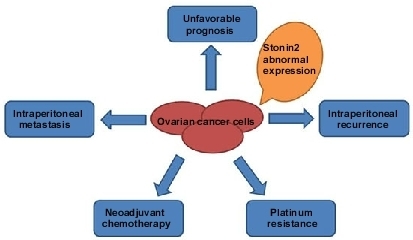
Stonin 2 Overexpression is Correlated with Unfavorable Prognosis and Tumor Invasion in Epithelial Ovarian Cancer
Abstract
:1. Introduction
2. Results
2.1. STON2 Expression is Higher in Ovarian Cancer Cell Lines than in Normal Ovarian Cell Lines
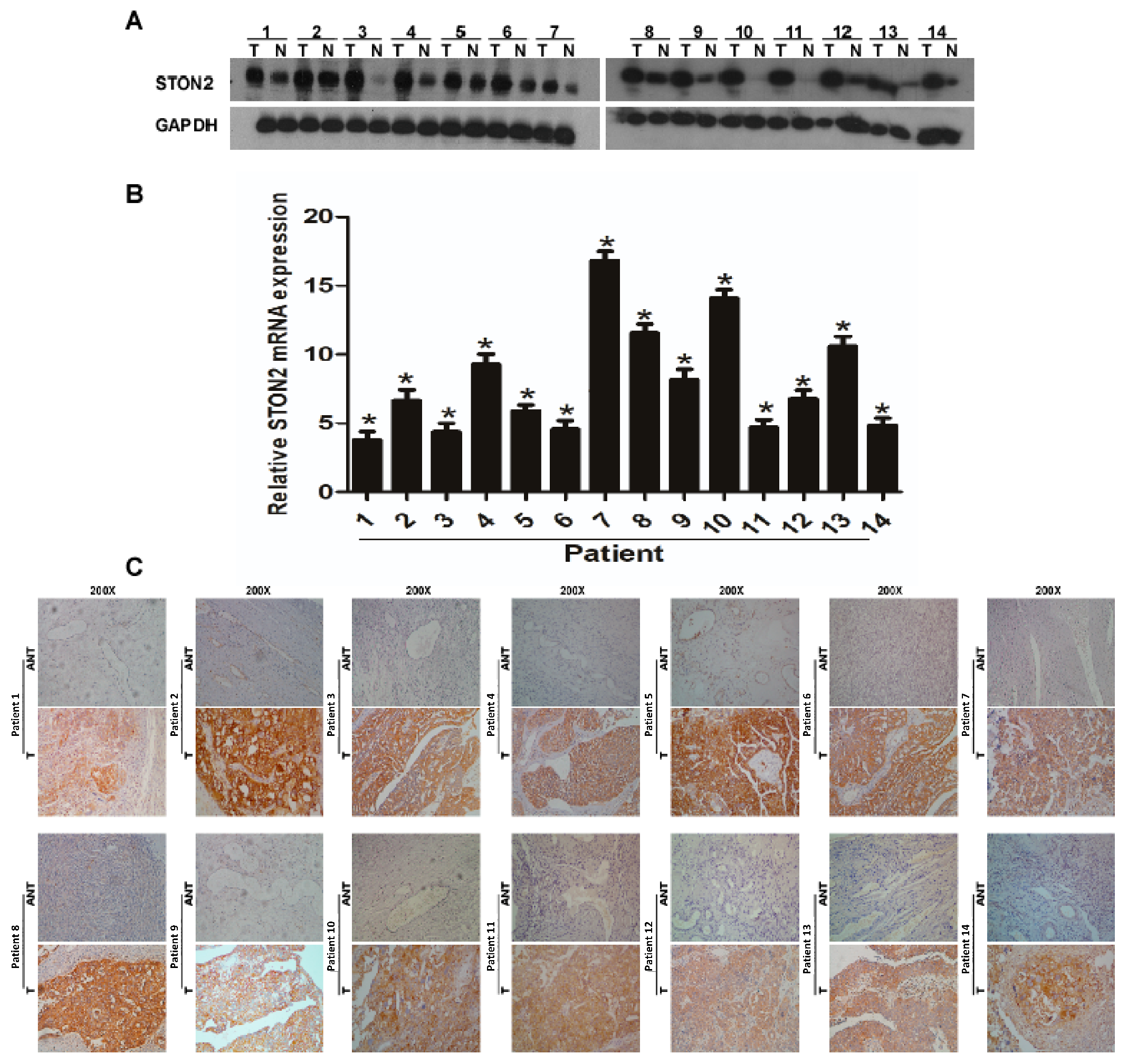
2.2. STON2 Expression Significantly Increased in Ovarian Cancer Tissues Compared with Normal Control Tissues
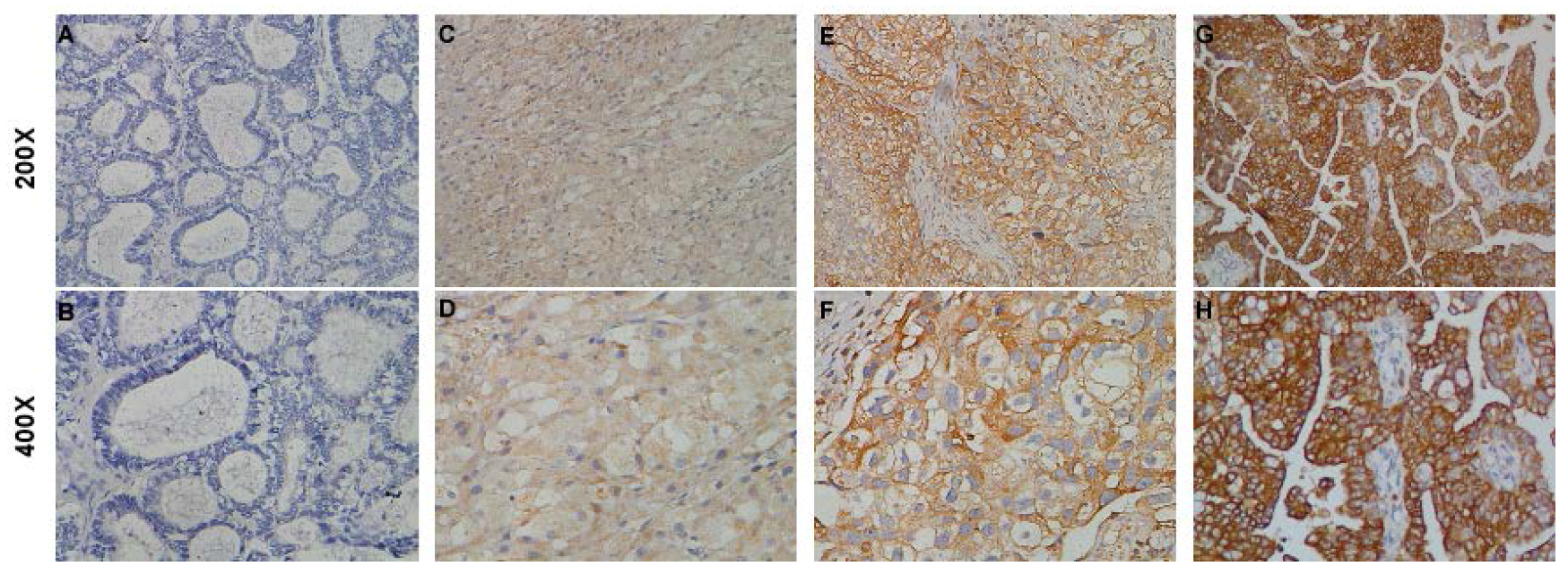
2.3. STON2 Overexpression Was Related with the Clinical Features of Ovarian Cancer
