Shear Bond Strength and Remineralisation Effect of a Casein Phosphopeptide-Amorphous Calcium Phosphate-Modified Glass Ionomer Cement on Artificial “Caries-Affected” Dentine
Abstract
:1. Introduction
2. Results
2.1. Shear Bond Strength Test and Failure Mode Analysis
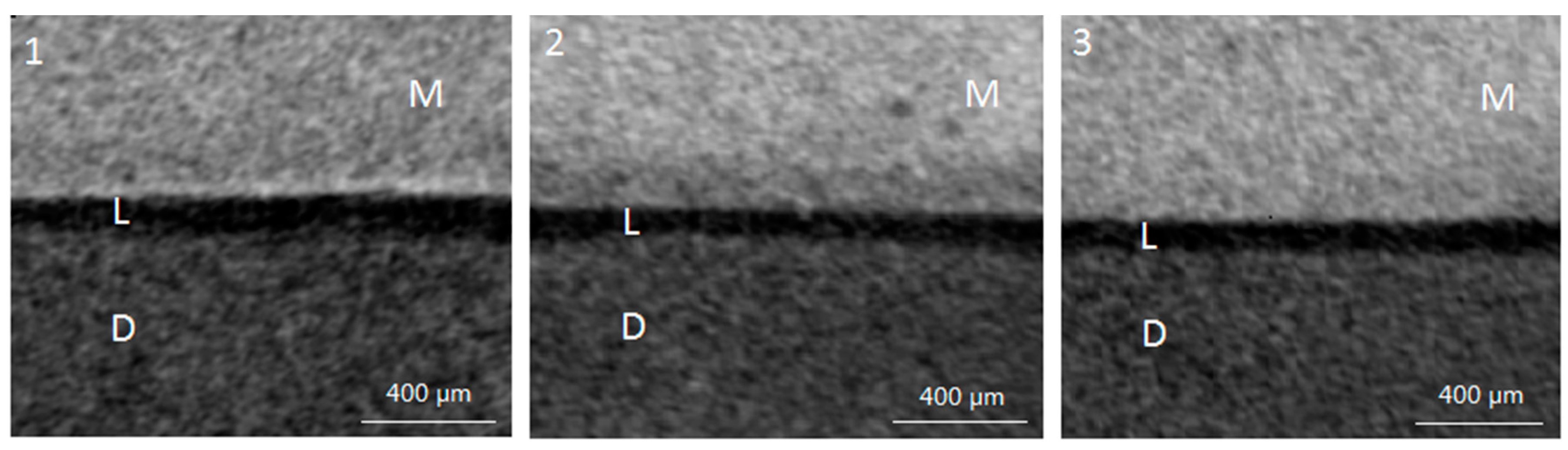
2.2. Lesion Depth from Micro-Computed Tomography (Micro-CT) Scan
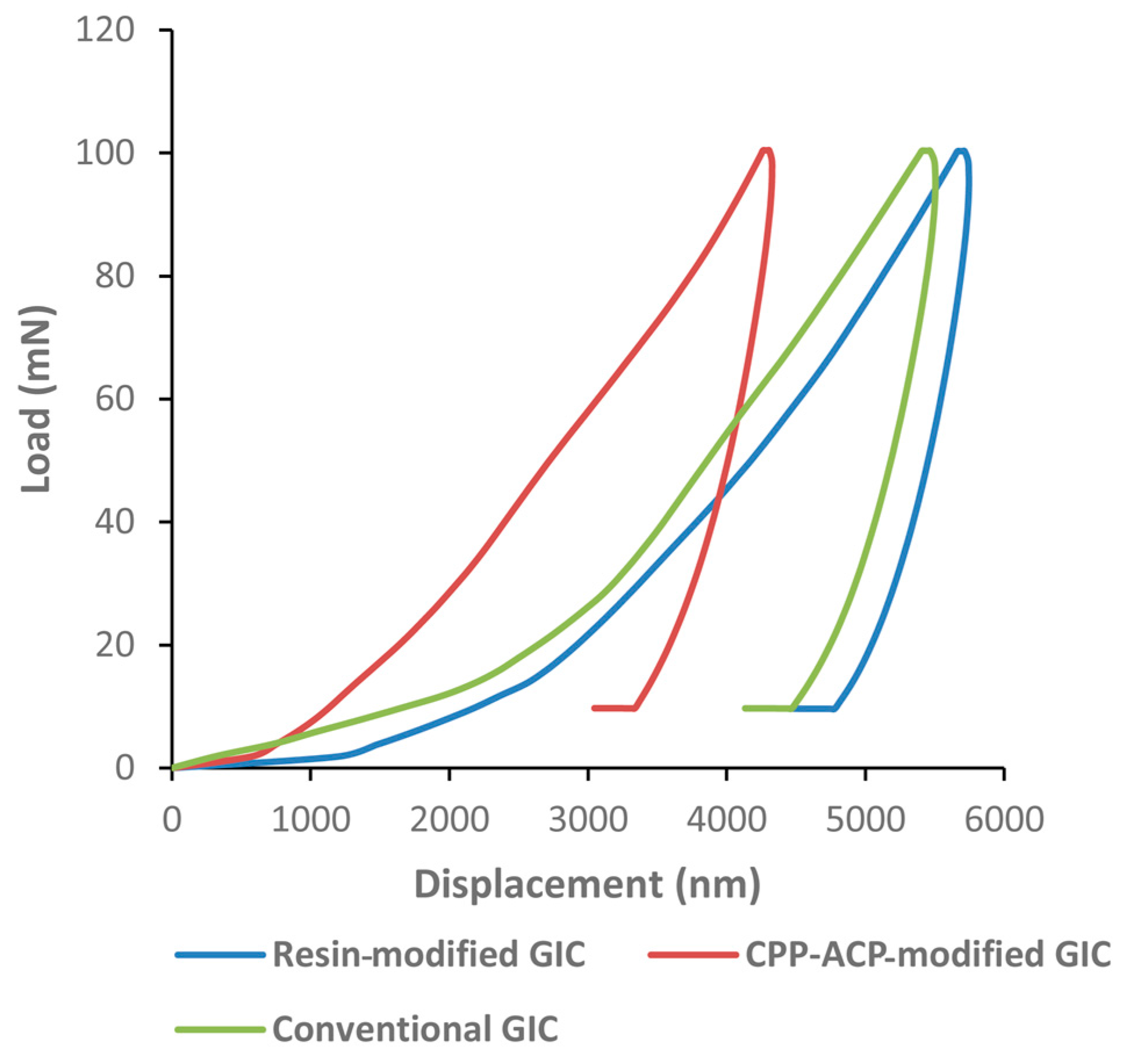
2.3. Nano-Hardness and Elastic Moduli from Nano-Indentation Test
3. Discussion
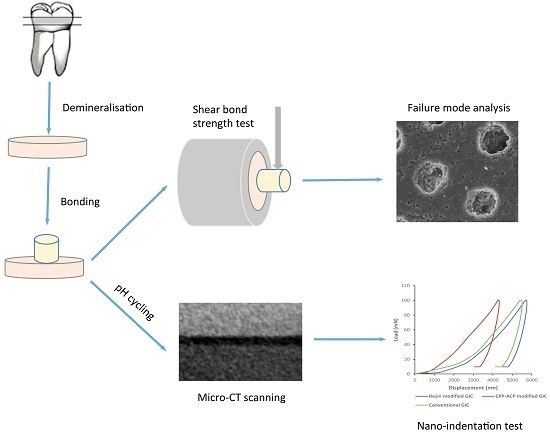
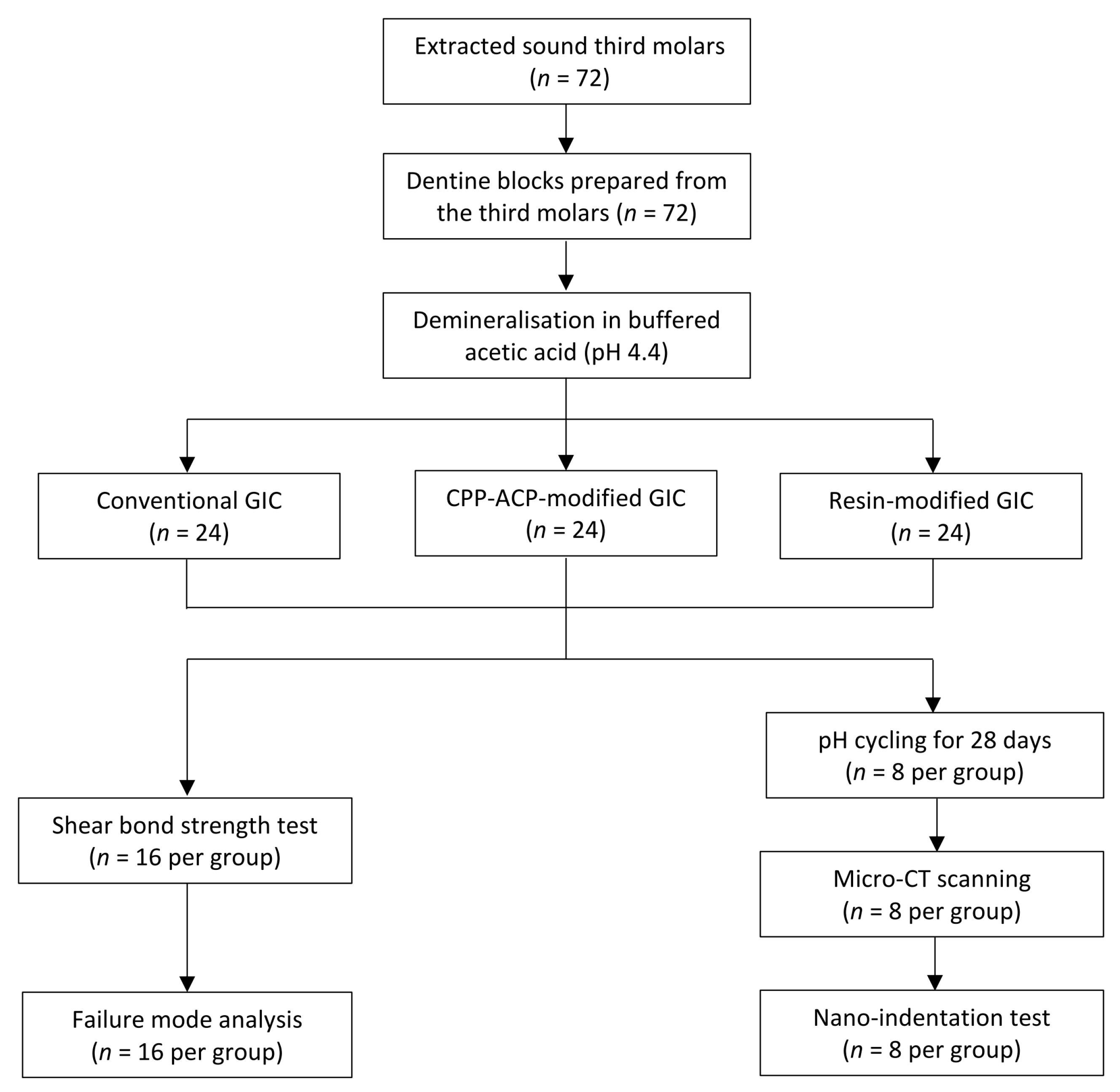
4. Materials and Methods
4.1. Sample Preparation
4.2. Shear Bond Strength Test and Failure Mode Analysis
4.3. Assessment of Remineralisation
4.3.1. pH Cycling
4.3.2. Micro-CT Scan
4.3.3. Mechanical Analysis (Nano-Indentation Test)
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CPP-ACP | Casein phosphopeptide-amorphous calcium phosphate |
| SBS | Shear bond strength |
| GIC | Glass ionomer cement |
| Micro-CT | Micro-computed tomography |
| HEMA | 2-hydroxyethyl methacrylate |
| SEM | Scanning electronic microscope |
References
- Fusayama, T. Two layers of carious dentin; diagnosis and treatment. Oper. Dent. 1979, 4, 63–70. [Google Scholar] [PubMed]
- Nakajima, M.; Kunawarote, S.; Prasansuttiporn, T.; Tagami, J. Bonding to caries-affected dentin. Jpn. Dent. Sci. Rev. 2011, 47, 102–114. [Google Scholar] [CrossRef]
