TrmFO, a Fibronectin-Binding Adhesin of Mycoplasma bovis
Abstract
:1. Introduction
2. Results
2.1. Bioinformatics Analysis
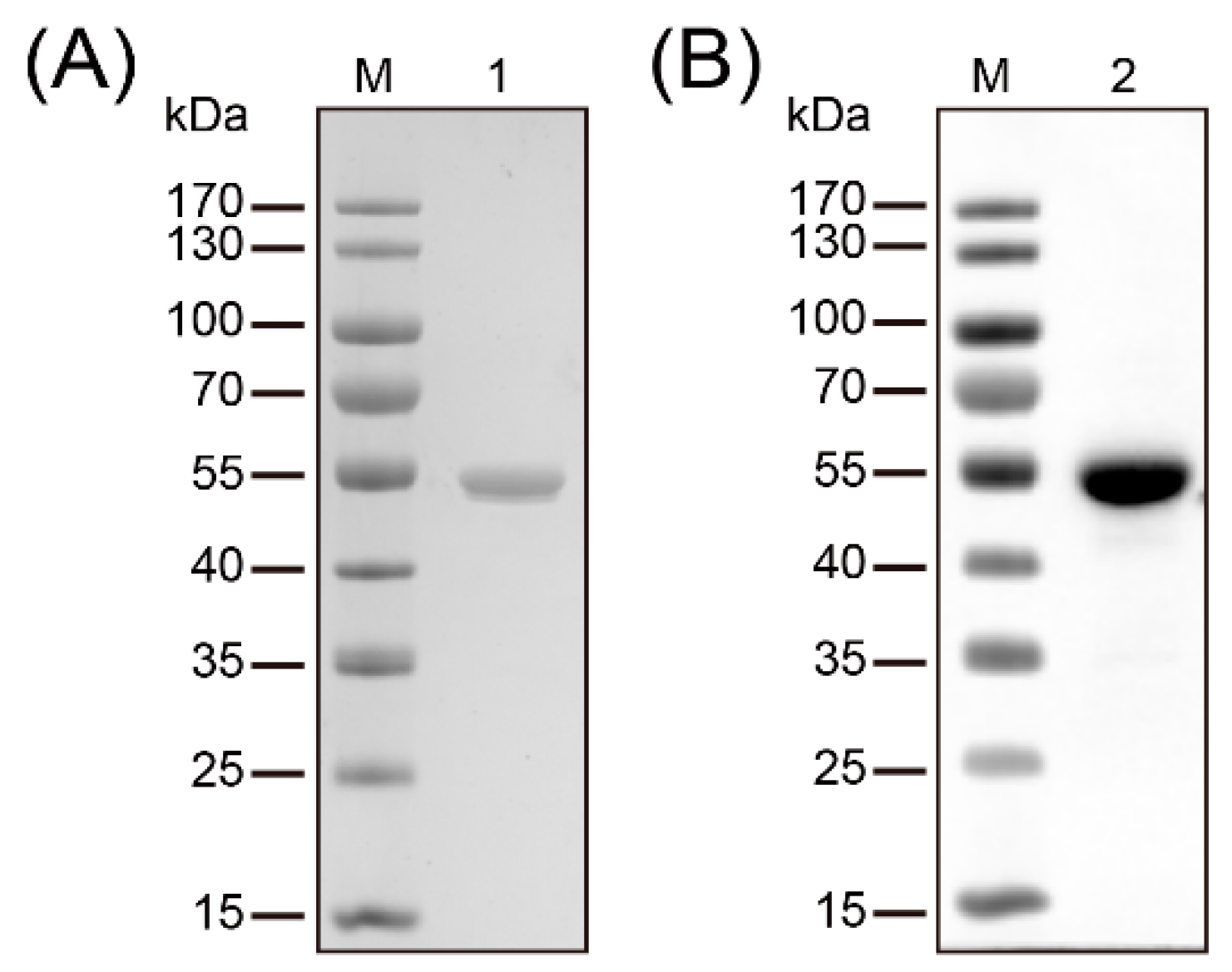
2.2. Expression and Purification of Recombinant TrmFO (rTrmFO)
2.3. Immunogenicity Analysis of rTrmFO