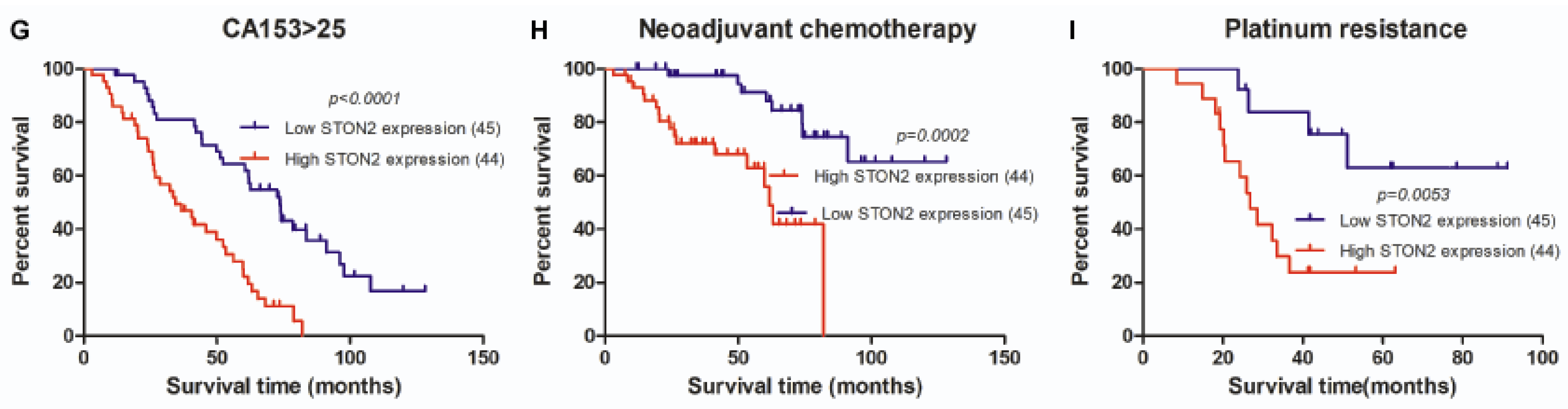
2.4. STON2 Overexpression Was Significantly Associated with a Poor Prognosis
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Tissue Specimens and Patient Information
4.3. RNA Extraction and Real-Time PCR (RT-PCR)
4.4. Western Blot Analysis
4.5. Immunohistochemistry
4.6 Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.E.; Zheng, W. Neoadjuvant chemotherapy for advanced ovarian cancer: The role of cytology in pretreatment diagnosis. Gynecol. Oncol. 2003, 90, 644–650. [Google Scholar] [CrossRef]
- McGuire, W.P., 3rd; Markman, M. Primary ovarian cancer chemotherapy: Current standards of care. Br. J. Cancer 2003, 89, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Weberpals, J.I.; Koti, M.; Squire, J.A. Targeting genetic and epigenetic alterations in the treatment of serous ovarian cancer. Cancer Genetics 2011, 204, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Howlander, N.; Noone, A.; Krapcho, M.; Garshell, J.; Miller, D.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975–2012; National Cancer Institute: Bethesda, MD, USA, 2015.