- Liu, Y.; Li, N.; Qi, Y.; Niu, L.N.; Elshafiy, S.; Mao, J.; Breschi, L.; Pashley, D.H.; Tay, F.R. The use of sodium trimetaphosphate as a biomimetic analog of matrix phosphoproteins for remineralization of artificial caries-like dentin. Dent. Mater. 2011, 27, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.P.; Li, N.; Niu, L.N.; Primus, C.M.; Ling, J.Q.; Pashley, D.H.; Tay, F.R. Remineralization of artificial dentinal caries lesions by biomimetically modified mineral trioxide aggregate. Acta Biomater. 2012, 8, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. Fluoride, remineralization and root caries. Am. J. Dent. 1994, 7, 271–274. [Google Scholar] [PubMed]
- Zafar, M.S.; Ahmed, N. Therapeutic roles of fluoride released from restorative dental materials. Fluoride 2015, 48, 184–194. [Google Scholar]
- Mei, M.L.; Zhao, I.S.; Ito, L.; Lo, E.C.M.; Chu, C.H. Prevention of secondary caries by silver diamine fluoride. Int. Dent. J. 2015, 66, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Xie, X.; Wang, Y.; Yin, W.; Antoun, J.S.; Farella, M.; Mei, L. Long-term remineralizing effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on early caries lesions in vivo: A systematic review. J. Dent. 2014, 42, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Prakash, V. CPP-ACP complex as a new adjunctive agent for remineralization: A review. Oral Health Prev. Dent. 2011, 9, 151–165. [Google Scholar] [PubMed]
- Reynolds, E.C.; Black, C.; Cai, F.; Cross, K.J.; Eakins, D.; Huq, N.L.; Morgan, M.V.; Nowicki, A.; Perich, J.W.; Riley, P. Advances in enamel remineralization: Casein phosphopeptide-amorphous calcium phosphate. J. Clin. Dent. 1999, 10, 86–88. [Google Scholar]