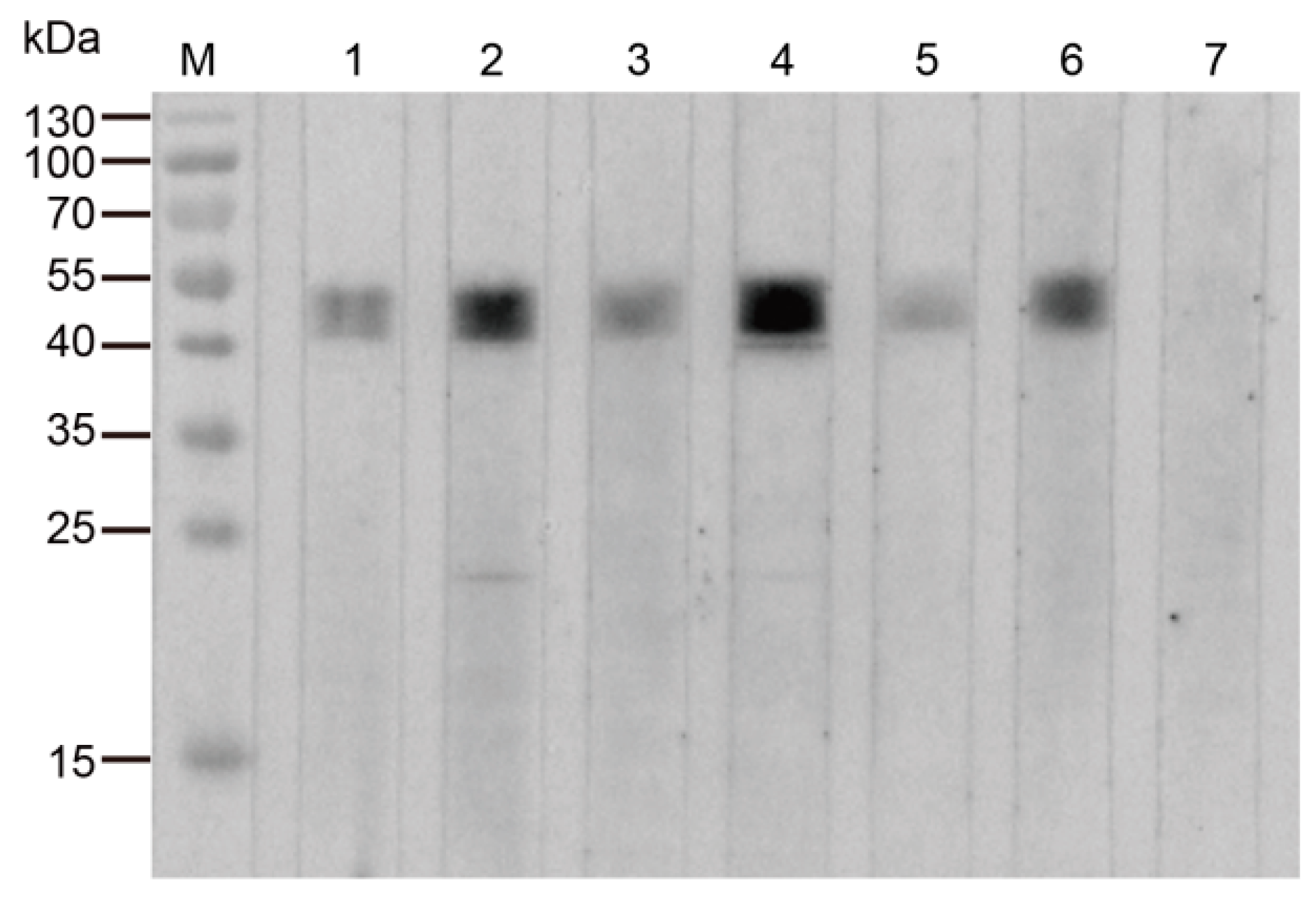
2.4. Expression of TrmFO in Different M. bovis Isolates
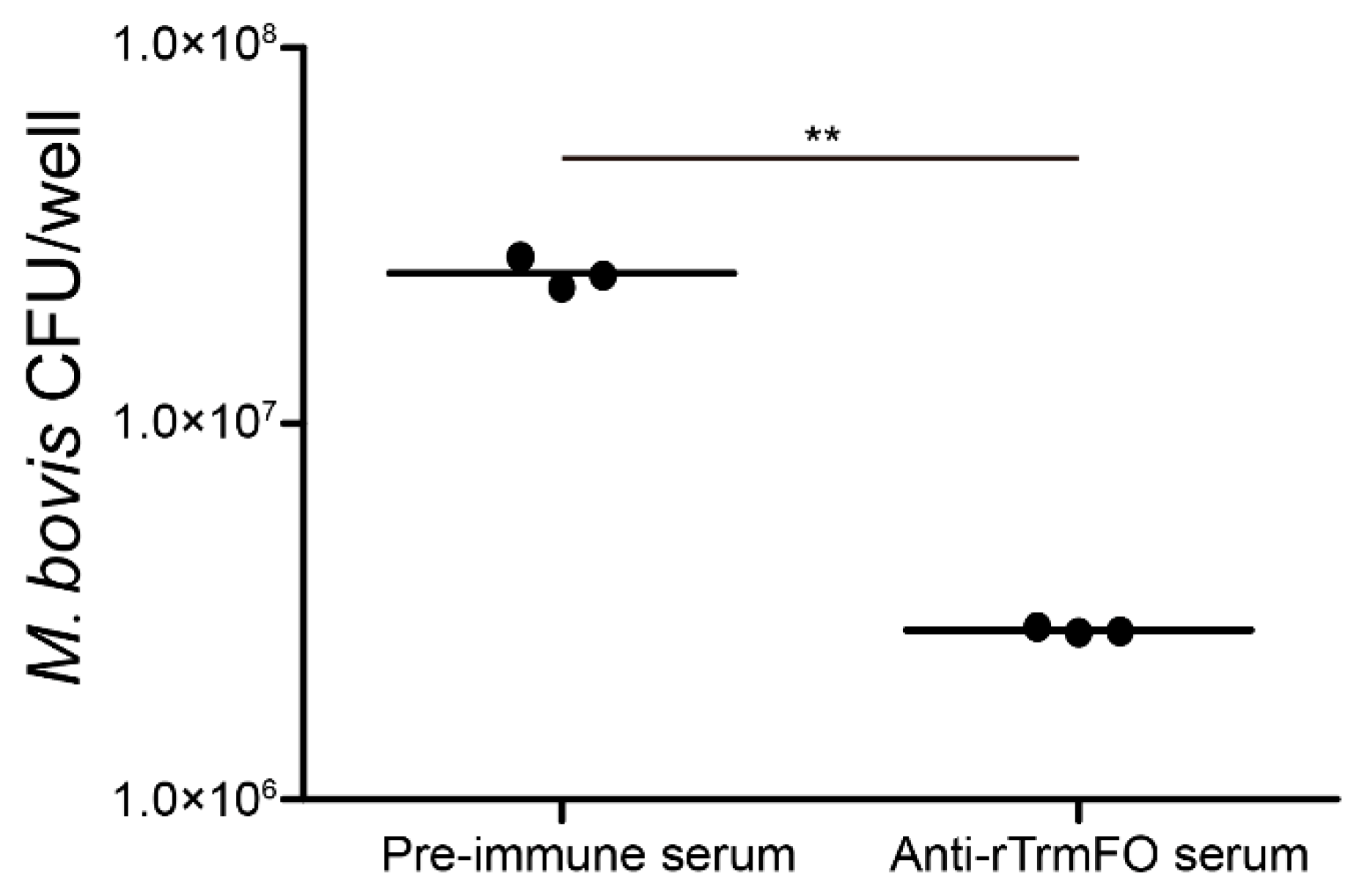
