The SGLT2 Inhibitor Luseogliflozin Rapidly Normalizes Aortic mRNA Levels of Inflammation-Related but Not Lipid-Metabolism-Related Genes and Suppresses Atherosclerosis in Diabetic ApoE KO Mice
Abstract
:1. Introduction
2. Results
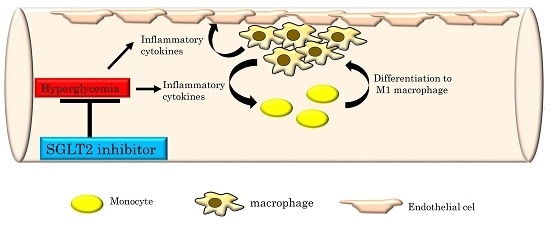
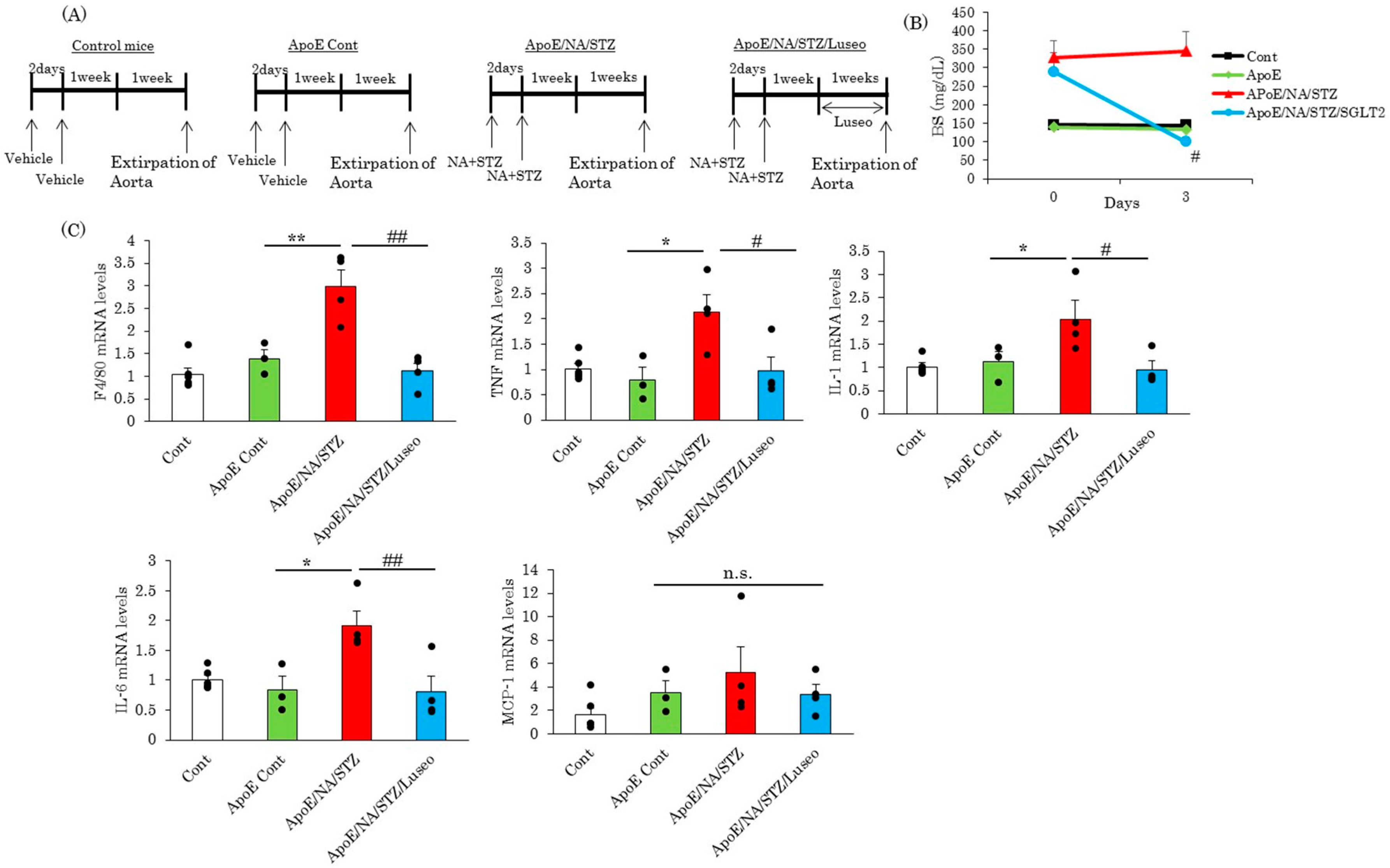
2.1. The SGLT2 Inhibitor, Luseogliflozin, Suppresses the Expression of Inflammatory Cytokines Induced by Short-Term Hyperglycemia
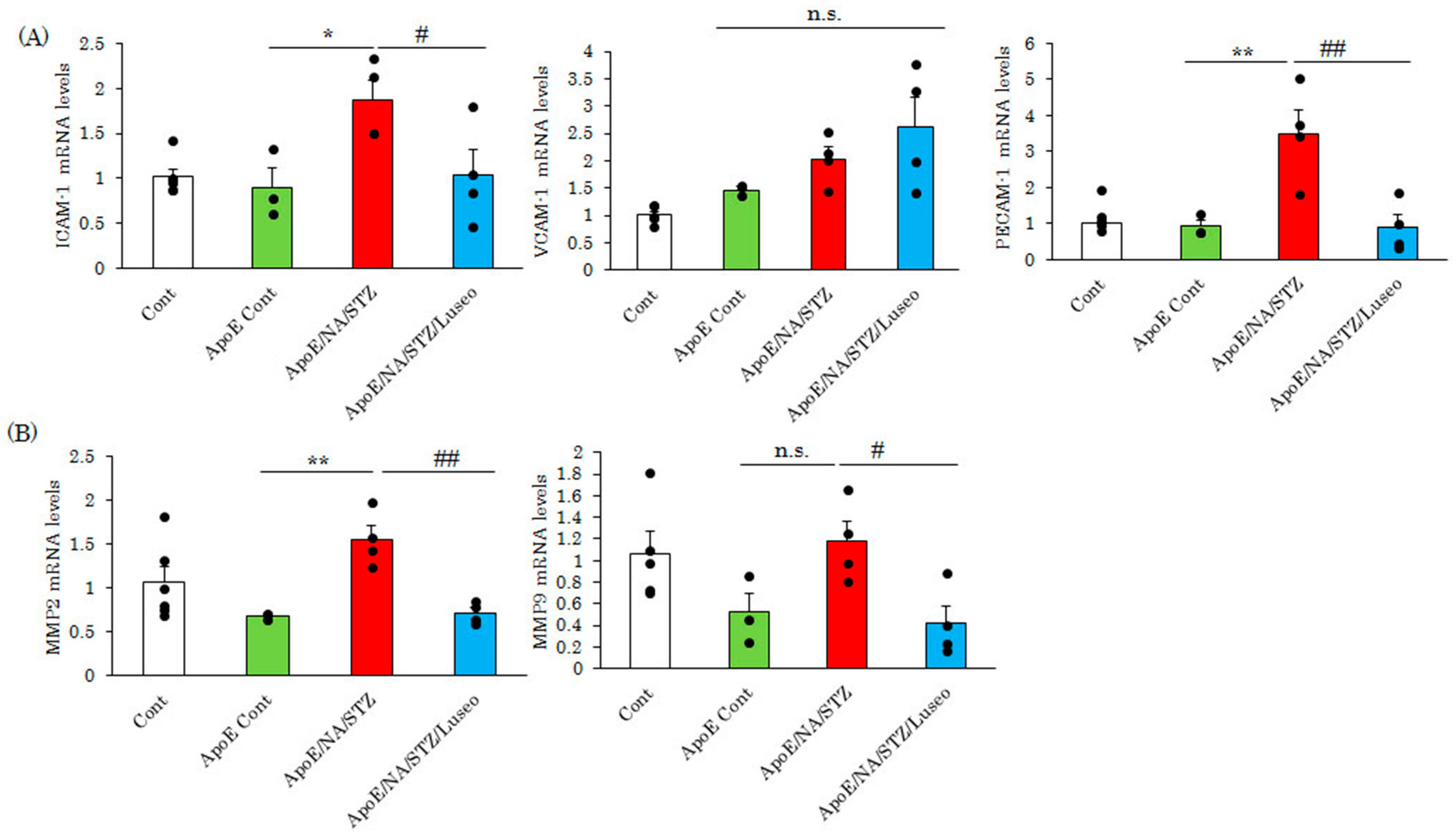
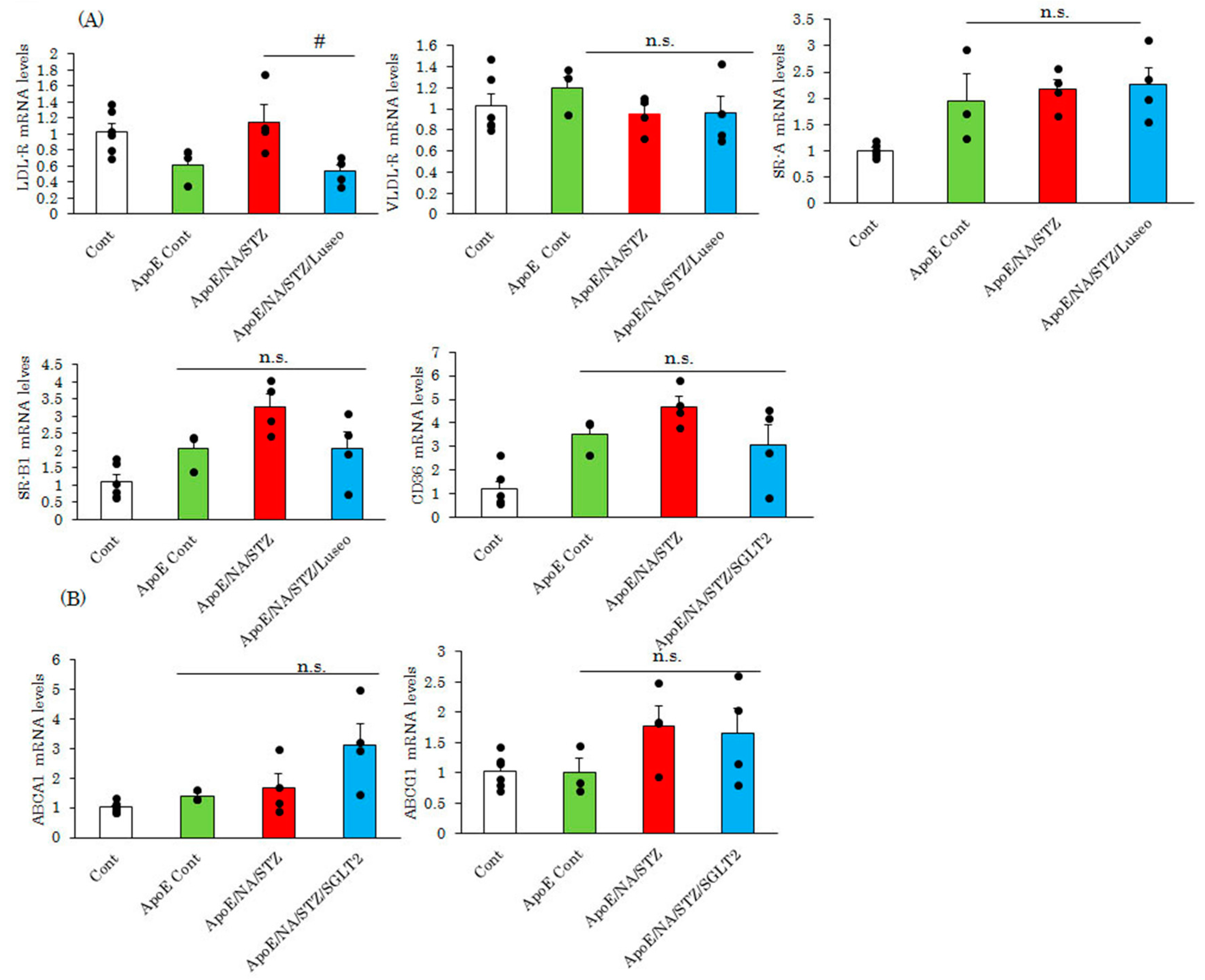
2.2. Luseogliflozin Treatment Normalized the Expression of Adhesion Molecules and Matrix Metalloproteinases, but Had Little Effect on Lipid Metabolism-Related Genes in the Aortas of NA/STZ-ApoE KO Mice
2.3. Luseogliflozin-Treatment Had No Effect on the Hyperlipidemia in ApoE KO Mice
2.4. Hyperglycemia Remarkably Exacerbates Atherosclerosis in ApoE KO Mice, but Luseogliflozin Prevents the Worsening of This Atherosclerosis
3. Discussion
4. Materials and Methods
4.1. Animals, Diets and Luseogliflozin Treatment
4.2. Evaluation of Atherosclerotic Area and Histochemical Studies
4.3. Measurements of Serum Parameters
4.4. Quantitative Real Time Reverse Transcription PCR
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.; Wiklund, U.; Zhao, Y.; Diederichsen, A.; Mickley, H.; Ovrehus, K.; Zamorano, J.; Gueret, P.; Schmermund, A.; Maffei, E.; et al. Gender and age effects on risk factor-based prediction of coronary artery calcium in symptomatic patients: A Euro-CCAD study. Athroscleoris 2016, 252, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Forlani, G.; Cerrelli, F.; Manini, R.; Natale, S.; Baraldi, L.; Ermini, G.; Savorani, G.; Zocchi, D.; Melchionda, N. WHO and ATP III proposals for the definition of the metabolic syndrome in patients with type 2 diabetes. Diabet. Med. 2004, 21, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Funahashi, T.; Nakamura, T. The concept of metabolic syndrome: Contribution of visceral fat accumulation and its molecular mechanism. J. Atheroscler. Thromb. 