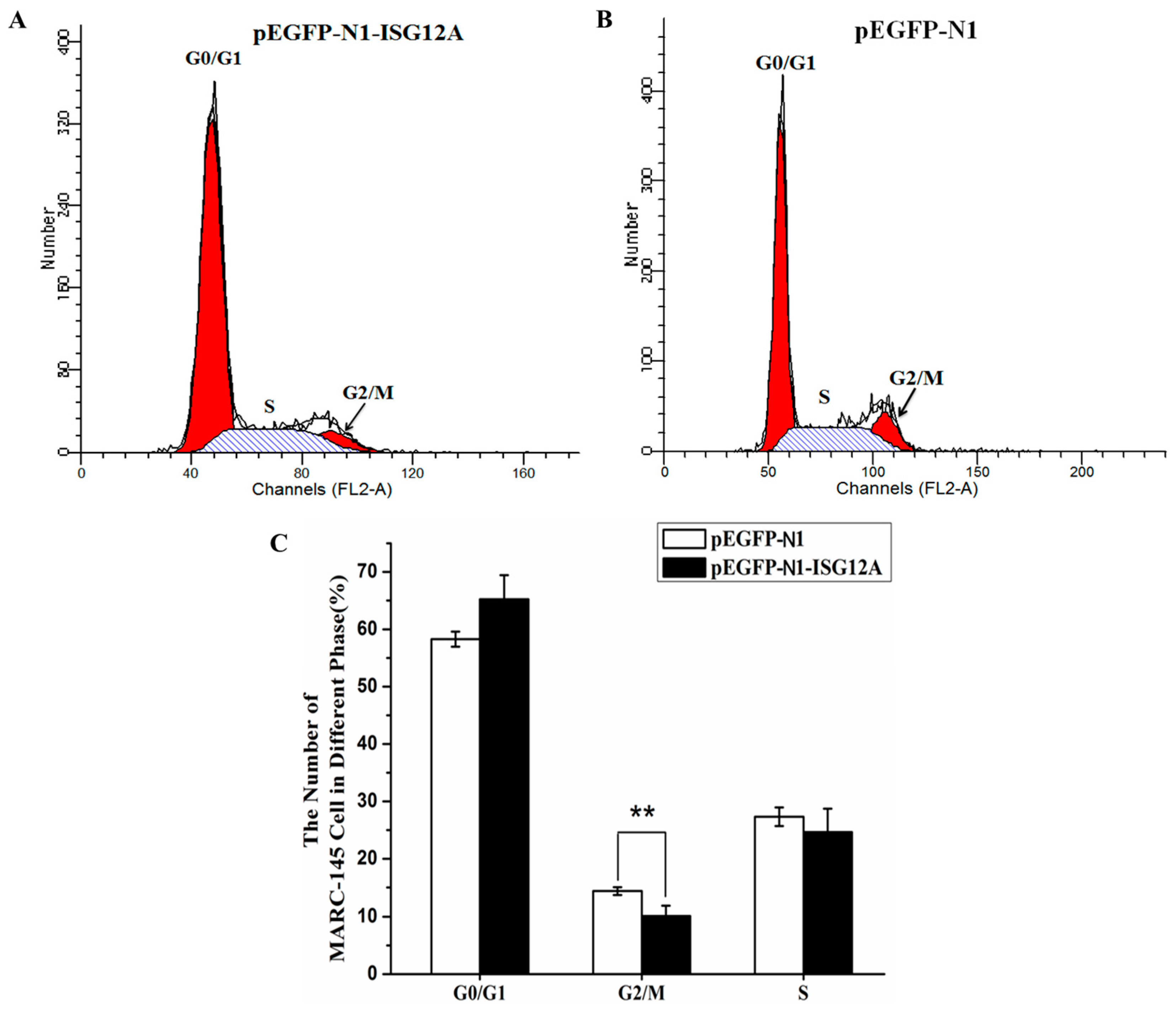
2.2. Porcine ISG12A Is Located in Mitochondria and Regulate Cell Cycle
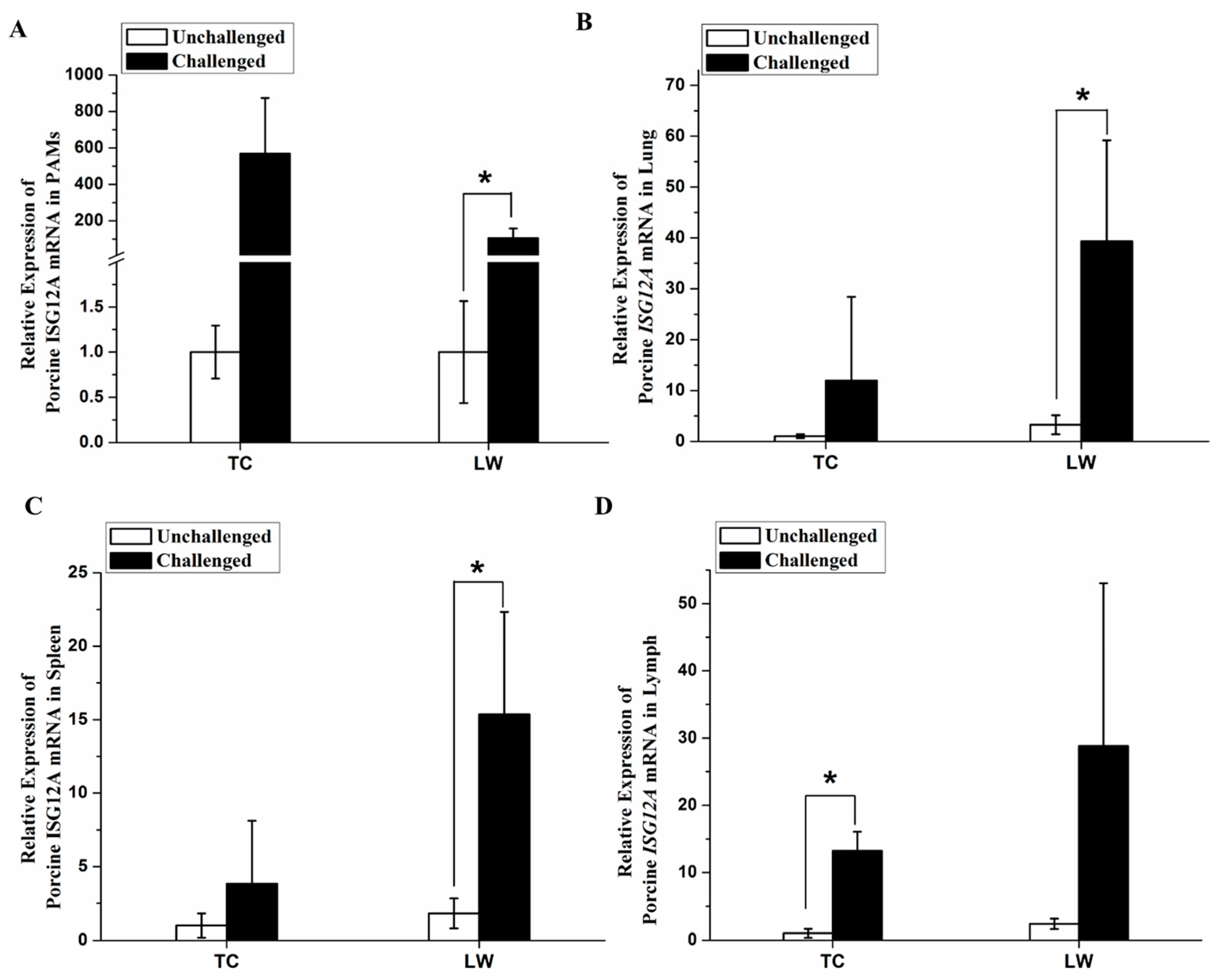
2.3. Increased Expression of Porcine ISG12A after PRRSV Challenge In Vivo
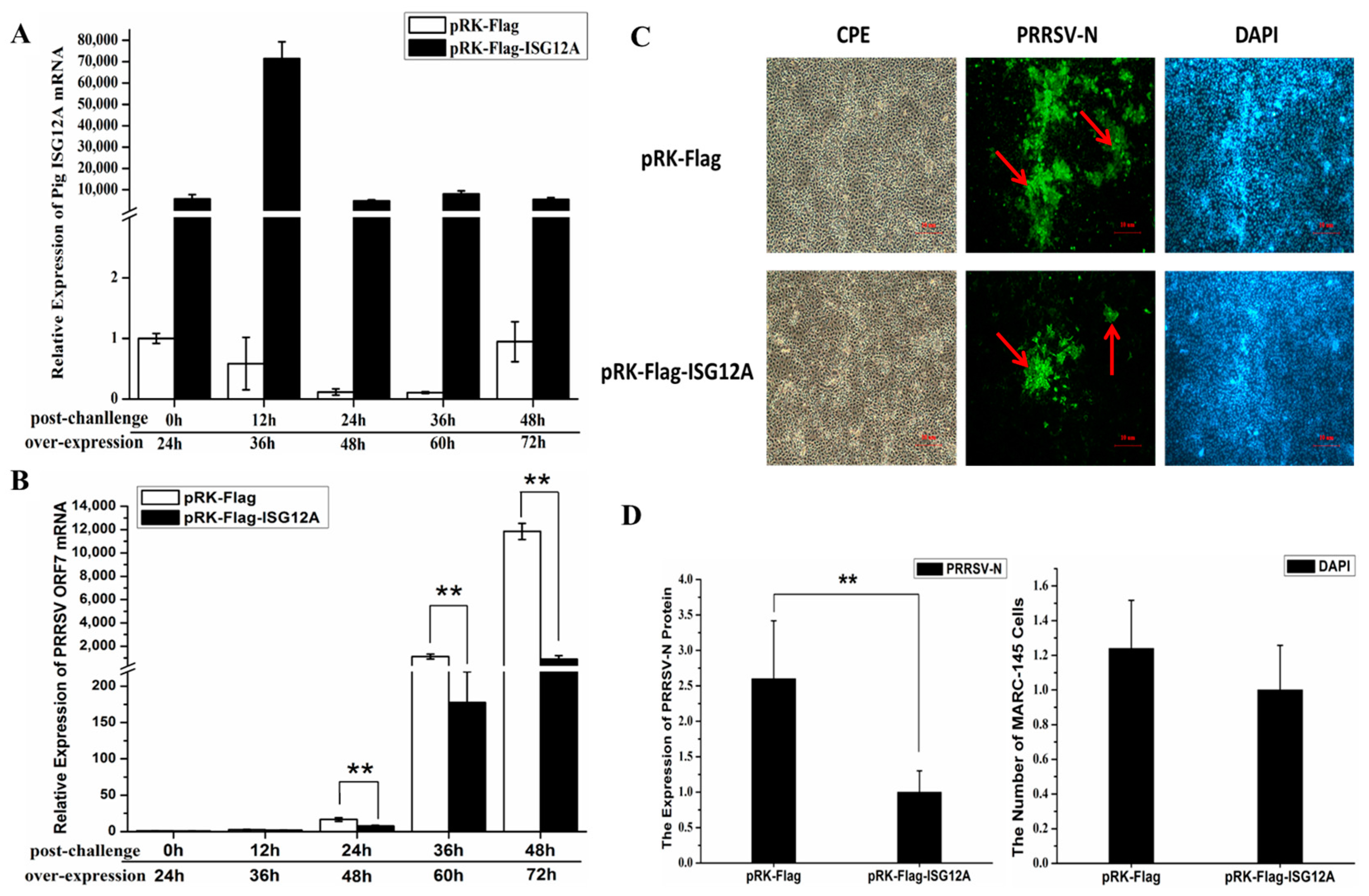
2.4. Porcine ISG12A Restricted PRRSV Replication in MARC-145 Cells
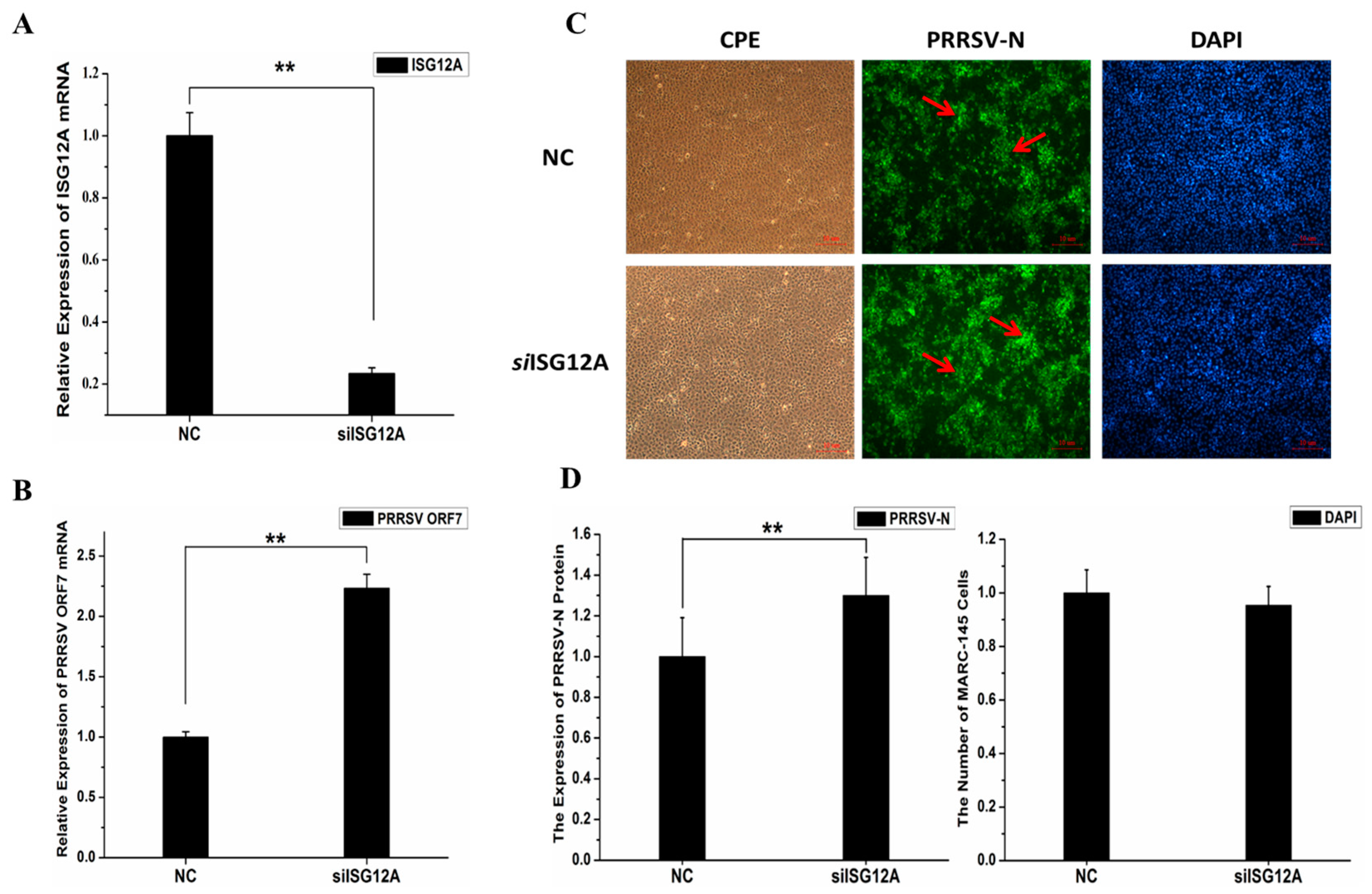
2.5. Knockdown of Endogenous ISG12A Enhanced PRRSV Replication in MARC-145 Cells
3. Discussion
4. Materials and Methods
4.1. Cells, Tissues, and Virus
4.2. Sequence Analysis
4.3. Expression Vectors
4.4. Confocal Microscopy
4.5. Flow Cytometry
4.6. Gene Expression in Cells and Tissues during PRRSV Challenge In Vivo
4.7. Transient Transfection and Real-Time Quantitative PCR
4.8. Immunofluorescence Assay
4.9. Statistical Tests
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Neumann, E.J.; Kliebenstein, J.B.; Johnson, C.D.; Mabry, J.W.; Bush, E.J.; Seitzinger, A.H.; Green, A.L.; Zimmerman, J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 2005, 227, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhao, T.; Nguyen, T.; Inui, K.; Ma, Y.; Nguyen, T.H.; Nguyen, V.C.; Liu, D.; Bui, Q.A.; To, L.T. Porcine respiratory and reproductive syndrome virus variants, Vietnam and China, 2007. Emerg. Infect. Dis. 2008, 14, 1774–1776. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Yu, X.; Zhao, T.; Feng, Y.; Cao, Z.; Wang, C.; Hu, Y.; Chen, X.; Hu, D.; Tian, X. Emergence of fatal PRRSV variants: Unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE 2007, 2, e526. [Google Scholar] [CrossRef] [PubMed]