- Reynolds, E.C.; Cain, C.J.; Webber, F.L.; Black, C.L.; Riley, P.F.; Johnson, I.H.; Perich, J.W. Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptides in the rat. J. Dent. Res. 1995, 74, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.C.; Cai, F.; Shen, P.; Walker, G. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J. Dent. Res. 2003, 82, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Reema, S.D.; Lahiri, P.K.; Roy, S.S. Review of casein phosphopeptides-amorphous calcium phosphate. Chin. J. Dent. Res. 2014, 17, 7–14. [Google Scholar] [PubMed]
- Dashper, S.G.; Catmull, D.V.; Liu, S.W.; Myroforidis, H.; Zalizniak, I.; Palamara, J.E.; Huq, N.L.; Reynolds, E.C. Casein Phosphopeptide-Amorphous Calcium Phosphate Reduces Streptococcus mutans Biofilm Development on Glass Ionomer Cement and Disrupts Established Biofilms. PLoS ONE 2016, 11, e0162322. [Google Scholar] [CrossRef] [PubMed]
- Zalizniak, I.; Palamara, J.E.; Wong, R.H.; Cochrane, N.J.; Burrow, M.F.; Reynolds, E.C. Ion release and physical properties of CPP-ACP-modified GIC in acid solutions. J. Dent. 2013, 41, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Mazzaoui, S.A.; Burrow, M.F.; Tyas, M.J.; Dashper, S.G.; Eakins, D.; Reynolds, E.C. Incorporation of casein phosphopeptide-amorphous calcium phosphate into a glass-ionomer cement. J. Dent. Res. 2003, 82, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Al Zraikat, H.; Palamara, J.E.; Messer, H.H.; Burrow, M.F.; Reynolds, E.C. The incorporation of casein phosphopeptide–Amorphous calcium phosphate into a glass ionomer cement. Dent. Mater. 