2011, 18, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The role of cytokines in the development of atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. JAMA 2016, 316, 1997–2007. [Google Scholar]
- Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar] [PubMed]
- Cornel, J.H.; Bakris, G.L.; Stevens, S.R.; Alvarsson, M.; Bax, W.A.; Chuang, L.M.; Engel, S.S.; Lopes, R.D.; McGuire, D.K.; Riefflin, A.; et al. Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: Outcomes from TECOS. Diabetes Care 2016, 39, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Azimova, K.; San Juan, Z.; Mukherjee, D. Cardiovascular safety profile of currently available diabetic drugs. Ochsner. J. 2014, 14, 616–632. [Google Scholar] [PubMed]
- Lee, G.; Oh, S.W.; Hwang, S.S.; Yoon, J.W.; Kang, S.; Joh, H.K.; Kwon, H.; Kim, J.; Park, D. Comparative effectiveness of oral antidiabetic drugs in preventing cardiovascular mortality and morbidity: A network mete-analysis. PLoS ONE 2017, 12, e0177646. [Google Scholar] [CrossRef] [PubMed]
- Oku, A.; Ueta, K.; Arakawa, K.; Ishihara, T.; Nawano, M.; Kuronuma, Y.; Matsumoto, M.; Saito, A.; Tsujihara, K.; Anai, M.; et al. T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes 1999, 48, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, A.; Kazuta, K.; Goto, K.; Yoshida, S.; Ueyama, E.; Utsuno, A. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes. Metab. 2015, 17, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Ogihara, T.; Katagiri, H.; Sakoda, H.; Ono, H.; Fujishiro, M.; Anai, M.; Kurihara, H.; Uchijima, Y. Glucose transporter and Na+/glucose cotransporter as molecular targets of anti-diabetic drugs. Curr. Med. Chem. 2004, 11, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; de Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Zinman, B. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 1801–1802. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Blhmki, E.; Hantel, S.; Matteus, M.; Biomath, D.; Devins, T.; Johansen, O.E.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Sonesson, C.; Johansson, P.A.; Johnsson, E.; Gause-Nilsson, I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: A meta-analysis. Cardiovasc. Diabetol. 2016, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.; Cavender, M.A.; Fu, A.Z.; Wilding, J.P.; Khunti, K.; Holl, R.W.; Norhammar, A.; Birkeland, K.I.; Jorgensen, M.; Thuresson, M.; et al. Lower risk of heart failure and death in patients initiated on SGLT-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL study. Circulation 2017, 136, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Reappraisal of the diuretic effect of empagliflozin in the EMPA-REG OUTCOME trial: Comparison with classic diuretics. Diabetes Metab. 2016, 42, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV protection in the EMPA-REG OUTCOME trial: A “thrifty substrate” hypothesis. Diabetes Care. 2016, 39, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Schmemund, A.; Achenbach, S.; Budde, T.; Buziashvili, Y.; Foster, A.; Friedrich, G.; Henein, M.; Kerhoff, G.; Knollmann, F.; Kukharchuk, V.; et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: A multicenter, randomized, double-blind trial. Circulation 2006, 113, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocok, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Luscher, T.F. Cardiovascular protection in the treatment of type 2 diabetes: A review of clinical trial results across drug classes. Am. J. Med. 2017, 130, S18–S29. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Bonnet, F.; Chabonnel, B.; Gomes, M.B.; Kosiborod, M.; Khunti, K.; Nicolucci, A.; Pocok, S.; Rathmann, W.; Shestakova, M.V.; et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: Rationale and methods of the DISCOVER observational study program. J. Diabetes Complicat. 2017, 31, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Qiang, S.; Nakatsu, Y.; Seno, Y.; Fujishiro, M.; Sakoda, H.; Kushiyama, A.; Mori, K.; Matsunaga, Y.; Yamamotoya, T.; Kamata, H.; et al. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol. Metab. Syndr. 2015, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Izumoto-Akita, T.; Tsunekawa, S.; Yamamoto, A.; Uenishi, E.; Ishikawa, K.; Ogata, H.; Iida, A.; Ikeniwa, M.; Hosokawa, K.; Niwa, Y.; et al. Secreted factors from dental pulp stem cells improve glucose intolerance in streptozotocin-induced diabetic mice by increasing pancreatic β-cell function. BMJ Open Diabetes Res. Care 2015, 3, e000128. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita, J.A.; Zhang, S.H.; Hagaman, J.R.; Oliver, P.M.; Maeda, N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA 1992, 89, 4471–4475. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of interleukin-1β decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Merhi-Soussi, F.; Kwak, B.R.; Magne, D.; Chadiichristos, C.; Berti, M.; Pelli, G.; James, R.W.; Mach, F.; Gabay, C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc. Res. 2005, 66, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Burger-Kentischer, A.; Gobel, H.; Kleemann, R.; Zernecke, A.; Bucala, R.; Leng, L.; Finkelmeier, D.; Geiger, G.; Schefer, H.E.; Schober, A.; et al. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF). Atherosclerosis 2006, 184, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Hori, O.; Chen, J.X.; Li, J.F.; Crandall, J.; Zhang, J.; Cao, R.; Yan, S.D.; Brett, J.; Stern, D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J. Clin. Investig. 