- Rossow, K.D. Porcine reproductive and respiratory syndrome. Vet. Pathol. 1998, 35, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Enjuanes, L.; Ziebuhr, J.; Snijder, E.J. Nidovirales: Evolving the largest RNA virus genome. Virus Res. 2006, 117, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2016). Arch. Virol. 2016, 161, 2921–2949. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, T.; Zhu, C.G.; Jin, Y.F.; Zhang, Y.Z. Genetic variation of Chinese PRRSV strains based on ORF5 sequence. Biochem. Genet. 2006, 44, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Paolini, R.; Bernardini, G.; Molfetta, R.; Santoni, A. NK cells and interferons. Cytokine Growth Factor Rev. 2015, 26, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Martensen, P.M.; Justesen, J. Small ISGs coming forward. J. Interferon Cytokine Res. 2004, 24, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J. Virol. 2007, 81, 12720–12729. [Google Scholar] [CrossRef] [PubMed]
- Knapp, S.; Yee, L.J.; Frodsham, A.J.; Hennig, B.J.W.; Hellier, S.; Zhang, L.; Wright, M.; Chiaramonte, M.; Graves, M.; Thomas, H.C. Polymorphisms in interferon-induced genes and the outcome of hepatitis C virus infection: Roles of MxA, OAS-1 and PKR. Genes Immun. 2003, 4, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, C.J.; Padalko, E. iNOS (NOS2) at a glance. J. Cell Sci. 2004, 117, 2865–2867. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, Y.; Ransburgh, R.; Snijder, E.J.; Fang, Y. Nonstructural protein 2 of porcine reproductive and respiratory syndrome virus inhibits the antiviral function of interferon-stimulated gene 15. J. Virol. 