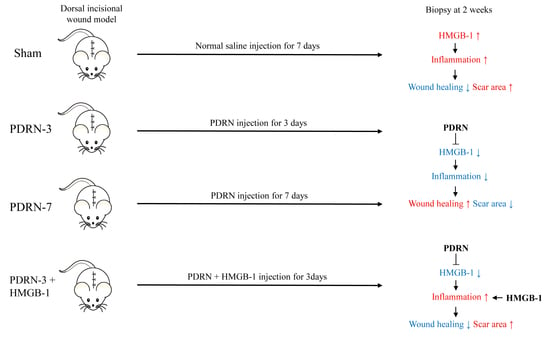
Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model
Abstract
:1. Introduction
2. Results
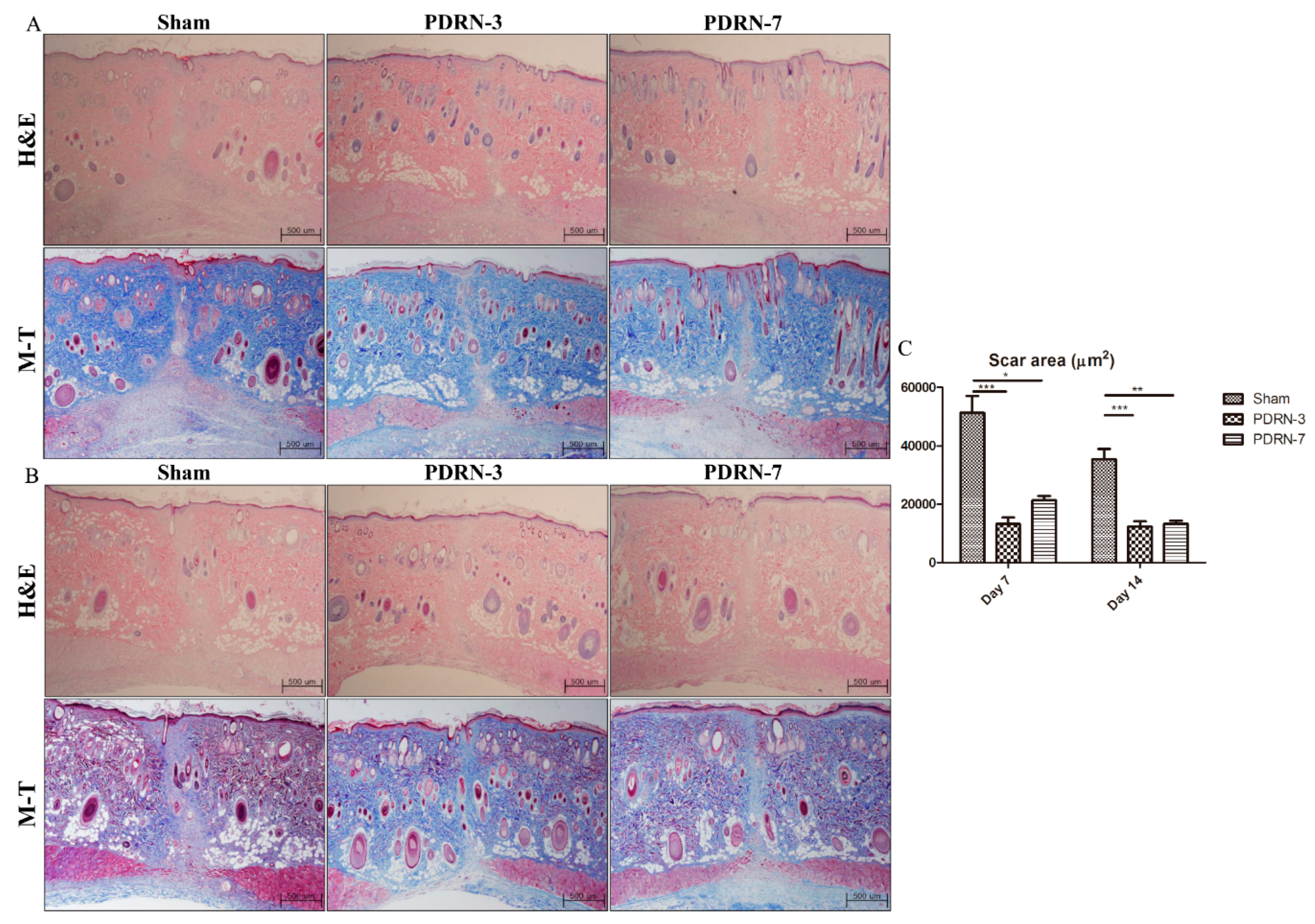
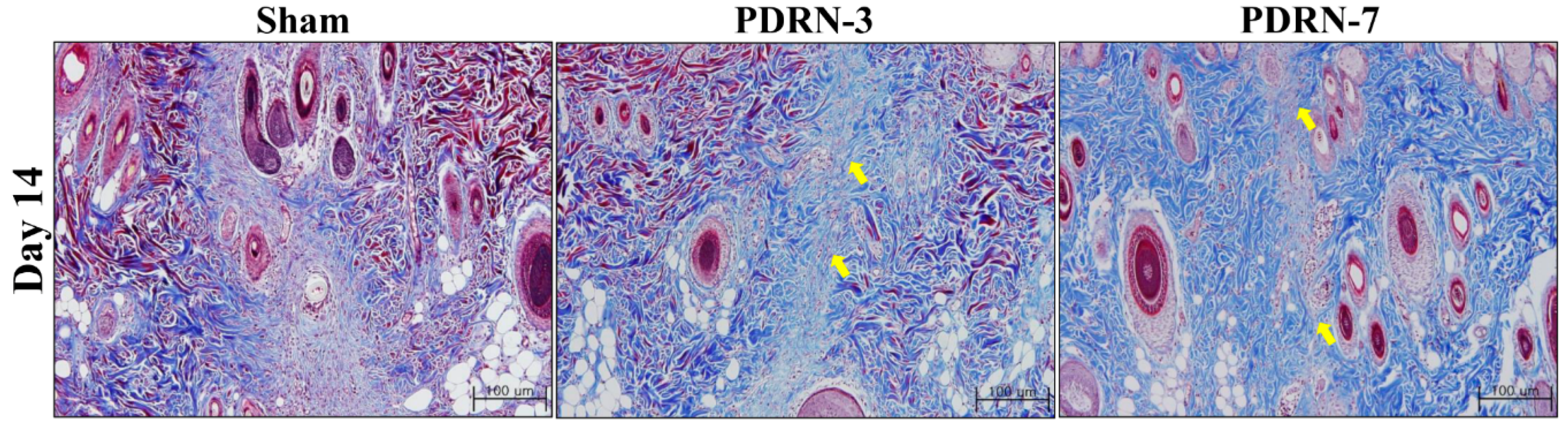
2.1. Polydeoxyribonucleotide Decreases Scar Size in Incisional Scar Tissue in Rats
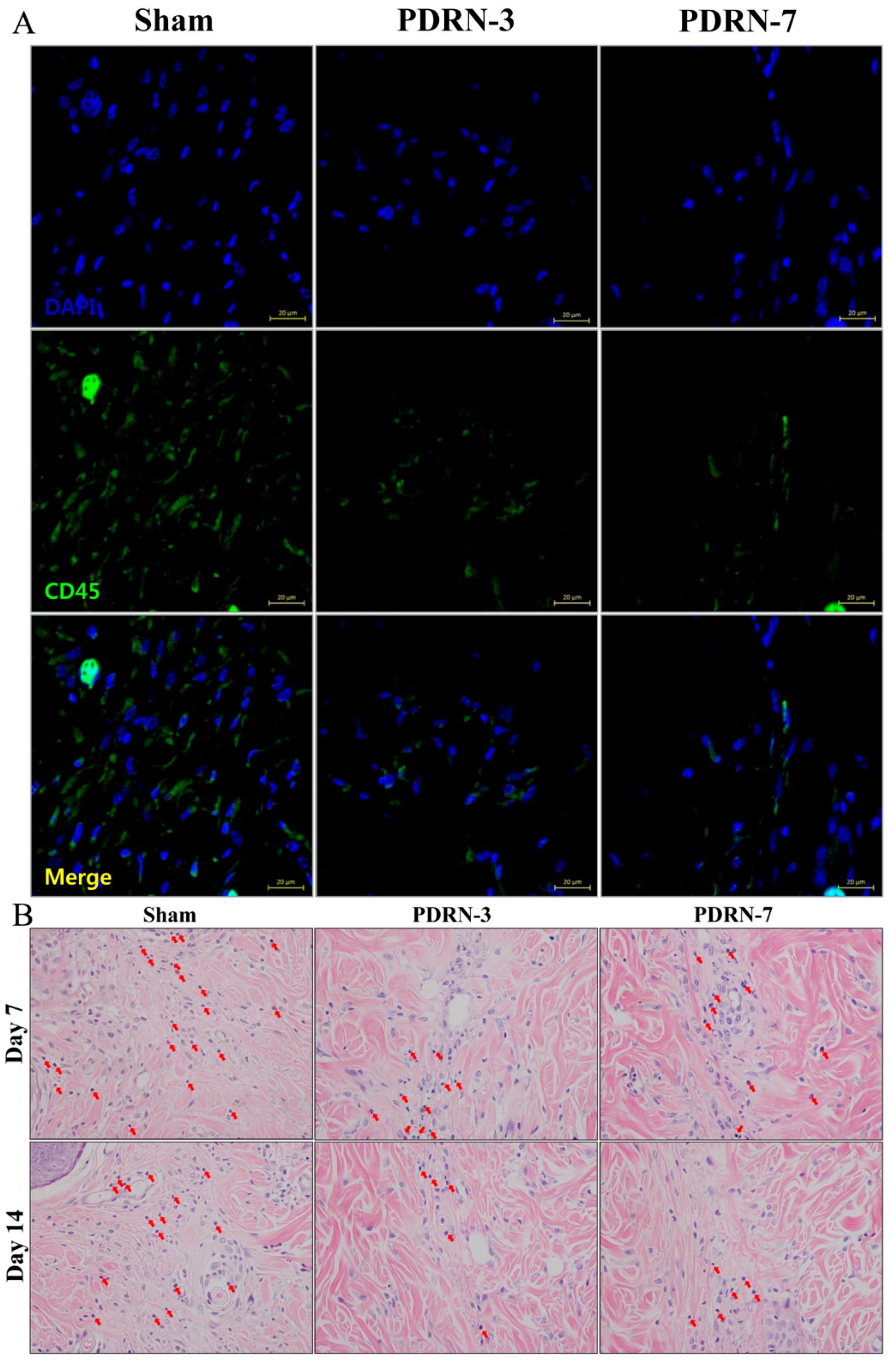
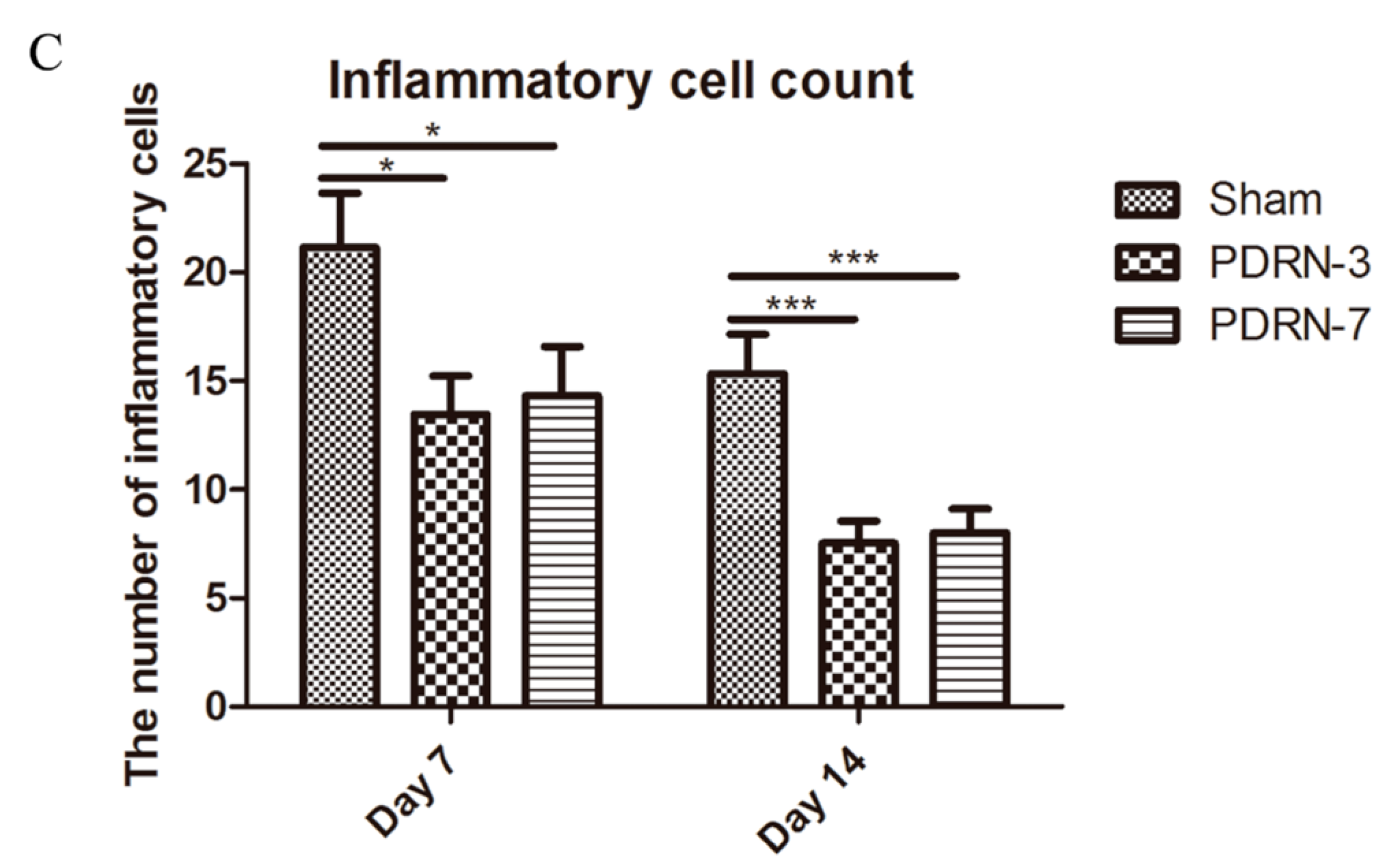
2.2. Polydeoxyribonucleotide Decreases Inflammatory Cell Infiltration in Incisional Scar Tissue in Rats
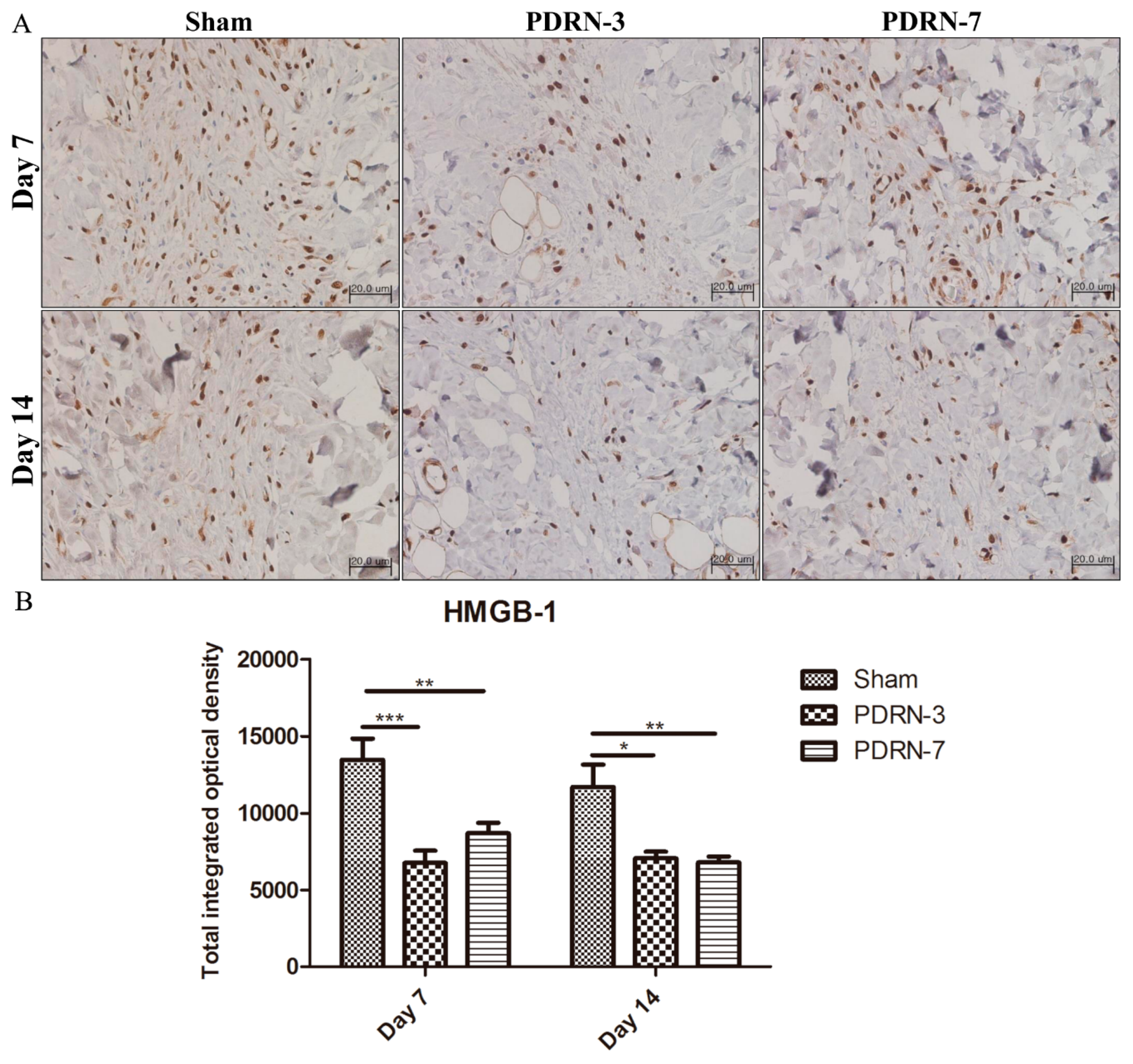
2.3. Polydeoxyribonucleotide Decreases HMGB-1 Expression in Incisional Scar Tissue in Rats
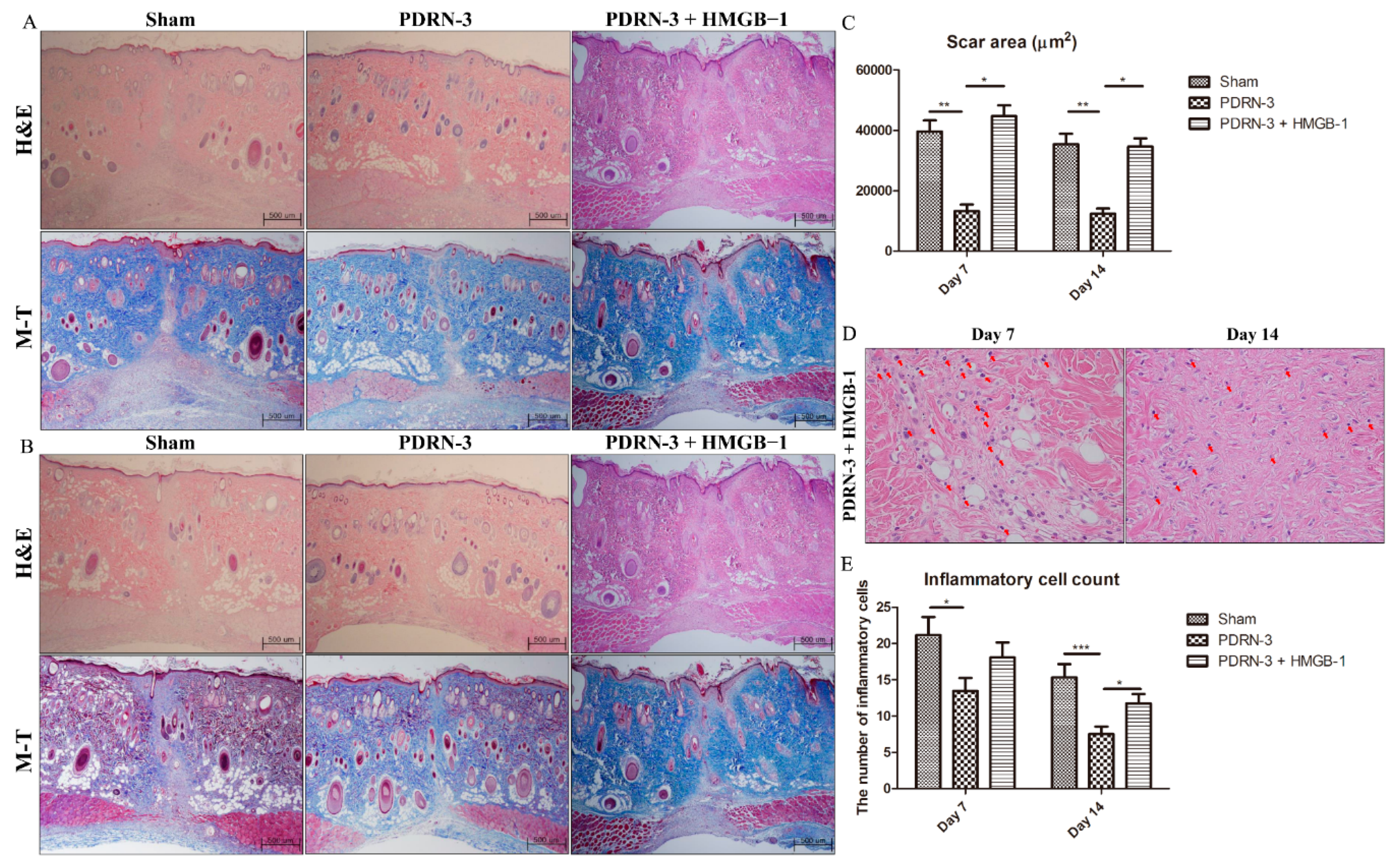
2.4. HMGB-1 Administration Reverses the Anti-Inflammatory and Collagen Synthesis Effects of PDRN
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Histologic Analysis
4.3. Immunohistochemistry for HMGB-1
4.4. Immunofluorescence Assay for CD45
4.5. Western Blots for Type I and Type III Collagen