2011, 27, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, I.S.; Mei, M.L.; Burrow, M.F.; Lo, E.C.M.; Chu, C.H. Prevention of secondary caries using silver diamine fluoride treatment and casein phosphopeptide-amorphous calcium phosphate modified glass-ionomer cement. J. Dent. 2017, 57, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kucukyilmaz, E.; Savas, S. Evaluation of shear bond strength, penetration ability, microleakage and remineralization capacity of glass ionomer-based fissure sealants. Eur. J. Paediatr. Dent. 2016, 17, 17–23. [Google Scholar] [PubMed]
- Koizumi, H.; Hamama, H.H.; Burrow, M.F. Evaluation of adhesion of a CPP-ACP-modified GIC to enamel, sound dentine, and caries-affected dentine. Int. J. Adhes. Adhes. 2016, 66, 176–181. [Google Scholar] [CrossRef]
- Choi, K.; Oshida, Y.; Platt, J.A.; Cochran, M.A.; Matis, B.A.; Yi, K. Microtensile bond strength of glass ionomer cements to artificially created carious dentin. Oper. Dent. 2006, 31, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Fritz, U.B.; Uno, S. Resin-modified glass ionomer cements: Bonding to enamel and dentin. Dent. Mater. 1996, 12, 161–166. [Google Scholar] [CrossRef]
- Dursun, E.; Nguyen, J.F.; Tang, M.L.; Attal, J.P.; Sadoun, M. HEMA release and degree of conversion from a resin-modified glass ionomer cement after various delays of light activation. Dent. Mater. 2016, 32, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Tanumiharja, M.; Burrow, M.F.; Tyas, M.J. Microtensile bond strengths of glass ionomer (polyalkenoate) cements to dentine using four conditioners. J. Dent. 2000, 28, 361–366. [Google Scholar] [CrossRef]