2012, 86, 3839–3850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Bai, J.; Du, Y.; Wang, X.; Liu, X.; Jiang, P. Poly(I:C) inhibits porcine reproductive and respiratory syndrome virus replication in MARC-145 cells via activation of IFIT3. Antivir. Res. 2013, 99, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Feng, N.; Li, Z.; Wang, P.; Qi, Z.; Liang, W.; Zhou, X.; Xu, X.; Liu, B. 2′,5′-oligoadenylate synthetase 1(OAS1) inhibits PRRSV replication in Marc-145cells. Antivir. Res. 2016, 132, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, U.B.; Wolf, C.; Mattei, M.-G.; Chenard, M.-P.; Bellocq, J.-P.; Chambon, P.; Rio, M.-C.; Basset, P. Identification of a new interferon-α-inducible gene (p27) on human chromosome 14q32 and its expression in breast carcinoma. Cancer Res. 1993, 53, 4096–4101. [Google Scholar] [PubMed]
- Parker, N.; Porter, A.C. Identification of a novel gene family that includes the interferon-inducible human genes 6–16 and ISG12. BMC Genom. 2004, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martensen, P.M.; Søgaard, T.M.M.; Gjermandsen, I.M.; Buttenschøn, H.N.; Rossing, A.B.; Bonnevie-Nielsen, V.; Rosada, C.; Simonsen, J.L.; Justesen, J. The interferon α induced protein ISG12 is localized to the nuclear membrane. Eur. J. Biochem. 2001, 268, 5947–5954. [Google Scholar] [CrossRef] [PubMed]
- Gjermandsen, I.M.; Justesen, J.; Martensen, P.M. The interferon-induced gene ISG12 is regulated by various cytokines as the gene 6–16 in human cell lines. Cytokine 2000, 12, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Makovitzki-Avraham, E.; Daniel-Carmi, V.; Alteber, Z.; Farago, M.; Tzehoval, E.; Eisenbach, L. The human ISG12a gene is a novel caspase dependent and p53 independent pro-apoptotic gene, that is overexpressed in breast cancer. Cell Biol. Int. Rep. 2013, 20, 37–46. [Google Scholar] [CrossRef]