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, J.; Jiao, H.; Honardoust, D.; Momtazi, M.; Shankowsky, H.A.; Tredget, E.E. Human hypertrophic scar-like nude mouse model: Characterization of the molecular and cellular biology of the scar process. Wound Repair Regen. 2011, 19, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Hori, K.; Ding, J.; Huang, Y.; Kwan, P.; Ladak, A.; Tredget, E.E. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J. Cell Physiol. 2011, 226, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef] [PubMed]
- Wulff, B.C.; Parent, A.E.; Meleski, M.A.; DiPietro, L.A.; Schrementi, M.E.; Wilgus, T.A. Mast cells contribute to scar formation during fetal wound healing. J. Investig. Dermatol. 2012, 132, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Dovi, J.V.; He, L.K.; DiPietro, L.A. Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 2003, 73, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.J.; Bayat, A. Genetic susceptibility to raised dermal scarring. Br. J. Dermatol. 2009, 161, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Bran, G.M.; Goessler, U.R.; Hormann, K.; Riedel, F.; Sadick, H. Keloids: Current concepts of pathogenesis (review). Int. J. Mol. Med. 2009, 24, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Shih, B.; Garside, E.; McGrouther, D.A.; Bayat, A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010, 18, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, A.; Mess, S.; Thomassen, J.M.; Kauffman, C.L.; Davison, S.P. Keloid pathogenesis and treatment. Plast. Reconstr. Surg. 2006, 117, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E.; Manfredi, A.A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007, 220, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, A.; Komura, K.; Iwata, Y.; Ogawa, F.; Hara, T.; Muroi, E.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; Fujimoto, M.; et al. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: Association with disease severity. J. Clin. Immunol. 2009, 29, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Algandaby, M.M.; Breikaa, R.M.; Eid, B.G.; Neamatallah, T.A.; Abdel-Naim, A.B.; Ashour, O.M. Icariin protects against thioacetamide-induced liver fibrosis in rats: Implication of anti-angiogenic and