- Mei, M.L.; Ito, L.; Cao, Y.; Li, Q.; Lo, E.C.M.; Chu, C.H. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J. Dent. 2013, 41, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Markovic, D.L.; Petrovic, B.B.; Peric, T.O. Fluoride content and recharge ability of five glassionomer dental materials. BMC Oral Health 2008, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Flaim, G.; Dickens, S.H. Remineralization of human natural caries and artificial caries-like lesions with an experimental whisker-reinforced ART composite. Acta Biomater. 2011, 7, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Epasinghe, D.J.; Yiu, C.K.; Burrow, M.F. Synergistic effect of Proanthocyanidin and CPP-ACFP on remineralization of artificial root caries. Aust. Dent. J. 2015, 60, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Ngan, A.H.W. A rate-jump method for characterization of soft tissues using nanoindentation techniques. Soft Matter 2012, 8, 5974–5979. [Google Scholar] [CrossRef] [Green Version]
- Ngan, A.H.W.; Tang, B. Viscoelastic effects during unloading in depth-sensing indentation. J. Mater. Res. 2002, 17, 2604–2610. [Google Scholar] [CrossRef] [Green Version]
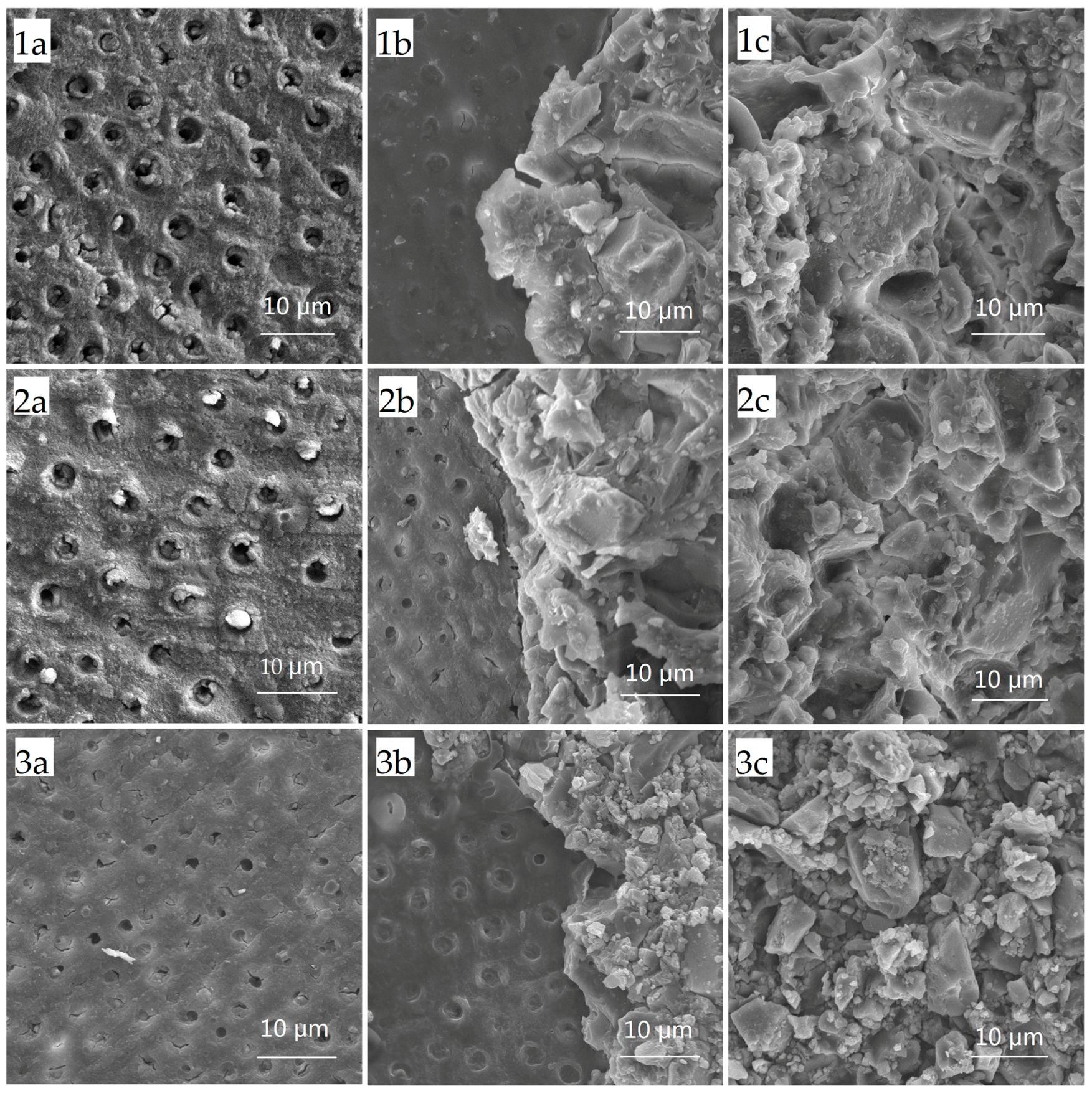
| Group | Failure Modes (n) | Total (n) | ||
|---|---|---|---|---|
| Cohesive | Adhesive | Mixed | ||
| 1 Conventional glass ionomer cements (GICs) | 4 | 2 | 10 | 16 |