- Ueland, F.R. A Perspective on Ovarian Cancer Biomarkers: Past, Present and Yet-To-Come. Diagnostics 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.; Zhang, Y.; Lu, T.; Ruan, Y.; Zhang, H.; Yan, J.; Li, L.; Sun, W.; Wang, L.; Yue, W.; et al. Positive association of the human STON2 gene with schizophrenia. Neuroreport 2011, 22, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Diril, M.K.; Wienisch, M.; Jung, N.; Klingauf, J.; Haucke, V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev. Cell 2006, 10, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Kaempf, N.; Kochlamazashvili, G.; Puchkov, D.; Maritzen, T.; Bajjalieh, S.M.; Kononenko, N.L.; Haucke, V. Overlapping functions of stonin 2 and SV2 in sorting of the calcium sensor synaptotagmin 1 to synaptic vesicles. Proc. Natl. Acad. Sci. USA 2015, 112, 7297–7302. [Google Scholar] [CrossRef] [PubMed]
- Traub, L.M. Tickets to ride: Selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 2009, 10, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.; Smith, M.; Merakovsky, J.; Coulson, M.; Hannan, F.; Kelly, L.E. The stoned locus of Drosophila melanogaster produces a dicistronic transcript and encodes two distinct polypeptides. Genetics 1996, 143, 1699–1711. [Google Scholar] [PubMed]
- Xiang, B.; Wu, J.Y.; Wang, Q.; Li, M.L.; Jiang, L.J.; Deng, W.; Chen, Z.F.; He, Z.L.; Huang, C.H.; Han, Y.; et al. Cortical surface area correlates with STON2 gene Ser307Pro polymorphism in first-episode treatment-naive patients with schizophrenia. PLoS ONE 2013, 8, e64090. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.F.; Xu, J.C.; Tereshchenko, Y.; Novak, D.; Schachner, M.; Kleene, R. Neural cell adhesion molecule modulates dopaminergic signaling and behavior by regulating dopamine D2 receptor internalization. J. Neurosci. 2009, 29, 14752–14763. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yang, F.; Papaleo, F.; Wang, H.X.; Gao, W.J.; Weinberger, D.R.; Lu, B. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc. Natl. Acad. Sci. USA 2009, 106, 19593–19598. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Smith, M.; Lu, C.; Shahzad, M.M.; Pena, G.N.; Allen, J.K.; Stone, R.L.; Mangala, L.S.; Han, H.D.; Kim, H.S.; Farley, D.; et al. Dopamine blocks stress-mediated ovarian carcinoma growth. Clin. Cancer Res. 2011, 17, 3649–3659. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.E.; Enquist, J.; Hopf, F.W.; Lee, J.H.; Gladher, F.; Kharazia, V.; Waldhoer, M.; Mailliard, W.S.; Armstrong, R.; Bonci, A.; et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 11521–11526. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yin, M.; Huang, B.; Wang, Y.; Li, X.; Lou, G. CLIC1 a novel biomarker of intraperitoneal metastasis in serous epithelial ovarian cancer. Tumour Biol. 2015, 36, 4175–4179. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, S.; Webb, P.M.; Blomfield, P.; Brand, A.H.; Friedlander, M.; Leung, Y.; Obermair, A.; Oehler, M.K.; Quinn, M.; Steer, C.; et al. Survival of Australian women with invasive epithelial ovarian cancer: A population-based study. Med. J. Aust. 2014, 201, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Nagao, S.; Kogiku, A.; Yamamoto, K.; Miwa, M.; Wakahashi, S.; Ichida, K.; Sudo, T.; Yamaguchi, S.; Fujiwara, K. A preoperative low cancer antigen 125 level (≤25.8 mg/dl) is a useful criterion to determine the optimal timing of interval debulking surgery following neoadjuvant chemotherapy in epithelial ovarian cancer. Jpn. J. Clin. Oncol. 2016, 46, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, L.E.; Rodriguez, G.; Hurteau, J.A. The role of neoadjuvant chemotherapy in treating advanced epithelial ovarian cancer. J. Surg. Oncol. 2010, 101, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Gore, M. Treatment of recurrent ovarian cancer relapsing 6–12 months post platinum-based chemotherapy. Crit. Rev. Oncol. Hematol. 2007, 64, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Chakroborty, D.; Sarkar, C.; Mitra, R.B.; Banerjee, S.; Dasgupta, P.S.; Basu, S. Depleted dopamine in gastric cancer tissues: Dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin. Cancer Res. 2004, 10, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Chakroborty, D.; Chowdhury, U.R.; Dasgupta, P.S.; Basu, S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin. Cancer Res. 2008, 14, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Nagy, J.A.; Pal, S.; Vasile, E.; Eckelhoefer, I.A.; Bliss, V.S.; Manseau, E.J.; Dasgupta, P.S.; Dvorak, H.F.; Mukhopadhyay, D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 2001, 7, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Smith, M.; Lee, S.J.; Lu, C.; Nagaraja, A.S.; He, G.; Rupaimoole, R.; Han, H.D.; Jennings, N.B.; Roh, J.W.; Nishimura, M.; et al. Biologic effects of dopamine on tumor vasculature in ovarian carcinoma. Neoplasia 2013, 15, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Sarkar, C.; Chakroborty, D.; Nagy, J.; Mitra, R.B.; Dasgupta, P.S.; Mukhopadhyay, D. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 2004, 64, 5551–5555. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Number of Cases (%) |
|---|---|
| Age (years) | |
| ≤50 | 35 (39.3) |
| >50 | 54 (60.7) |
| FIGO stage | |
| I | 17 (19.3) |
| II | 10 (11.4) |
| III | 53 (60.2) |
| IV | 8 (9.1) |
| Histological type | |
| Serous adenocarcinoma | 69 (77.5) |
| Mucoid adenocarcinoma | 15 (16.9) |
| Endometrial adenocarcinoma | 3 (3.4) |
| Clear cell carcinoma | 2 (2.2) |
| Lymph node metastasis | |
| Absent | 29 (65.9) |
| Present | 15 (34.1) |
| Intraperitoneal metastasis | |
| No | 25 (28.1) |
| Yes | 64 (71.9) |
| Intestinal metastasis | |
| No Yes | 56 (62.9) 33 (37.1) |
| Expression of STON2 | |
| Low or none High | 45 (50.6) 44 (49.4) |
| Vital status (at last follow-up) | |
| Alive | 42 (47.2) |
| Dead | 47 (52.8) |
| Intraperitoneal recurrence | |
| No | 48 (69.6) |
| Yes | 21 (30.4) |
| Distant recurrence | |
| No | 57 (82.6) |
| Yes | 12 (17.4) |
| Residual tumor size (cm) | |
| ≤1 | 75 (84.3) |
| >1 | 14 (15.7) |
| Differentiation grade | |
| G1/G2 | 13 (27.1) |
| G3 | 35 (72.9) |
| Neoadjuvant chemotherapy | |
| No | 64 (71.9) |
| Yes | 25 (28.1) |
| Postoperative chemotherapy | |
| No | 7 (7.9) |
| Yes | 82 (92.1) |
| HIPEC | |
| No | 62 (71.3) |
| Yes | 25 (28.7) |