2.5. Mycoplasmacidal Activity of Rabbit Anti-rTrmFO Serum
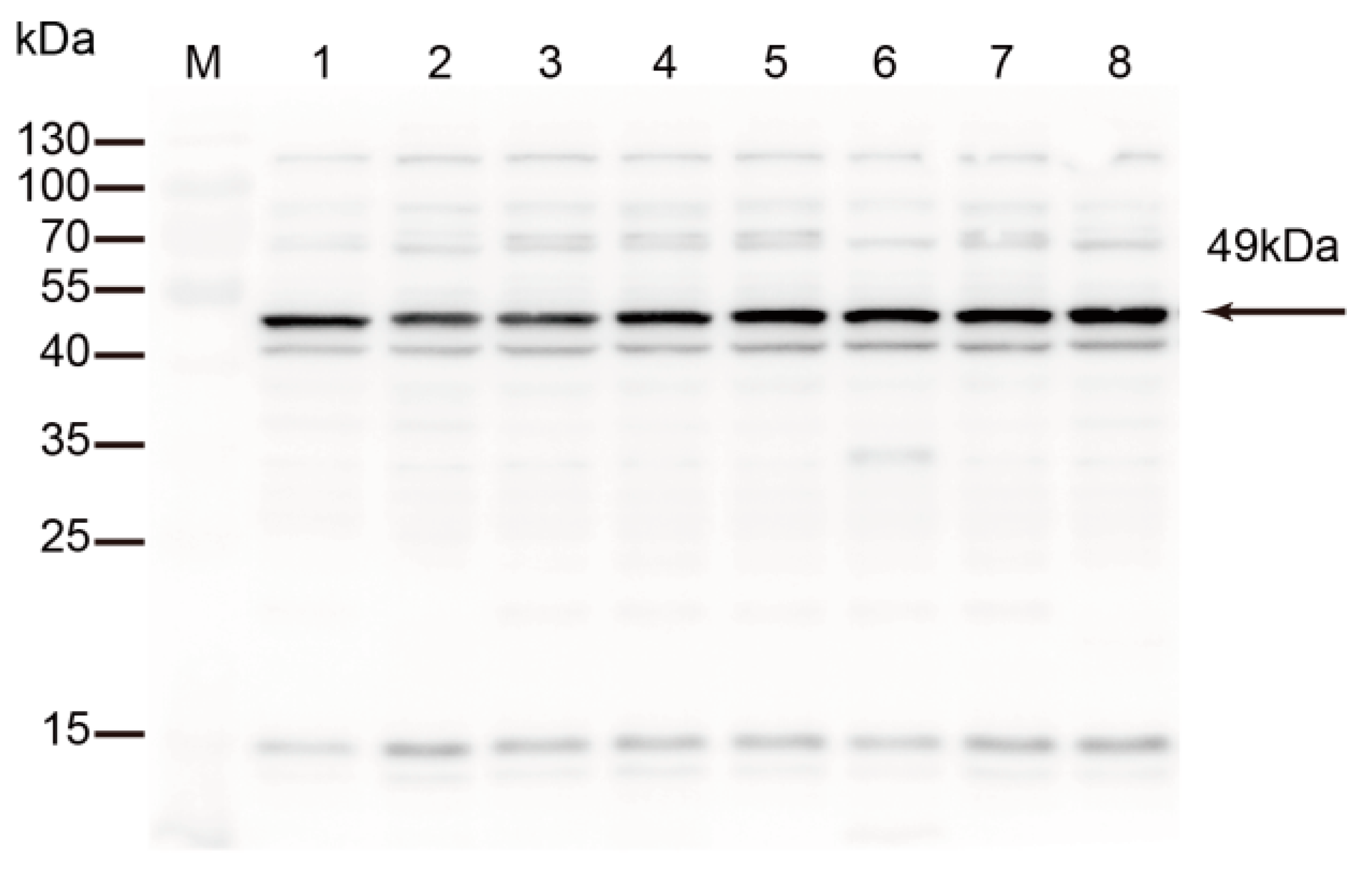
