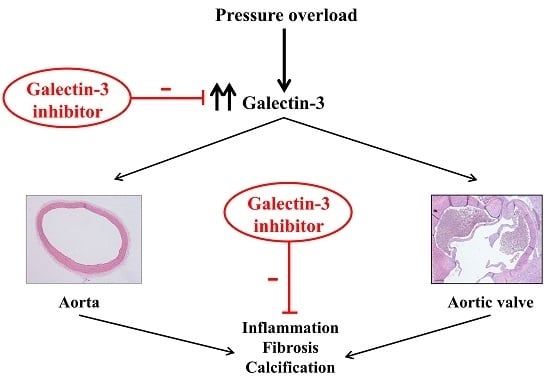
Beneficial Effects of Galectin-3 Blockade in Vascular and Aortic Valve Alterations in an Experimental Pressure Overload Model
Abstract
:1. Introduction
2. Results
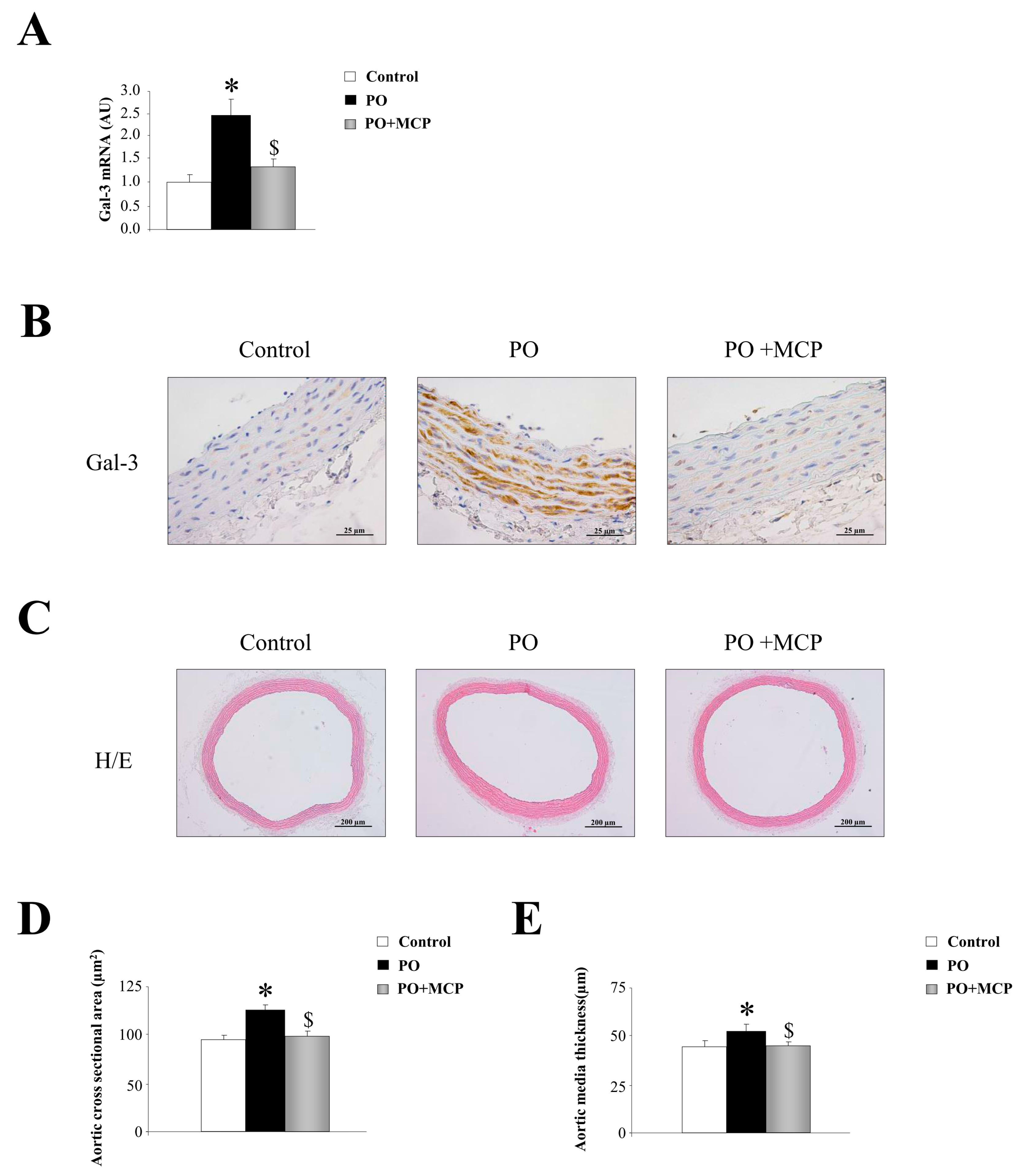
2.1. Aortic Structural Changes Are Improved by Galectin-3 (Gal-3) Blockade in Pressure Overload Rats
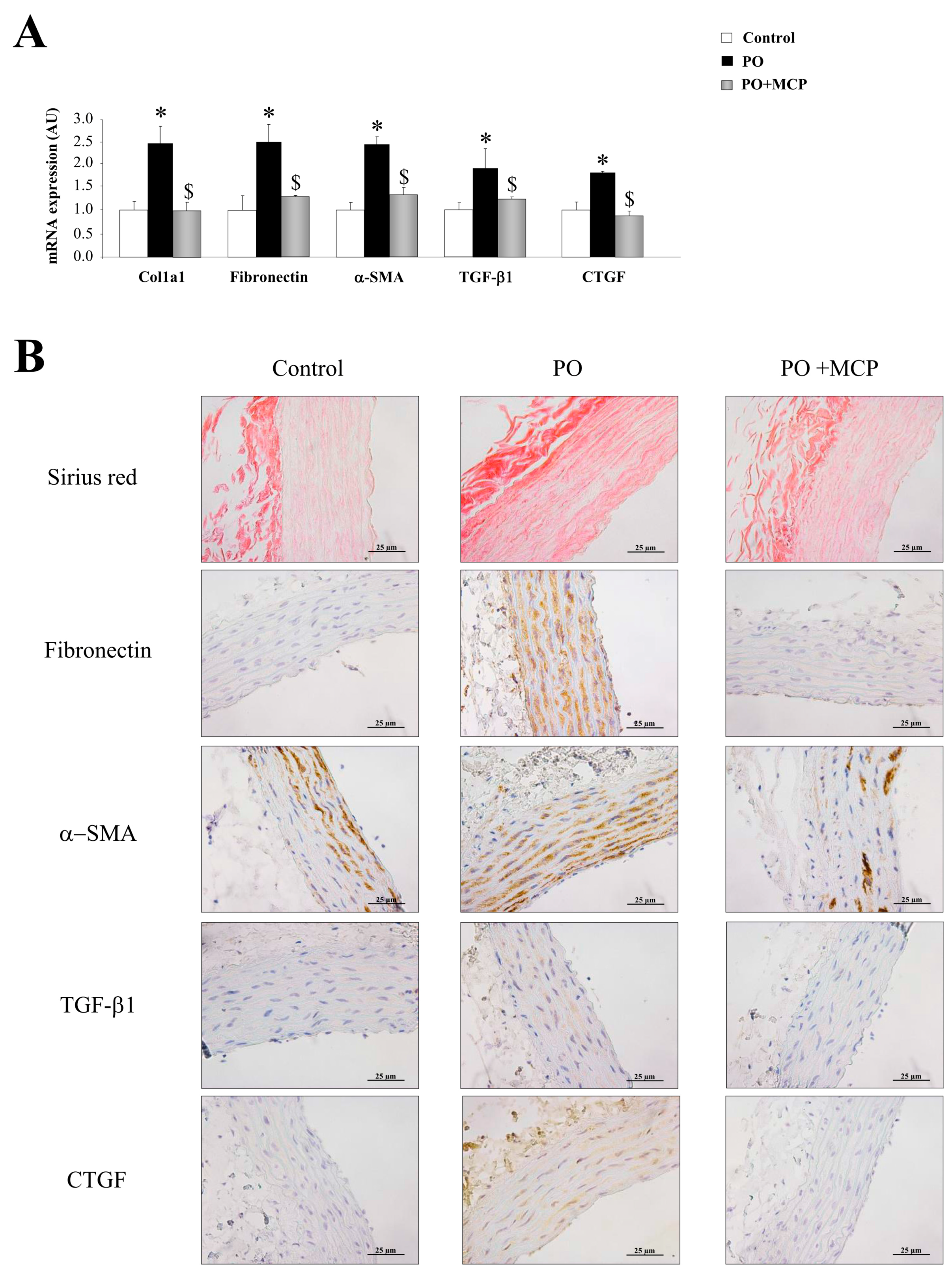
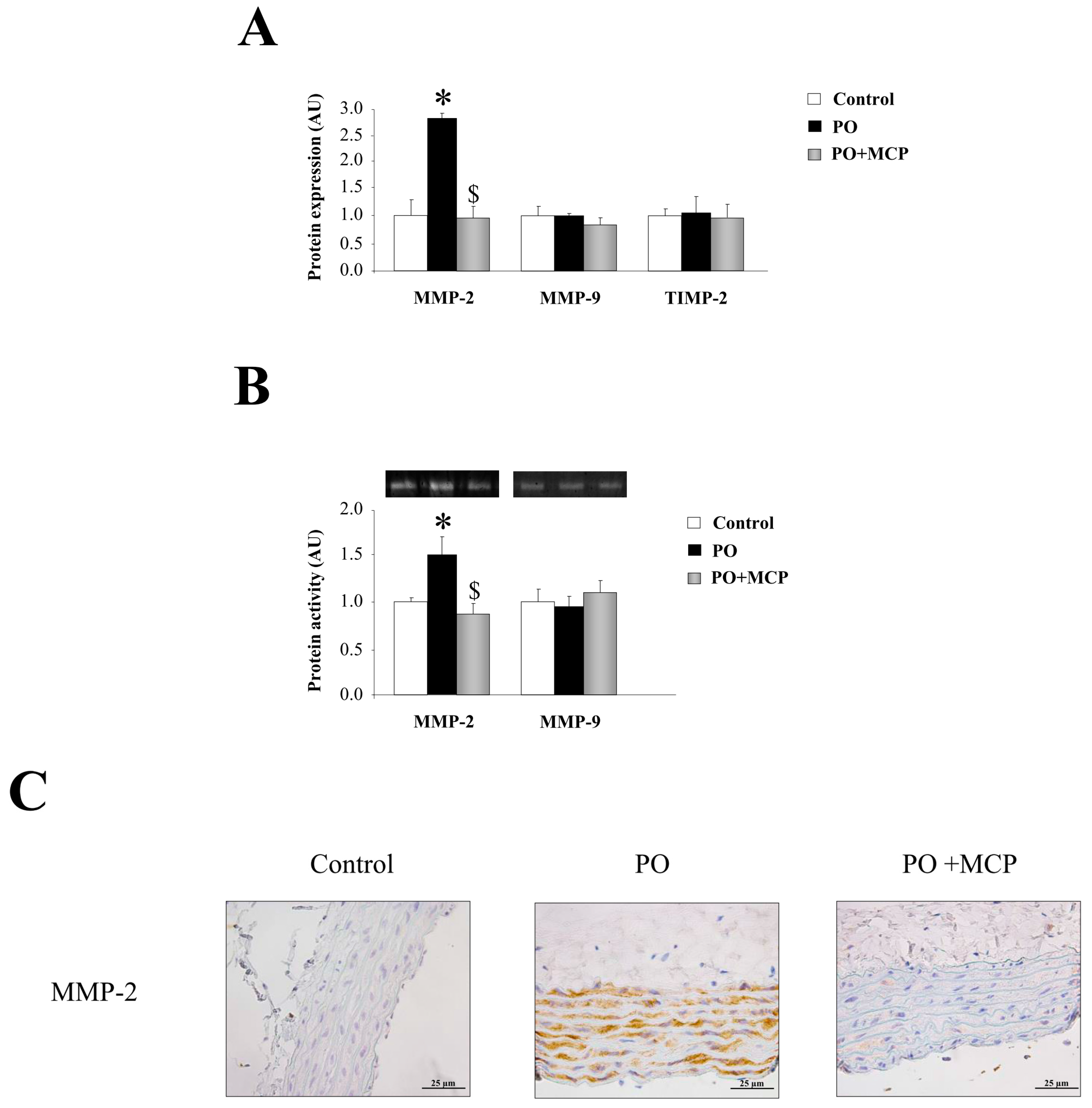
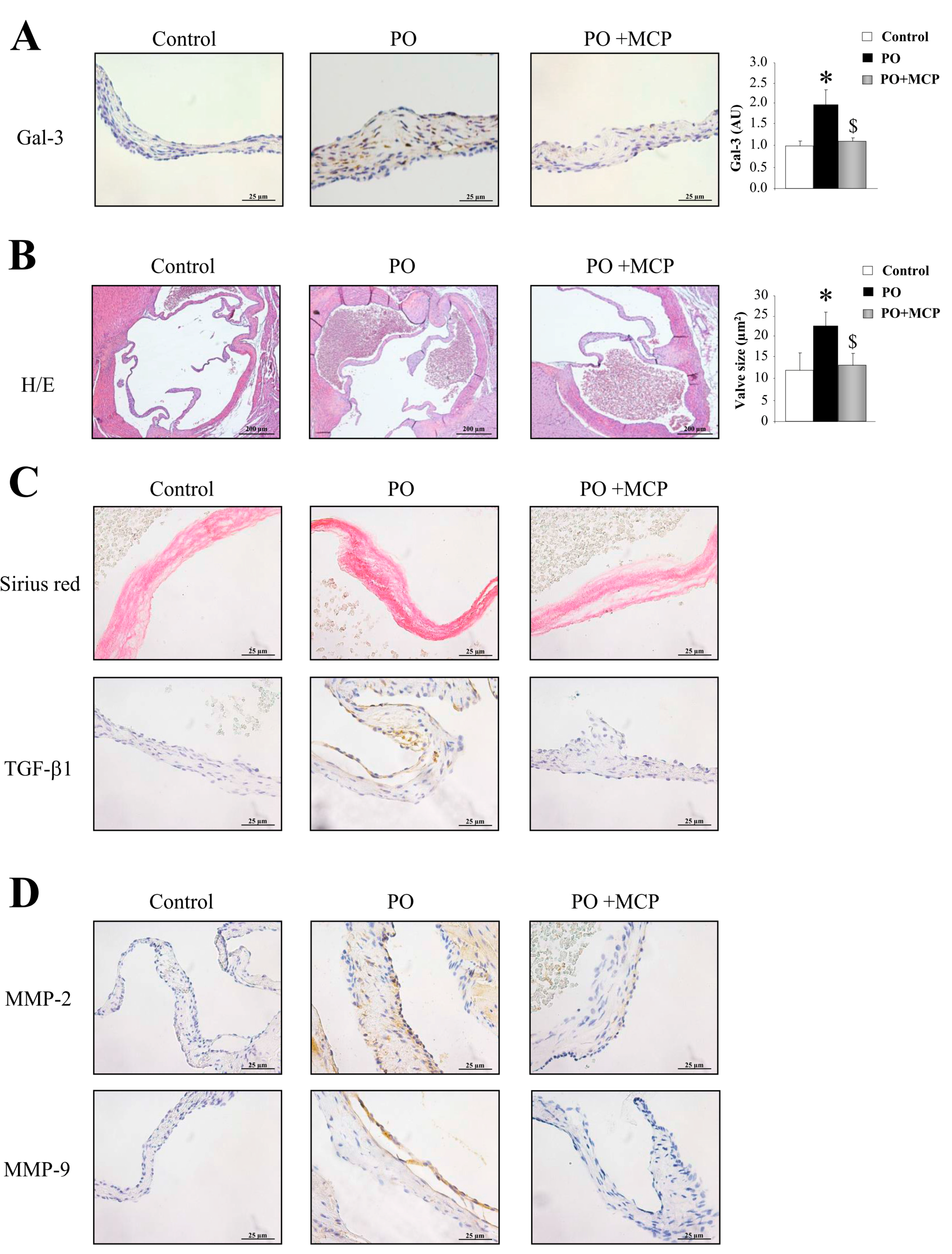
2.2. Gal-3 Pharmacological Blockade Diminishes Aortic Remodeling in Pressure Overload (PO) Rats
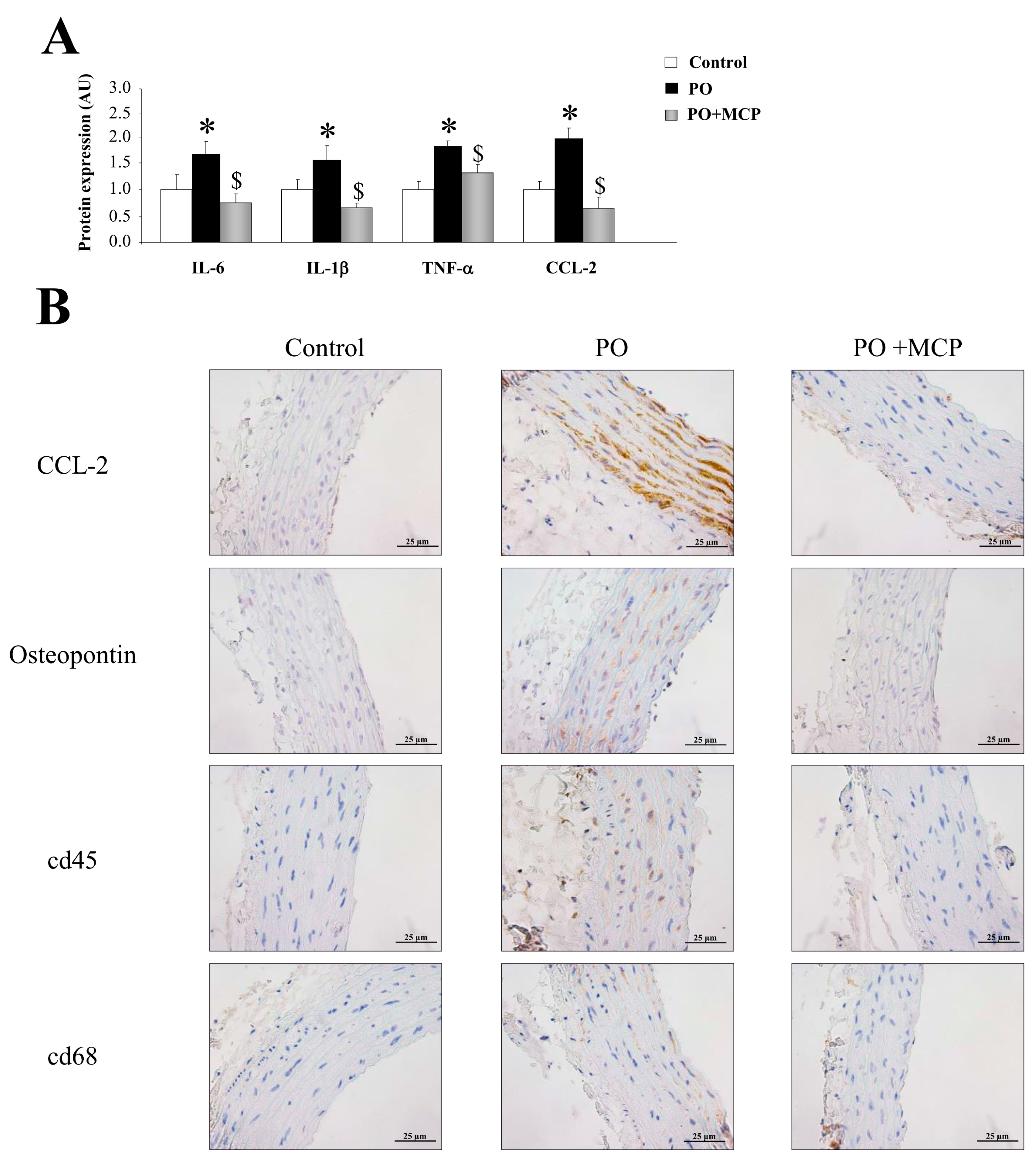
2.3. Inhibition of Gal-3 Attenuates Vascular Inflammation in PO Rats
2.4. Gal-3 Blockade Reduces Aortic Valve (AV) Thickening and Fibrosis in PO Rats
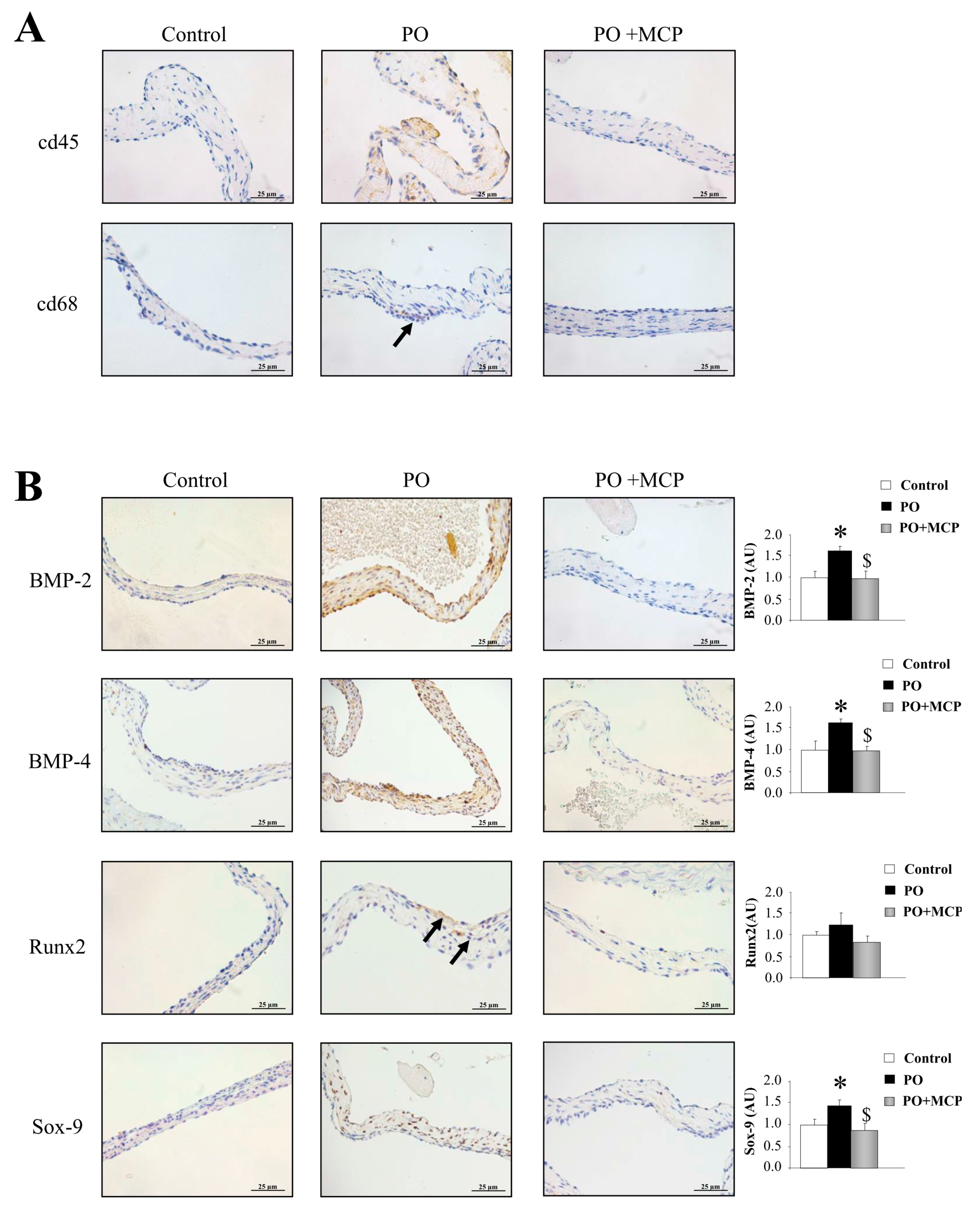
2.5. Gal-3 Inhibition Decreases AV Inflammation and Calcification in PO Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Histological Analysis
4.3. Real-Time Reverse Transcription Polymerase Chain Reaction (PCR)
4.4. ELISA
4.5. Gelatin Zymography
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heusch, G.; Libby, P.; Gersh, B.; Yellon, D.; Bohm, M.; Lopaschuk, G.; Opie, L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014, 