| Ascites see tumor cells (+) | |
| No | 21 (39.6) |
| Yes | 32 (60.4) |
| Cytoreductive surgery | |
| No | 12 (13.5) |
| Yes | 77 (86.5) |
| Platinum resistance * | |
| No | 14 (45.2) |
| Yes | 17 (54.8) |
| CA125 (U/mL) | |
| ≤35 | 5 (5.7) |
| >35 | 82 (94.3) |
| CA199 (U/mL) | |
| ≤35 | 59 (67.0) |
| >35 | 29 (33.0) |
| CA153 (U/mL) ≤25 >25 NSE (U/mL) ≤15.2 >15.2 CEA (U/mL) ≤5.0 >5.0 β-HCG (U/mL) ≤3.0 >3.0 | 20 (23.3) 66 (76.7) 25 (37.3) 42 (62.7) 63 (81.8) 14 (18.2) 39 (78.0) 11 (22.0) |
| ≤25 | 20 (23.3) |
| >25 | 66 (76.7) |
| NSE (U/mL) ≤15.2 >15.2 CEA (U/mL) ≤5.0 >5.0 β-HCG (U/mL) ≤3.0 >3.0 | 25 (37.3) 42 (62.7) 63 (81.8) 14 (18.2) 39 (78.0) 11 (22.0) |
| Characteristic | Total | STON2 | Chi-squared Test p Value | Fisher’s Exact Test p Value | ||
|---|---|---|---|---|---|---|
| No or Weak Expression | Moderate or Strong Expression | |||||
| Age (years) | ≤50 | 35 | 19 (21.3) | 16 (18.0) | 0.572 | 0.666 |
| >50 | 54 | 26 (29.2) | 28 (31.5) | |||
| Histological type | Serous adenocarcinoma | 69 | 35 (39.3) | 34 (38.2) | 0.940 | - |
| Mucoid adenocarcinoma | 15 | 8 (9.0) | 7 (7.9) | |||
| Endometrial adenocarcinoma | 3 | 1 (1.1) | 2 (2.2) | |||
| Clear cell carcinoma | 2 | 1 (1.1) | 1 (1.1) | |||
| FIGO stage | I | 17 | 12 (13.6) | 5 (5.7) | 0.199 | - |
| II | 10 | 3 (3.4) | 7 (8.0) | |||
| III | 53 | 25 (28.4) | 28 (31.8) | |||
| IV | 8 | 4 (4.5) | 4 (4.5) | |||
| Lymph node metastasis | Absent | 29 | 19 (43.2) | 10 (22.7) | 0.431 | 0.521 |
| Present | 15 | 8 (18.2) | 7 (15.9) | |||
| Intraperitoneal metastasis | No | 25 | 18 (20.2) | 7 (7.9) | 0.011 | 0.018 |
| Yes | 64 | 27 (30.3) | 37 (41.6) | |||
| Intestinal metastasis | No | 56 | 35 (39.3) | 21 (23.6) | 0.003 | 0.004 |
| Yes | 33 | 10 (11.2) | 23 (25.8) | |||
| Vital status (at last follow-up) | Alive Dead | 42 47 | 27 (30.3) 18 (20.2) | 15 (16.9) 29 (32.6) | 0.014 | 0.020 |
| Intraperitoneal recurrence | No Yes | 48 21 | 31 (44.9) 17 (24.6) | 6 (8.7) 15 (21.7) | 0.006 | 0.008 |
| Distant recurrence | No Yes | 57 12 | 30 (43.5) 27 (39.1) | 7 (10.1) 5 (7.2) | 0.719 | 0.761 |
| Residual tumor size (cm) | ≤1 | 75 | 40 (44.9) | 35 (39.3) | 0.226 | 0.258 |
| Differentiation grade | >1 G1/G2 G3 | 14 13 35 | 5 (5.6) 9 (18.8) 15 (31.2) | 9 (10.1) 4 (8.3) 20 (41.7) | 0.104 | 0.193 |
| Neoadjuvant chemotherapy Postoperative chemotherapy | No Yes No Yes | 64 25 7 82 | 37 (41.6) 8 (9.0) 3 (3.4) 42 (47.2) | 27 (30.3) 17 (19.1) 4 (4.5) 40 (44.9) | 0.029 0.671 | 0.035 0.714 |
| Platinum resistance | No Yes | 14 17 | 9 (29.0) 4 (12.9) | 5 (16.1) 13 (41.9) | - | 0.033 |
| HIPEC | No | 62 | 29 (33.3) | 33 (37.9) | 0.436 | 0.484 |
| Yes | 25 | 14 (16.1) | 11 (12.6) | |||
| Ascites with tumor cells (+) | No | 21 | 15 (28.3) | 6 (11.3) | 0.016 | 0.024 |
| Yes | 32 | 12 (22.6) | 20 (37.7) | |||
| Cytoreductive surgery | No | 12 | 7 (7.9) | 5 (5.6) | 0.563 | 0.484 0.758 |
| Yes | 77 | 38 (42.7) | 39 (43.8) | |||
| CA125 (U/mL) | ≤35 >35 | 5 82 | 4 (4.6) 39 (44.8) | 1 (1.1) 43 (49.4) | 0.159 | 0.202 |
| CA199 (U/mL) | ≤35 | 59 | 26 (29.5) | 33 (37.5) | 0.112 | 0.173 |
| >35 | 29 | 18 (20.5) | 11 (12.5) | |||
