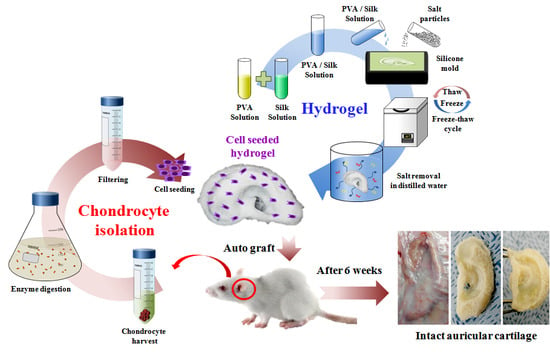
Artificial Auricular Cartilage Using Silk Fibroin and Polyvinyl Alcohol Hydrogel
Abstract
:1. Introduction
2. Results
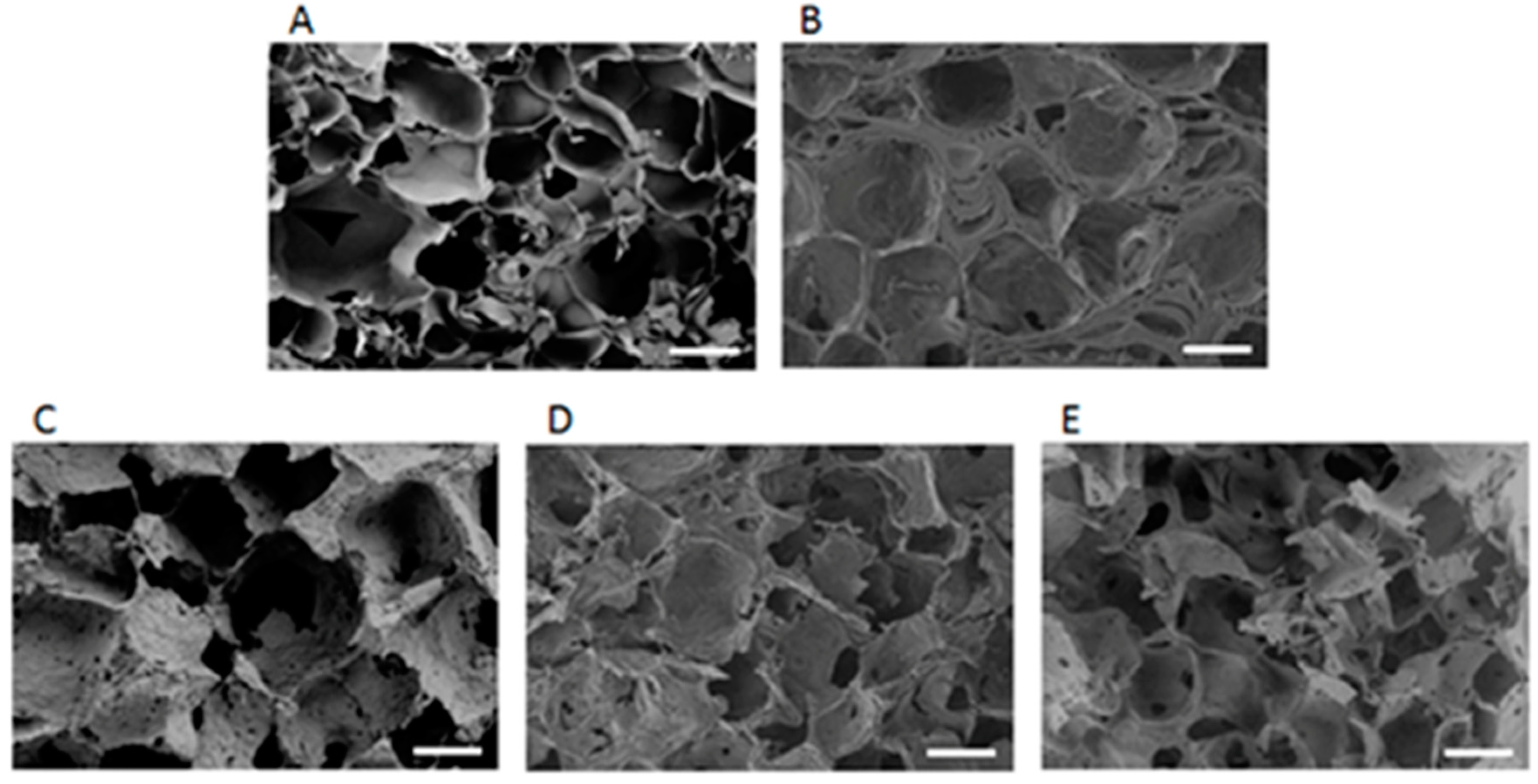
2.1. Structural and Morphological Characteristics
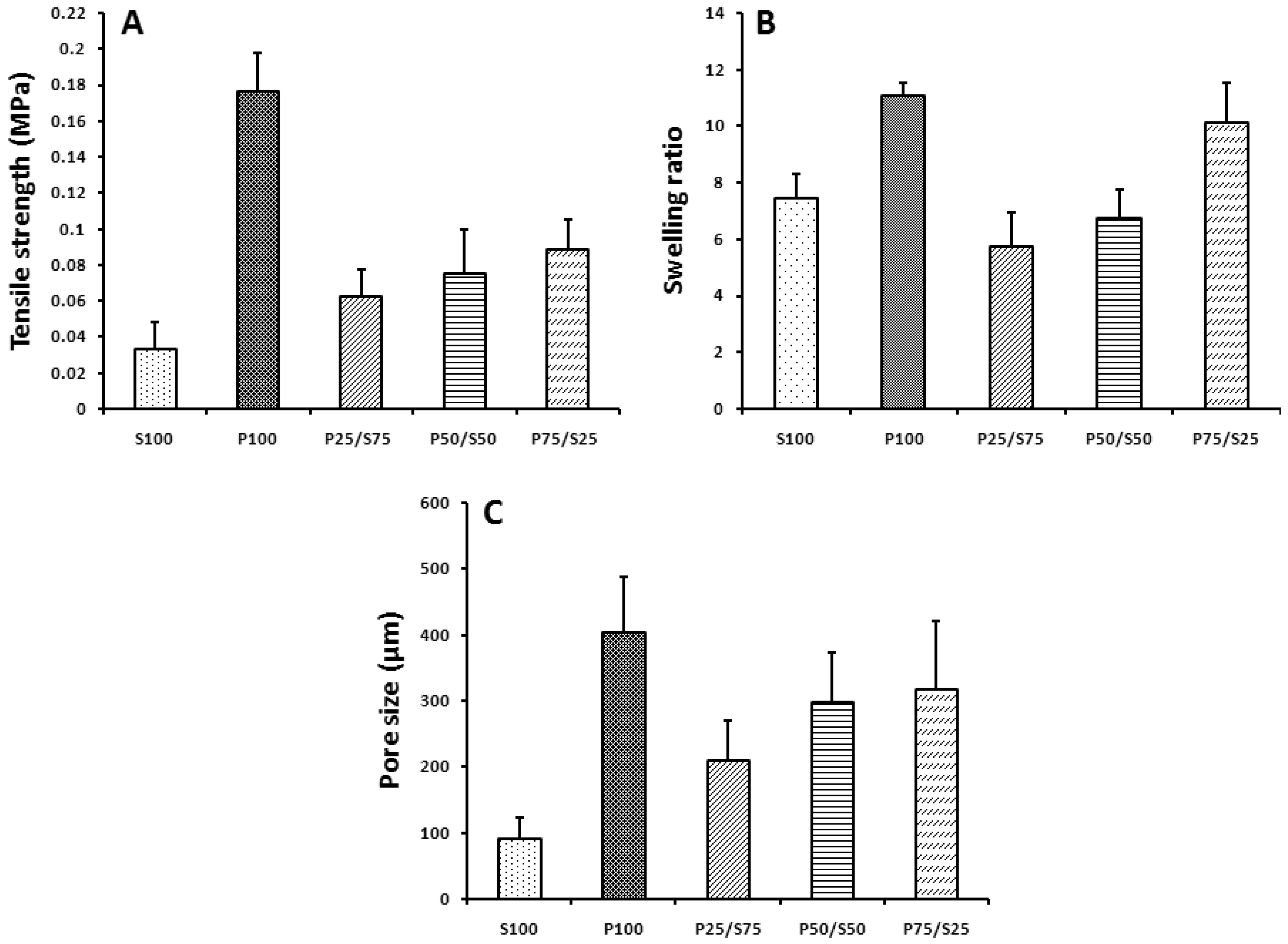
2.2. Mechanical Properties, Swelling Ratio, and Pore Size
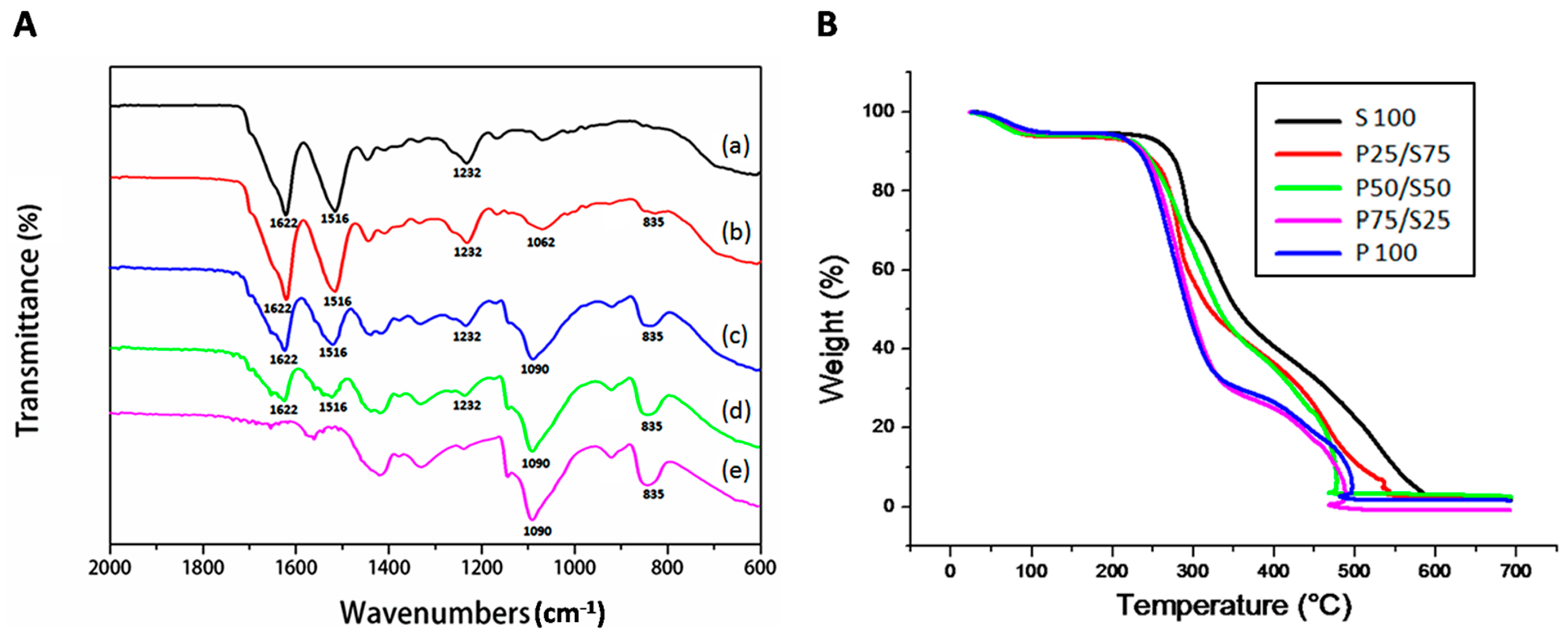
2.3. FTIR and TGA
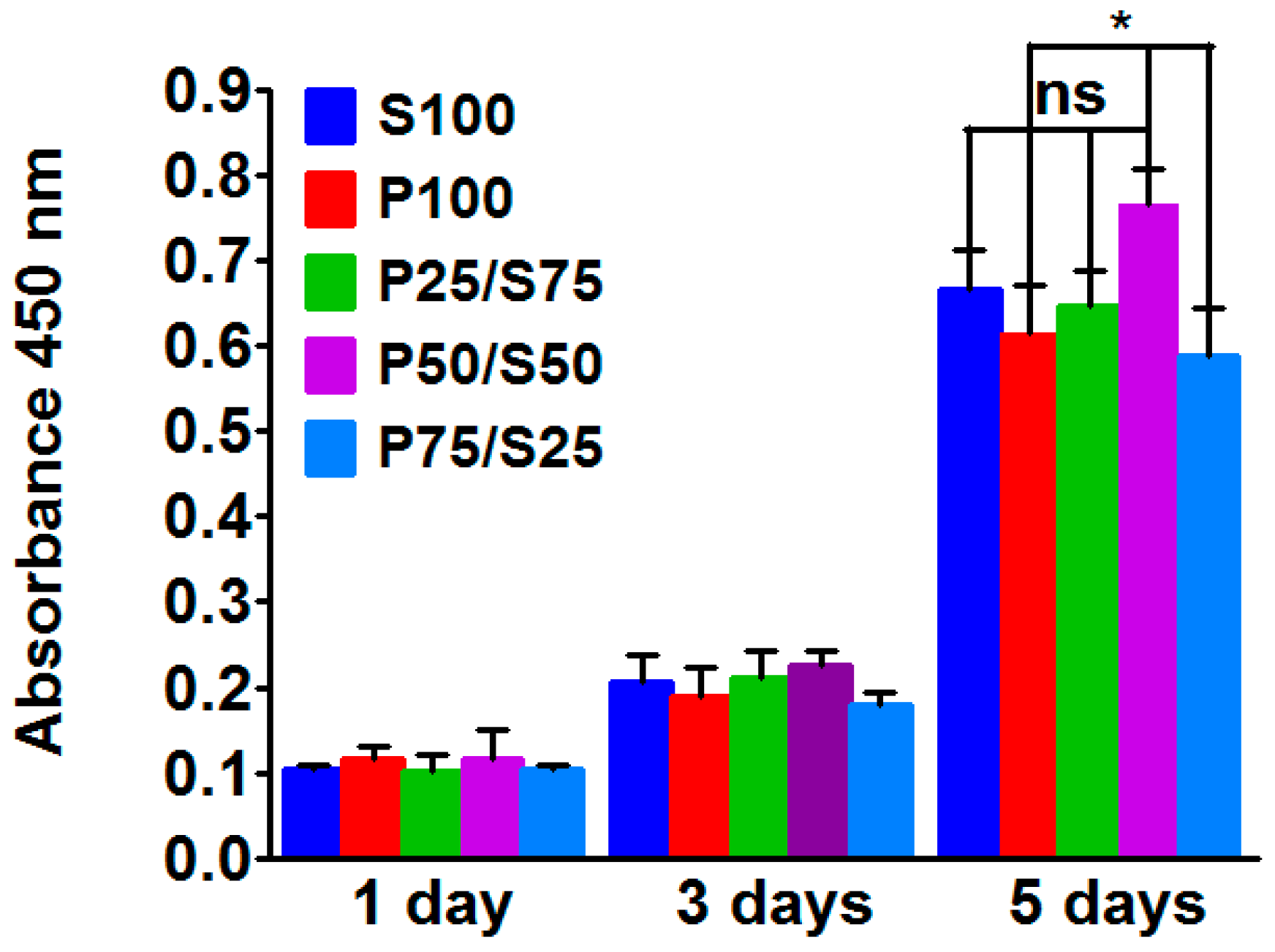
2.4. Biocompatibility of the Hydrogels
2.5. In Vitro Chondrogenesis
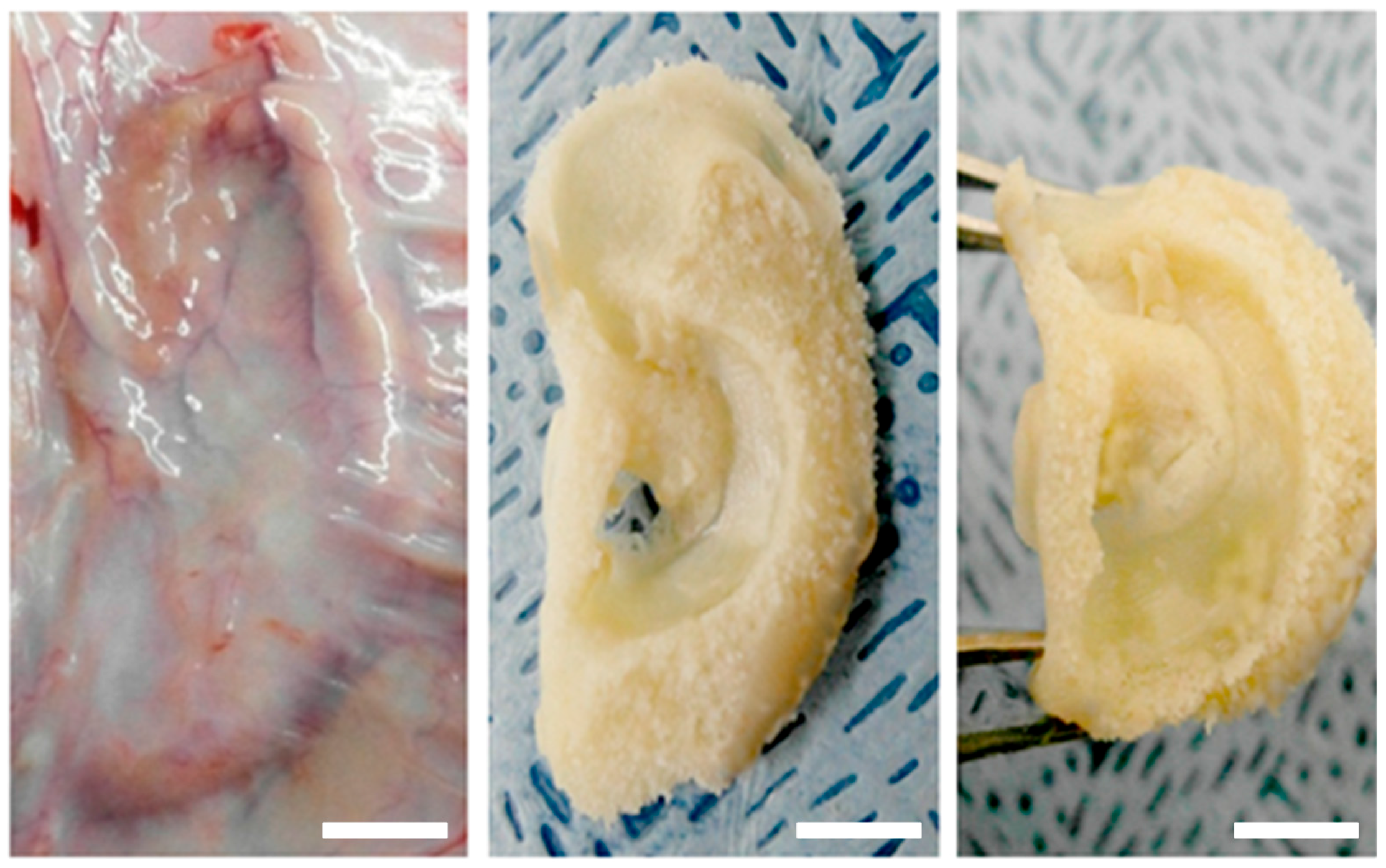
2.6. In Vivo Engineered Cartilage
3. Discussion
4. Materials and Methods
4.1. Preparation of SF and PVA Solutions
4.2. Preparation of the 3D Ear Mold
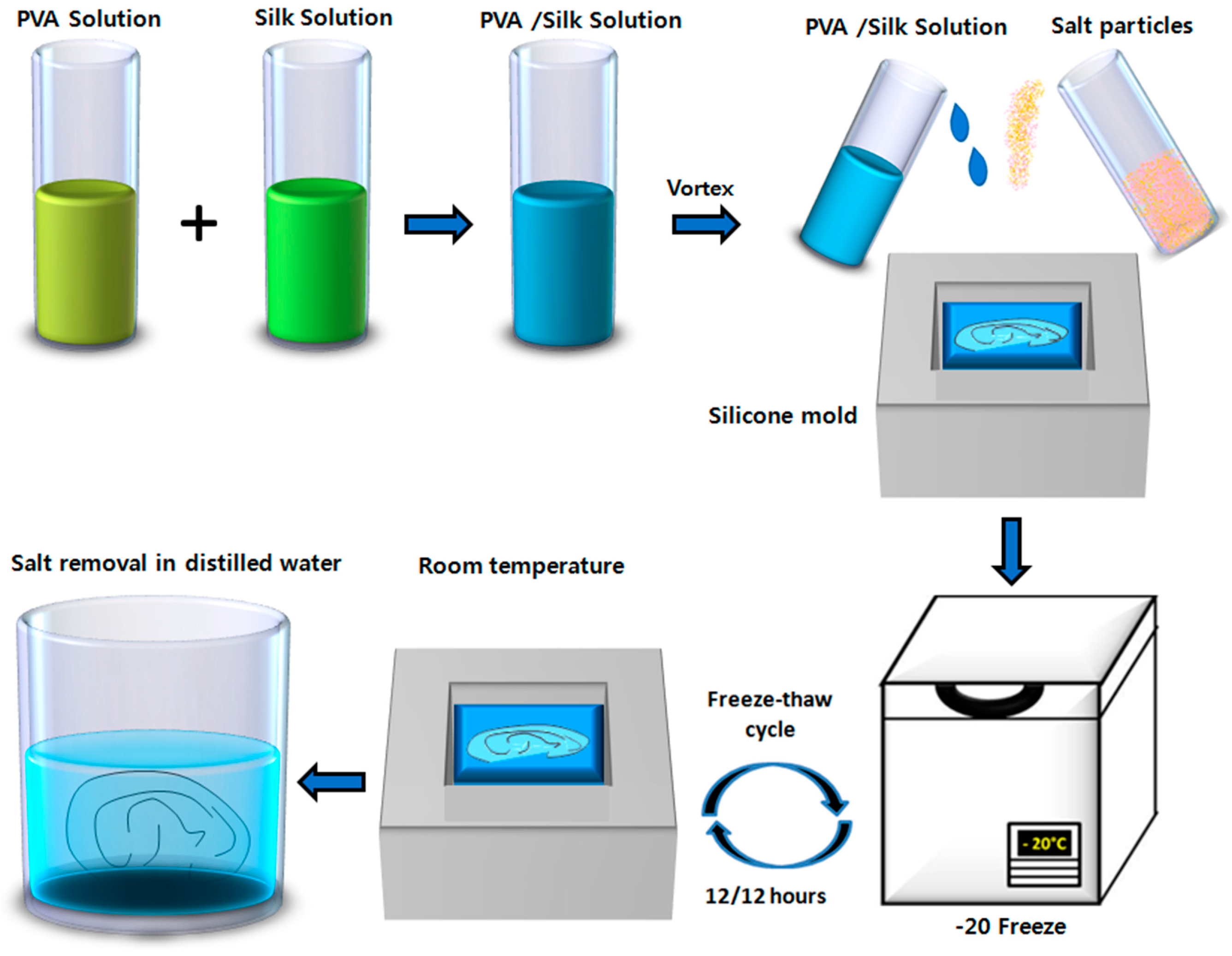
4.3. Fabrication of Hydrogel
4.4. Analysis of Mechanical Properties (Tensile Strenth)
4.5. Swelling Ratio and TGA Analysis
4.6. SEM and FTIR Analysis
4.7. Chondrocyte Isolation and Culture
4.8. Cell Seeded Hydrogels
4.9. Animal Study
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nimeskern, L.; Utomo, L.; Lehtoviita, I.; Fessel, G.; Snedeker, J.G.; van Osch, G.J.; Muller, R.; Stok, K.S. Tissue composition regulates distinct viscoelastic responses in auricular and articular cartilage. J. Biomech. 2016, 49, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Feng, B.; Zheng, R.; Lu, Y.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, Y.; Zhang, W.J. Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials 2013, 34, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Otto, I.A.; Melchels, F.P.; Zhao, X.; Randolph, M.A.; Kon, M.; Breugem, C.C.; Malda, J. Auricular reconstruction using biofabrication-based tissue engineering strategies. Biofabrication 2015, 7, 032001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Blasioli, D.J.; Kim, H.J.; Kim, H.S.; Kaplan, D.L. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials 2006, 27, 4434–4442. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Weder, C.; Foster, E.J. Directed cell growth in multi-zonal scaffolds for cartilage tissue engineering. Biomaterials 2016, 74, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Amadori, S.; Torricelli, P.; Panzavolta, S.; Parrilli, A.; Fini, M.; Bigi, A. Highly porous gelatin reinforced 3D scaffolds for articular cartilage regeneration. Macromol. Biosci. 2015, 15, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Jiang, Z.; James, T.; Kong, Y.L.; Malatesta, K.A.; Soboyejo, W.O.; Verma, N.; Gracias, D.H.; McAlpine, M.C. 3D printed bionic ears. NANO Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef] [PubMed]
- Nimeskern, L.; Martinez Avila, H.; Sundberg, J.; Gatenholm, P.; Muller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Xue, J.X.; Zhang, W.J.; Zhou, G.D.; Liu, W.; Cao, Y. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials 2011, 32, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Zhou, G.; Li, Q.; Liu, W.; Yu, Z.; Luo, X.; Jiang, T.; Zhang, W.; Cao, Y. In vitro engineering of human ear-shaped cartilage assisted with CAD/CAM technology. Biomaterials 2010, 31, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Schué, F. Encyclopedia of Controlled Drug Delivery; Wiley and Sons: New York, NY, USA, 2002. [Google Scholar]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.H.; Yodmuang, S.; Wang, X.; Sun, L.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk hydrogel for cartilage tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.M.; Choi, M.J.; Sultan, M.T.; Yang, J.W.; Ju, H.W.; Lee, J.M.; Park, H.J.; Park, Y.R.; Kim, S.H.; Kim, D.W.; et al. Novel fabrication method of the peritoneal dialysis filter using silk fibroin with urease fixation system. J. Biomed. Mater. Res. B Appl. Biomater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Borer, M.E.; Wenk, E.; Meinel, L.; Lacroix, C. Development of silk fibroin-based beads for immobilized cell fermentations. J. Microencapsul. 2010, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; St John, P.; Kaplan, D.L. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials 2008, 29, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Horan, R.L.; Antle, K.; Collette, A.L.; Wang, Y.; Huang, J.; Moreau, J.E.; Volloch, V.; Kaplan, D.L.; Altman, G.H. In vitro degradation of silk fibroin. Biomaterials 2005, 26, 3385–3393. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Nerem, R.M. Progress in tissue engineering and regenerative medicine. Proc. Natl. Acad. Sci. USA 2010, 107, 3285–3286. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, E.; Schoen, F.J. Cardiovascular tissue engineering. Cardiovasc. Pathol. 2002, 11, 305–317. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bella, E.; Lee, C.S.; Migliaresi, C.; Pelcastre, L.; Schwartz, Z.; Boyan, B.D.; Motta, A. The synergistic effects of 3-D porous silk fibroin matrix scaffold properties and hydrodynamic environment in cartilage tissue regeneration. Biomaterials 2010, 31, 4672–4681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, W.; Ma, Z.; Wu, C.; Fang, W.; Liu, G.; Xiao, Y. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater. 2010, 6, 3021–3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Kundu, S.C. Biospinning by silkworms: Silk fiber matrices for tissue engineering applications. Acta Biomater. 2010, 6, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Chae, T.; Sheikh, F.A.; Ju, H.W.; Moon, B.M.; Park, H.J.; Park, Y.R.; Park, C.H. Three dimensional poly(epsilon-caprolactone) and silk fibroin nanocomposite fibrous matrix for artificial dermis. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Poole-Warren, L.A.; Martens, P.; Kundu, S.C. Silk fibroin/poly(vinyl alcohol) photocrosslinked hydrogels for delivery of macromolecular drugs. Acta Biomater. 