383, 1933–1943. [Google Scholar] [CrossRef]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Barwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The euro heart survey on valvular heart disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Ajith Kumar, G.S.; Binil Raj, S.K.S.; Sanjay, G. Ascending aortic constriction in rats for creation of pressure overload cardiac hypertrophy model. J. Vis. Exp. 2014, 88, 50983. [Google Scholar]
- Gould, S.T.; Srigunapalan, S.; Simmons, C.A.; Anseth, K.S. Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ. Res. 2013, 113, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, J.; Burg, N.; Yoshinaga, K.; Janczak, C.A.; Rifkin, D.B.; Coller, B.S. In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-β1. Blood 2008, 112, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- Mourino-Alvarez, L.; Baldan-Martin, M.; Gonzalez-Calero, L.; Martinez-Laborde, C.; Sastre-Oliva, T.; Moreno-Luna, R.; Lopez-Almodovar, L.F.; Sanchez, P.L.; Fernandez-Aviles, F.; Vivanco, F.; et al. Patients with calcific aortic stenosis exhibit systemic molecular evidence of ischemia, enhanced coagulation, oxidative stress and impaired cholesterol transport. Int. J. Cardiol. 2016, 225, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kaden, J.J.; Dempfle, C.E.; Grobholz, R.; Tran, H.T.; Kilic, R.; Sarikoc, A.; Brueckmann, M.; Vahl, C.; Hagl, S.; Haase, K.K.; et al. Interleukin-1β promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis 2003, 170, 205–211. [Google Scholar] [CrossRef]
- Malek, A.M.; Gibbons, G.H.; Dzau, V.J.; Izumo, S. Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J. Clin. Investig. 1993, 92, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Furtak, V.; Lukyanov, P. Extracellular functions of galectin-3. Glycoconj. J. 2004, 19, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; Andre, S.; Crijns, H.J.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Sadaba, J.R.; Martinez-Martinez, E.; Arrieta, V.; Alvarez, V.; Fernandez-Celis, A.; Ibarrola, J.; Melero, A.; Rossignol, P.; Cachofeiro, V.; Lopez-Andres, N. Role for galectin-3 in calcific aortic valve stenosis. J. Am. Heart Assoc. 2016, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, V.; Martinez-Martinez, E.; Ibarrola, J.; Alvarez, V.; Sadaba, R.J.; Garcia-Pena, A.; Fernandez-Celis, A.; Cachofeiro, V.; Rossignol, P.; Lopez-Andres, N. A role for galectin-3 in the development of early molecular alterations in short-term aortic