| CA153 (U/mL) | ≤25 >25 | 20 66 | 14 (16.3) 29 (33.7) | 6 (7.0) 37 (43.0) | 0.041 | 0.072 |
| NSE (U/mL) | ≤15.2 >15.2 | 25 42 | 13 (19.4) 18 (26.9) | 12 (17.9) 24 (35.8) | 0.468 | 0.613 |
| CEA (U/mL) | ≤5.0 >5.0 | 63 14 | 28 (36.4) 9 (11.7) | 35 (45.5) 5 (6.5) | 0.179 | 0.240 |
| β-HCG (U/mL) | ≤3.0 >3.0 | 39 11 | 19 (38.0) 6 (12.0) | 20 (40.0) 5 (10.0) | 0.733 | 1.000 |
| Variable | STON2 Expression | |
|---|---|---|
| Spearman’s Correlation Coefficient | p Value | |
| Age | 0.060 | 0.577 |
| Histological type | 0.011 | 0.915 |
| FIGO stage | 0.096 | 0.373 |
| Intraperitoneal metastasis | 0.268 | 0.011 |
| Lymph node metastasis | 0.119 | 0.443 |
| Intestinal metastasis | 0.311 | 0.003 |
| Vital status (at last follow-up) | 0.259 | 0.014 |
| Intraperitoneal Recurrence | 0.332 | 0.005 |
| Distant Recurrence | −0.043 | 0.724 |
| Residual tumor size (cm) | 0.128 | 0.231 |
| Differentiation grade | 0.234 | 0.109 |
| Neoadjuvant chemotherapy | 0.232 | 0.029 |
| Postoperative chemotherapy | −0.045 | 0.675 |
| HIPEC | −0.08 | 0.442 |
| Ascites with tumor cells (+) Cytoreductive surgery Platinum Resistance CA125 (U/mL) CA199 (U/mL) CA153 (U/mL) NSE (U/mL) CEA (U/mL) β-HCG (U/mL) | 0.332 0.061 0.411 0.151 −0.169 0.220 0.089 −0.153 −0.048 | 0.015 0.568 0.022 0.163 0.115 0.042 0.475 0.184 0.739 |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Number of Patients | p | Regression Coefficient (SE) | p | Relative Risk | 95% Confidence Interval | |
| STON2 | 0.001 | 2.834 (0.312) | 0.001 | 37.631 | 4.794–295.37 | |
| Low expression | 45 | |||||
| High expression | 44 | |||||
| Intraperitoneal metastasis | 0.000 | 9.351 (0.599) | 0.358 | 0.355 | 0.039–3.238 | |
| No | 25 | |||||
| Yes | 64 | |||||
| Intestinal metastasis | 0.000 | 3.048 (0.300) | 0.603 | 1.556 | 0.294–8.246 | |
| No Yes | 56 33 | |||||
| Intraperitoneal recurrence No Yes | 48 21 | 0.000 | 4.494 (0.354) | 0.007 | 13.871 | 2.067–93.098 |
| Neoadjuvant chemotherapy No Yes | 64 25 | 0.016 | 2.060 (0.301) | 0.234 | 2.744 | 0.520–14.473 |
| Ascites with tumor cells (+) No Yes Platinum Resistance No Yes | 21 77 14 17 | 0.002 0.000 | 4.711 (0.509) 5.694 (0.493) | 0.896 0.004 | 1.196 24.220 | 0.082–17.515 2.794–209.925 |
| CA153 (U/mL) ≤25 >25 | 20 66 | 0.004 | 4.628 (0.526) | 0.074 | 0.113 | 0.010–1.235 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhang, W.; Li, H.; Niu, C.; Ou, Y.; Song, L.; Zhang, Y. Stonin 2 Overexpression is Correlated with Unfavorable Prognosis and Tumor Invasion in Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 1653. https://doi.org/10.3390/ijms18081653
Sun X, Zhang W, Li H, Niu C, Ou Y, Song L, Zhang Y. Stonin 2 Overexpression is Correlated with Unfavorable Prognosis and Tumor Invasion in Epithelial Ovarian Cancer. International Journal of Molecular Sciences. 2017; 18(8):1653. https://doi.org/10.3390/ijms18081653
Chicago/Turabian StyleSun, Xiaoying, Weijing Zhang, Han Li, Chunhao Niu, Yulan Ou, Libing Song, and Yanna Zhang. 2017. "Stonin 2 Overexpression is Correlated with Unfavorable Prognosis and Tumor Invasion in Epithelial Ovarian Cancer" International Journal of Molecular Sciences 18, no. 8: 1653. https://doi.org/10.3390/ijms18081653