2012, 8, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Price, R.; Gustafson, J.; Greish, K.; Cappello, J.; McGill, L.; Ghandehari, H. Comparison of silk-elastinlike protein polymer hydrogel and poloxamer in matrix-mediated gene delivery. Int. J. Pharm. 2012, 427, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Q.; Cheng, N.; Tao, X.; Zhang, Z.; Sun, X.; Zhang, Q. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Yodmuang, S.; McNamara, S.L.; Nover, A.B.; Mandal, B.B.; Agarwal, M.; Kelly, T.A.; Chao, P.H.; Hung, C.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015, 11, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Nair, P.D. Polyvinyl alcohol-poly(caprolactone) semi IPN scaffold with implication for cartilage tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Geever, L.M.; Kennedy, J.E.; Higginbotham, C.L.; Cahill, P.A.; McGuinness, G.B. Thermal behavior and mechanical properties of physically crosslinked PVA/Gelatin hydrogels. J. Mech. Behav. Biomed. Mater. 2010, 3, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Laurencin, S.J.; Lowman, A.M. Characterization of the behavior of porous hydrogels in model osmotically-conditioned articular cartilage systems. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Katta, J.K.; Marcolongo, M.; Lowman, A.; Mansmann, K.A. Friction and wear behavior of poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for articular cartilage replacement. J. Biomed. Mater. Res. A 