- Yang, D.; Meng, X.; Xue, B.; Liu, N.; Wang, X.; Zhu, H. miR-942 mediates hepatitis C virus-induced apoptosis via regulation of ISG12a. PLoS ONE 2014, 9, e94501. [Google Scholar] [CrossRef] [PubMed]
- Sudini, K.R. A Role for Interferon Stimulated Gene 12a (ISG12a) in Vesicular Stomatitis Virus Antiviral Responses. Ph.D. Thesis, University of Toledo, Toledo, OH, USA, 2012. [Google Scholar]
- Liu, N.; Long, Y.; Liu, B.; Yang, D.; Li, C.; Chen, T.; Wang, X.; Liu, C.; Zhu, H. ISG12a mediates cell response to Newcastle disease viral infection. Virology 2014, 462–463, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Thyrell, L.; Erickson, S.; Zhivotovsky, B.; Pokrovskaja, K.; Sangfelt, O.; Castro, J.; Einhorn, S.; Grandér, D. Mechanisms of Interferon-α induced apoptosis in malignant cells. Oncogene 2002, 21, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- García-Nicolás, O.; Baumann, A.; Vielle, N.J.; Gómez-Laguna, J.; Quereda, J.J.; Pallarés, F.J.; Ramis, G.; Carrasco, L.; Summerfield, A. Virulence and genotype-associated infectivity of interferon-treated macrophages by porcine reproductive and respiratory syndrome viruses. Virus Res. 2014, 179, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Genini, S.; Delputte, P.L.; Malinverni, R.; Cecere, M.; Stella, A.; Nauwynck, H.J.; Giuffra, E. Genome-wide transcriptional response of primary alveolar macrophages following infection with porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2008, 89, 2550–2564. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Yang, D.; Wang, J.; Xu, Y.; Wang, X.; Qin, Y.; Tian, R.; Chen, S.; Xie, Q.; Liu, N. ISG12a restricts hepatitis C virus infection through ubiquitination-dependent degradation pathway. J. Virol. 2016, 90, 6832–6845. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, R.; Ozono, E.; Fujisawa, J.; Ikeda, M.A.; Okamura, N.; Huang, Y.; Ohtani, K. Activation of the cyclin D2 and CDK6 genes through NF-κB is critical for cell-cycle progression induced by HTLV-I Tax. Oncogene 2008, 27, 5635–5642. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Halder, S.; Upadhyay, S.K.; Lu, J.; Kumar, P.; Murakami, M.; Cai, Q.; Robertson, E.S. Epstein-barr virus nuclear antigen 3C facilitates G1-S transition by stabilizing and enhancing the function of cyclin D1. PLoS Pathog. 2011, 7, e1001275. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, D.; Giri, S.; Prasanth, S.G.; Yoo, D. Differential host cell gene expression and regulation of cell cycle progression by nonstructural protein 11 of porcine reproductive and respiratory syndrome virus. BioMed Res. Int. 2014, 2014, 430508. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Barbosa, J.A.; Labrie, J.; Beaudry, F.; Gagnon, C.A.; Jacques, M. Actinobacillus pleuropneumoniae induces SJPL cell cycle arrest in G2/M-phase and inhibits porcine reproductive and respiratory syndrome virus replication. Virol. J. 2015, 12, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Zhai, S.; Zhou, X.; Lin, P.; Jiang, T.; Hu, X.; Jiang, Y.; Wu, B.; Zhang, Q.; Xu, X. Correction: Molecular characterization