anti-autophagic properties. Pharmacol. Rep. 2017, 69, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Irrera, N.; D’Ascola, A.; Avenoso, A.; Nastasi, G.; Campo, G.; Micali, A.; Bagnato, G.; Minutoli, L.; et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A2A receptor. Arthritis Rheum. 2011, 63, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- Rork, T.H.; Wallace, K.L.; Kennedy, D.P.; Marshall, M.A.; Lankford, A.R.; Linden, J. Adenosine A2A receptor activation reduces infarct size in the isolated, perfused mouse heart by inhibiting resident cardiac mast cell degranulation. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1825–H1833. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Oteri, G.; Pisano, M.; Polito, F.; Irrera, N.; Minutoli, L.; Squadrito, F.; Altavilla, D. Adenosine receptor stimulation by polynucleotides (PDRN) reduces inflammation in experimental periodontitis. J. Clin. Periodontol. 2013, 40, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Altavilla, D.; Minutoli, L.; Migliorato, A.; Squadrito, F. Polydeoxyribonucleotide (PDRN) restores blood flow in an experimental model of peripheral artery occlusive disease. J. Vasc. Surg. 2008, 48, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Galeano, M.; Squadrito, F.; Minutoli, L.; Polito, F.; Dye, J.F.; Clayton, E.A.; Calò, M.; Venuti, F.S.; Vaccaro, M.; et al. Polydeoxyribonucleotide improves angiogenesis and wound healing in experimental thermal injury. Crit. Care Med. 2008, 36, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Sini, P.; Denti, A.; Cattarini, G.; Daglio, M.; Tira, M.E.; Balduini, C. Effect of polydeoxyribonucleotides on human fibroblasts in primary culture. Cell Biochem. Funct. 1999, 17, 107–114. [Google Scholar] [CrossRef]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calo, M.; Cascio, P.L.; d’Alcontres, F.S.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Fernandez, P.; Cronstein, B.N. Adenosine in inflammatory joint diseases. Purinergic Signal. 