stenosis. Clin. Sci. 2017, 131, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Baldenhofer, G.; Zhang, K.; Spethmann, S.; Laule, M.; Eilers, B.; Leonhardt, F.; Sanad, W.; Dreger, H.; Sander, M.; Grubitzsch, H.; et al. Galectin-3 predicts short- and long-term outcome in patients undergoing transcatheter aortic valve implantation (TAVI). Int. J. Cardiol. 2014, 177, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Ricci, C.; Blasetti Fantauzzi, C.; Salvi, L.; Pesce, C.M.; Relucenti, M.; Familiari, G.; Taurino, M.; Pugliese, G. The galectin-3/RAGE dyad modulates vascular osteogenesis in atherosclerosis. Cardiovasc. Res. 2013, 100, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; de Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Lopez-Andres, N.; Jurado-Lopez, R.; Rousseau, E.; Bartolome, M.V.; Fernandez-Celis, A.; Rossignol, P.; Islas, F.; Antequera, A.; Prieto, S.; et al. Galectin-3 participates in cardiovascular remodeling associated with obesity. Hypertension 2015, 66, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, N.K.; Foley, J.B.; O’Briain, D.S.; Crean, P.; Kelleher, D.; Walsh, M. Adhesion molecules in nonrheumatic aortic valve disease: Endothelial expression, serum levels and effects of valve replacement. J. Am. Coll. Cardiol. 2000, 36, 2257–2262. [Google Scholar] [CrossRef]