1995, 96, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Johansson, F.; Kramer, F.; Barnhart, S.; Kanter, J.E.; Vaisar, T.; Merrill, R.D.; Geng, L.; Oka, K.; Chen, L.; Chait, A.; et al. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc. Natl. Acad. Sci. USA 2008, 105, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- Branen, L.; Hovgaard, L.; Nitulescu, M.; Bengtsson, E.; Nilsson, J.; Joving, S. Inhibition of tumor necrosis factor-α reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Shuto, Y.; Asai, A.; Nagao, M.; Sugihara, H.; Oikawa, S. Repetitive glucose spikes accelerate atherosclerotic lesion formation in C57BL/6 mice. PLoS ONE 2015, 10, e0136840. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017. [CrossRef] [PubMed]
- Yao, D.; Brownlee, M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.; Warnholtz, A.; et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001, 88, E14–E22. [Google Scholar] [CrossRef] [PubMed]
- Wendt, T.; Haria, E.; Bucciarelli, L.; Qu, W.; Lu, Y.; Rong, L.L.; Jekins, D.G.; Stein, G.; Schmidt, A.M.; Yan, S.F. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis 2006, 185, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Ouyamng, X.; Lei, X.; Wu, M.; Chen, L.; Wu, Q.; Deng, W.; Liang, Z. The SGLT-2 Inhibitor dapagliflozin has a therapeutic effect on atherosclerosis in diabetic ApoE-/-mice. Mediators Inflamm. 2016, 2016, 6305735. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Oh, T.J.; Lee, G.; Maeng, H.J.; Lee, D.H.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lee, H.S.; Park, K.S.; et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE-/-mice fed a western diet. Diabetologia 2017, 60, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Salim, H.M.; Fukuda, D.