2007, 3, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Squadrito, F.; Polito, F.; Irrera, N.; Calo, M.; Lo Cascio, P.; Galeano, M.; Cava, L.L.; Minutoli, L.; Marini, H.; et al. Activation of adenosine A2A receptors restores the altered cell-cycle machinery during impaired wound healing in genetically diabetic mice. Surgery 2011, 149, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, G.G.; Maan, Z.N.; Wong, V.W.; Duscher, D.; Hu, M.S.; Zielins, E.R.; Wearda, T.; Muhonen, E.; McArdle, A.; Tevlin, R.; et al. Scarless wound healing: Chasing the holy grail. Plast. Reconstr. Surg. 2015, 135, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Szpaderska, A.M.; DiPietro, L.A. Inflammation in surgical wound healing: Friend or foe? Surgery 2005, 137, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Harada, T.; Morikawa, S.; Kato, Y. Expression of leukocyte common antigen (CD45) on various human leukemia/lymphoma cell lines. Acta. Pathol. Jpn. 1990, 40, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, T.; Beltrame, M.; Ferrari, S.; Bianchi, M.E. Hmg4, a new member of the Hmg1/2 gene family. Genomics 1998, 49, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Trowbridge, R.M.; Ayoub, N.T.; Agrawal, D.K. High-mobility group box protein-1, matrix metalloproteinases, and vitamin D in keloids and hypertrophic scars. Plast. Reconstr. Surg. Glob. Open 2015, 3, e425. [Google Scholar] [CrossRef] [PubMed]
- Dardenne, A.D.; Wulff, B.C.; Wilgus, T.A. The alarmin HMGB-1 influences healing outcomes in fetal skin wounds. Wound Repair Regen. 2013, 21, 282–291. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, W.; Yang, C.E.; Roh, T.S.; Kim, J.H.; Lee, J.H.; Lee, W.J. Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model. Int. J. Mol. Sci. 2017, 18, 1698. https://doi.org/10.3390/ijms18081698
Jeong W, Yang CE, Roh TS, Kim JH, Lee JH, Lee WJ. Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model. International Journal of Molecular Sciences. 2017; 18(8):1698. https://doi.org/10.3390/ijms18081698
Chicago/Turabian StyleJeong, Woonhyeok, Chae Eun Yang, Tai Suk Roh, Jun Hyung Kim, Ju Hee Lee, and Won Jai Lee. 2017. "Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model" International Journal of Molecular Sciences 18, no. 8: 1698. https://doi.org/10.3390/ijms18081698