| 2 CPP-ACP-modified GICs | 3 | 2 | 11 | 16 |
| 3 Resin-modified GICs | 8 | 1 | 7 | 16 |
| Mechanical Properties | Group 1 Conventional GICs | Group 2 CPP-ACP-Modified GICs | Group 3 Resin-Modified GICs | p Value | Bonferroni Comparison |
|---|---|---|---|---|---|
| Nano-hardness (GPa) | 0.85 ± 0.22 | 1.14 ± 0.21 | 0.81 ± 0.09 | 0.003 | Groups 1, 3 < Group 2 |
| Elastic moduli (GPa) | 1.70 ± 0.33 | 2.35 ± 0.44 | 1.59 ± 0.13 | <0.001 | Groups 1, 3 < Group 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, I.S.; Mei, M.L.; Zhou, Z.L.; Burrow, M.F.; Lo, E.C.-M.; Chu, C.-H. Shear Bond Strength and Remineralisation Effect of a Casein Phosphopeptide-Amorphous Calcium Phosphate-Modified Glass Ionomer Cement on Artificial “Caries-Affected” Dentine. Int. J. Mol. Sci. 2017, 18, 1723. https://doi.org/10.3390/ijms18081723
Zhao IS, Mei ML, Zhou ZL, Burrow MF, Lo EC-M, Chu C-H. Shear Bond Strength and Remineralisation Effect of a Casein Phosphopeptide-Amorphous Calcium Phosphate-Modified Glass Ionomer Cement on Artificial “Caries-Affected” Dentine. International Journal of Molecular Sciences. 2017; 18(8):1723. https://doi.org/10.3390/ijms18081723
Chicago/Turabian StyleZhao, Irene Shuping, May Lei Mei, Zhuo Long Zhou, Michael Francis Burrow, Edward Chin-Man Lo, and Chun-Hung Chu. 2017. "Shear Bond Strength and Remineralisation Effect of a Casein Phosphopeptide-Amorphous Calcium Phosphate-Modified Glass Ionomer Cement on Artificial “Caries-Affected” Dentine" International Journal of Molecular Sciences 18, no. 8: 1723. https://doi.org/10.3390/ijms18081723