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Glycemic control with ipragliflozin, a novel selective SGLT2 inhibitor, ameliorated endothelial dysfunction in streptozotocin-induced diabetic mouse. Front. Cardiovasc. Med. 2016, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, H.; Oi, T.; Hashimoto-Tsuchiya, Y.; Arai, M.; Kawakita, Y.; Fukasawa, Y.; Iida, I.; Hagima, N.; Takeuchi, H.; Chino, Y.; et al. (1S)-1,5-anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-d-glucito l (TS-071) is a potent, selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment. J. Med. Chem. 2010, 53, 3247–3261. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatsu, Y.; Kokubo, H.; Bumdelger, B.; Yoshizumi, M.; Yamamotoya, T.; Matsunaga, Y.; Ueda, K.; Inoue, Y.; Inoue, M.-K.; Fujishiro, M.; et al. The SGLT2 Inhibitor Luseogliflozin Rapidly Normalizes Aortic mRNA Levels of Inflammation-Related but Not Lipid-Metabolism-Related Genes and Suppresses Atherosclerosis in Diabetic ApoE KO Mice. Int. J. Mol. Sci. 2017, 18, 1704. https://doi.org/10.3390/ijms18081704
Nakatsu Y, Kokubo H, Bumdelger B, Yoshizumi M, Yamamotoya T, Matsunaga Y, Ueda K, Inoue Y, Inoue M-K, Fujishiro M, et al. The SGLT2 Inhibitor Luseogliflozin Rapidly Normalizes Aortic mRNA Levels of Inflammation-Related but Not Lipid-Metabolism-Related Genes and Suppresses Atherosclerosis in Diabetic ApoE KO Mice. International Journal of Molecular Sciences. 2017; 18(8):1704. https://doi.org/10.3390/ijms18081704
Chicago/Turabian StyleNakatsu, Yusuke, Hiroki Kokubo, Batmunkh Bumdelger, Masao Yoshizumi, Takeshi Yamamotoya, Yasuka Matsunaga, Koji Ueda, Yuki Inoue, Masa-Ki Inoue, Midori Fujishiro, and et al. 2017. "The SGLT2 Inhibitor Luseogliflozin Rapidly Normalizes Aortic mRNA Levels of Inflammation-Related but Not Lipid-Metabolism-Related Genes and Suppresses Atherosclerosis in Diabetic ApoE KO Mice" International Journal of Molecular Sciences 18, no. 8: 1704. https://doi.org/10.3390/ijms18081704