of transcriptome-wide interactions between highly pathogenic porcine reproductive and respiratory syndrome virus and porcine alveolar macrophages in vivo. Int. J. Biol. Sci. 2011, 7, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Rosebeck, S.; Leaman, D.W. Mitochondrial localization and pro-apoptotic effects of the interferon-inducible protein ISG12a. Apoptosis 2008, 13, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Cheriyath, V.; Leaman, D.W.; Borden, E.C. Emerging roles of FAM14 family members (G1P3/ISG 6–16 and ISG12/IFI27) in innate immunity and cancer. J. Interferon Cytokine Res. 2011, 31, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhang, N.; Xu, L.; Zhou, L.; Ge, X.; Guo, X.; Yang, H. Induction of apoptosis by the nonstructural protein 4 and 10 of porcine reproductive and respiratory syndrome virus. PLoS ONE 2016, 11, e0156518. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiao, S.; Wang, Y.; Xu, S.; Jiang, Y.; Chen, H.; Fang, L. Immunogenicity of the highly pathogenic porcine reproductive and respiratory syndrome virus GP5 protein encoded by a synthetic ORF5 gene. Vaccine 2009, 27, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Li, Z.; Wang, P.; Fan, P.; Zhang, Y.; Zhang, Q.; Wang, Y.; Xu, X.; Liu, B. Differences of immune responses between Tongcheng (Chinese local breed) and Large White pigs after artificial infection with highly pathogenic porcine reproductive and respiratory syndrome virus. Virus Res. 2016, 215, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCt) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence (5′–3′) | T (°C) | Application |