- Hampton, C.; Rosa, R.; Campbell, B.; Kennan, R.; Gichuru, L.; Ping, X.; Shen, X.; Small, K.; Madwed, J.; Lynch, J.J. Early echocardiographic predictors of outcomes in the mouse transverse aortic constriction heart failure model. J. Pharmacol. Toxicol. Methods 2017, 84, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.Q.; Geng, L.; Prakash, S.K.; Cao, J.M.; Guo, S.; Villamizar, C.; Kwartler, C.S.; Peters, A.M.; Brasier, A.R.; Milewicz, D.M. Aortic remodeling after transverse aortic constriction in mice is attenuated with AT1 receptor blockade. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Souders, C.A.; Borg, T.K.; Banerjee, I.; Baudino, T.A. Pressure overload induces early morphological changes in the heart. Am. J. Pathol. 2012, 181, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Engebretsen, K.V.; Waehre, A.; Bjornstad, J.L.; Skrbic, B.; Sjaastad, I.; Behmen, D.; Marstein, H.S.; Yndestad, A.; Aukrust, P.; Christensen, G.; et al. Decorin, lumican, and their gag chain-synthesizing enzymes are regulated in myocardial remodeling and reverse remodeling in the mouse. J. Appl. Physiol. 2013, 114, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, M.; Korkmaz-Icoz, S.; Li, S.; Nemeth, B.T.; Hegedus, P.; Brlecic, P.; Matyas, C.; Zorn, M.; Merkely, B.; Karck, M.; et al. Myocardial reverse remodeling after pressure unloading is associated with maintained cardiac mechanoenergetics in a rat model of left ventricular hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Libhaber, E.; Woodiwiss, A.J.; Raymond, A.; Gomes, M.; Maseko, M.J.; Sareli, P.; Norton, G.R. Independent associations of circulating galectin-3 concentrations with aortic pulse wave velocity and wave reflection in a community sample. Hypertension 2015, 65, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Lala, R.I.; Darabantiu, D.; Pilat, L.; Puschita, M. Galectin-3: A link between myocardial and arterial stiffening in patients with acute decompensated heart failure? Arq. Bras. Cardiol. 2016, 106, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Calvier, L.; Fernandez-Celis, A.; Rousseau, E.; Jurado-Lopez, R.; Rossoni, L.V.; Jaisser, F.; Zannad, F.; Rossignol, P.; Cachofeiro, V.; et al. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension 2015, 66, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sadaba, J.R.; Zannad, F.; Rossignol, P.; Lopez-Andres, N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska, B.; Wieczorek-Surdacka, E.; Kruszelnicka, O.; Chyrchel, B.; Surdacki, A.; Dudek, D. Clinical correlates and prognostic value of plasma galectin-3 levels in degenerative aortic stenosis: A single-center prospective study of patients referred for invasive treatment. Int. J. Mol. Sci. 2017, 18, 947. [Google Scholar] [CrossRef] [PubMed]
- Back, M.; Gasser, T.C.; Michel, J.B.; Caligiuri, G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc. Res. 2013, 99, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, D.; van Almen, G.C.; van Aelst, L.N.; van Cleemput, J.; Droogne, W.; Jin, Y.; van de Werf, F.; Carmeliet, P.; Vanhaecke, J.; Papageorgiou, A.P.; et al. Matricellular proteins and matrix metalloproteinases mark the inflammatory and fibrotic response in human cardiac allograft rejection. Eur. Heart J. 2013, 34, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, Z.Z.; Chen, L.J.; Yu, H.M.; Guo, S.J.; Xu, Y.L.; Oudit, G.Y.; Zhang, Y.; Chang, Q.; Song, B.; et al. Ascending aortic adventitial remodeling and fibrosis are ameliorated with apelin-13 in rats after TAC via suppression of the miRNA-122 and LGR4-β-catenin signaling. Peptides 2016, 86, 85–94. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarrola, J.; Martínez-Martínez, E.; Sádaba, J.R.; Arrieta, V.; García-Peña, A.; Álvarez, V.; Fernández-Celis, A.; Gainza, A.; Rossignol, P.; Cachofeiro Ramos, V.; et al. Beneficial Effects of Galectin-3 Blockade in Vascular and Aortic Valve Alterations in an Experimental Pressure Overload Model. Int. J. Mol. Sci. 2017, 18, 1664. https://doi.org/10.3390/ijms18081664
Ibarrola J, Martínez-Martínez E, Sádaba JR, Arrieta V, García-Peña A, Álvarez V, Fernández-Celis A, Gainza A, Rossignol P, Cachofeiro Ramos V, et al. Beneficial Effects of Galectin-3 Blockade in Vascular and Aortic Valve Alterations in an Experimental Pressure Overload Model. International Journal of Molecular Sciences. 2017; 18(8):1664. https://doi.org/10.3390/ijms18081664
Chicago/Turabian StyleIbarrola, Jaime, Ernesto Martínez-Martínez, J. Rafael Sádaba, Vanessa Arrieta, Amaia García-Peña, Virginia Álvarez, Amaya Fernández-Celis, Alicia Gainza, Patrick Rossignol, Victoria Cachofeiro Ramos, and et al. 2017. "Beneficial Effects of Galectin-3 Blockade in Vascular and Aortic Valve Alterations in an Experimental Pressure Overload Model" International Journal of Molecular Sciences 18, no. 8: 1664. https://doi.org/10.3390/ijms18081664