2007, 83, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.A.; Doty, S.B.; Torzilli, P.A.; Thornton, S.; Lowman, A.M.; Thomas, J.D.; Warren, R.; Wright, T.M.; Myers, E. Nondegradable hydrogels for the treatment of focal cartilage defects. J. Biomed. Mater. Res. A 2007, 83, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.; Twigg, P.; Egan, A.; Moody, A.; Smith, A.; Eagland, D.; Crowther, N.; Britland, S. Poly(vinyl alcohol) hydrogel as a biocompatible viscoelastic mimetic for articular cartilage. Biotechnol. Prog. 2006, 22, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Laurencin, S.J.; Charlton, D.; Maher, S.A.; Lowman, A.M. Superporous hydrogels for cartilage repair: Evaluation of the morphological and mechanical properties. Acta Biomater. 2008, 4, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Chang, Y.S.; Oka, M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 2005, 26, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Shieh, S.J.; Terada, S.; Vacanti, J.P. Tissue engineering auricular reconstruction: In vitro and in vivo studies. Biomaterials 2004, 25, 1545–1557. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Banerjee, R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Annabi, N. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.C.; Campbell, G.; Spadiccino, A.; Robertson, M.; Curtis, A.S. The influence of microscale topography on fibroblast attachment and motility. Biomaterials 2004, 25, 5781–5788. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Mithieux, S.M.; Boughton, E.A.; Ruys, A.J.; Weiss, A.S.; Dehghani, F. Synthesis of highly porous crosslinked elastin hydrogels and their interaction with fibroblasts in vitro. Biomaterials 2009, 30, 4550–4557. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; Dutta, N.K.; Elvin, C.M.; Choudhury, N.R. Fabrication of highly elastic resilin/silk fibroin based hydrogel by rapid photo-crosslinking reaction. J. Mater. Chem. B 2015, 3, 6576–6579. [Google Scholar] [CrossRef]
- Ji, C.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. 2002, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, O.J.; Lee, M.C.; Moon, B.M.; Ju, H.W.; Lee, J.; Kim, J.H.; Kim, D.W.; Park, C.H. Fabrication of 3D porous silk scaffolds by particulate (salt/sucrose) leaching for bone tissue reconstruction. Int. J. Biol. Macromol. 2015, 78, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Neo, P.Y.; Shi, P.; Goh, J.C.; Toh, S.L. Characterization and mechanical performance study of silk/PVA cryogels: Towards nucleus pulposus tissue engineering. Biomed. Mater. 2014, 9, 065002. [Google Scholar] [CrossRef] [PubMed]
- Tangerino Filho, E.P.; Fachi, J.L.; Vasconcelos, I.C.; Dos Santos, G.M.; Mendonca, F.A.; de Aro, A.A.; Pimentel, E.R.; Esquisatto, M.A. Effects of microcurrent therapy on excisional elastic cartilage defects in young rats. Tissue Cell 2016, 48, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Imada, M.; Ikeda, M.; Sueno, K.; Arikuni, T.; Kida, A. A morphological study of age changes in adult human auricular cartilage with special emphasis on elastic fibers. Laryngoscope 2001, 111, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Dozin, B.; Giannoni, P.; Quarto, R. Tissue engineering and cell therapy of cartilage and bone. Matrix. Biol. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Sasaki, S.; Murakami, T.; Suzuki, A. Frictional properties of physically cross-linked PVA hydrogels as artificial cartilage. Biosurface Biotribol. 2016, 2, 11–17. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.M.; Sultan, M.T.; Kim, S.H.; Kumar, V.; Yeon, Y.K.; Lee, O.J.; Park, C.H. Artificial Auricular Cartilage Using Silk Fibroin and Polyvinyl Alcohol Hydrogel. Int. J. Mol. Sci. 2017, 18, 1707. https://doi.org/10.3390/ijms18081707
Lee JM, Sultan MT, Kim SH, Kumar V, Yeon YK, Lee OJ, Park CH. Artificial Auricular Cartilage Using Silk Fibroin and Polyvinyl Alcohol Hydrogel. International Journal of Molecular Sciences. 2017; 18(8):1707. https://doi.org/10.3390/ijms18081707
Chicago/Turabian StyleLee, Jung Min, Md. Tipu Sultan, Soon Hee Kim, Vijay Kumar, Yeung Kyu Yeon, Ok Joo Lee, and Chan Hum Park. 2017. "Artificial Auricular Cartilage Using Silk Fibroin and Polyvinyl Alcohol Hydrogel" International Journal of Molecular Sciences 18, no. 8: 1707. https://doi.org/10.3390/ijms18081707