|---|---|---|---|
| pISG12A-PF1 | ATCTCGAGTATGGCCATGGTAGCCACCC | 58 | Expression vector construction |
| pISG12A-PR1 | GTAAGCTTCAAGCCCACTATTTTGGTAC | ||
| pISG12A-PF2 | GCCACCCAAGCAGTCTTATCCTC | 60 | qRT-PCR |
| pISG12A-PR2 | TGTCCCCAGCAAGGATCCCA | ||
| mISG12A-PF3 | GTGGCCTCCGCTTTCACCT | 60 | qRT-PCR |
| mISG12A-PR3 | TTCCCGTCGCAGTGAAACCC | ||
| ORF7-PF | CCCATTTCCCTCTAGCGACT | 60 | qRT-PCR |
| ORF7-PR | GCGTCGGCAAACTAAACTCCA | ||
| mGAPDH-PF | TGGGGAAGGTGAAGGTCGG | 60 | qRT-PCR |
| mGAPDH-PR | TCCTGGAAGATGGTGATGGG | ||
| pGAPDH-PF | CGTCCCTGAGACACGATGGT | 60 | qRT-PCR |
| pGAPDH-PR | GCCTTGACTGTGCCGTGGAAT | ||
| ISG12A-siRNA | GCUUUCACCUCAUCAGCAGUG | Macaque ISG12A siRNA | |
| NC-siRNA | UUCUUCGAACGUGUCACGUTT | Negative control siRNA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, L.; Zhou, X.; Liang, W.; Liu, J.; Liu, B. Porcine Interferon Stimulated Gene 12a Restricts Porcine Reproductive and Respiratory Syndrome Virus Replication in MARC-145 Cells. Int. J. Mol. Sci. 2017, 18, 1613. https://doi.org/10.3390/ijms18081613
Ji L, Zhou X, Liang W, Liu J, Liu B. Porcine Interferon Stimulated Gene 12a Restricts Porcine Reproductive and Respiratory Syndrome Virus Replication in MARC-145 Cells. International Journal of Molecular Sciences. 2017; 18(8):1613. https://doi.org/10.3390/ijms18081613
Chicago/Turabian StyleJi, Likai, Xiang Zhou, Wan Liang, Jianjian Liu, and Bang Liu. 2017. "Porcine Interferon Stimulated Gene 12a Restricts Porcine Reproductive and Respiratory Syndrome Virus Replication in MARC-145 Cells" International Journal of Molecular Sciences 18, no. 8: 1613. https://doi.org/10.3390/ijms18081613