2.6. Subcellular Localization of TrmFO in M. bovis
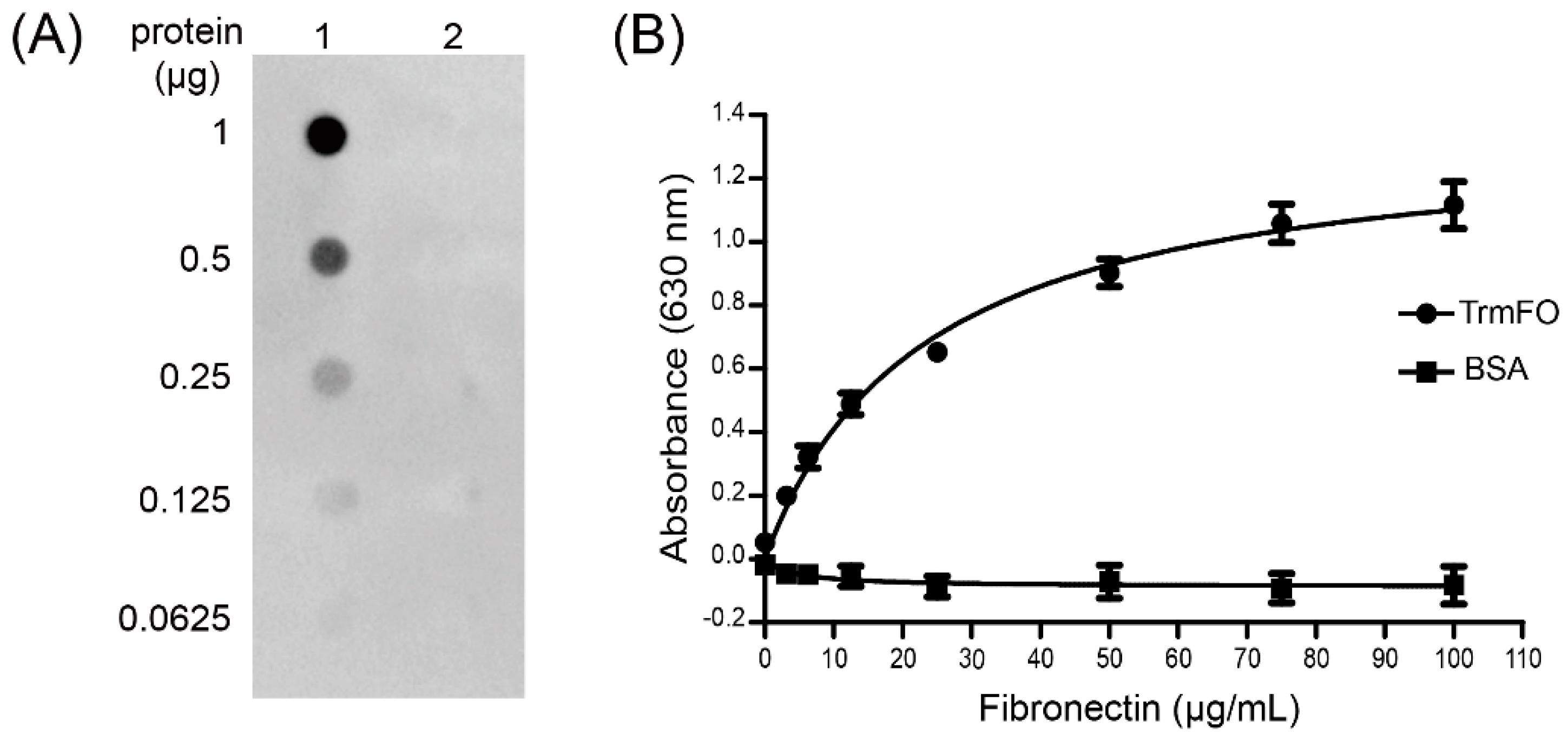
2.7. Fibronectin (Fn)-Binding Ability of rTrmFO
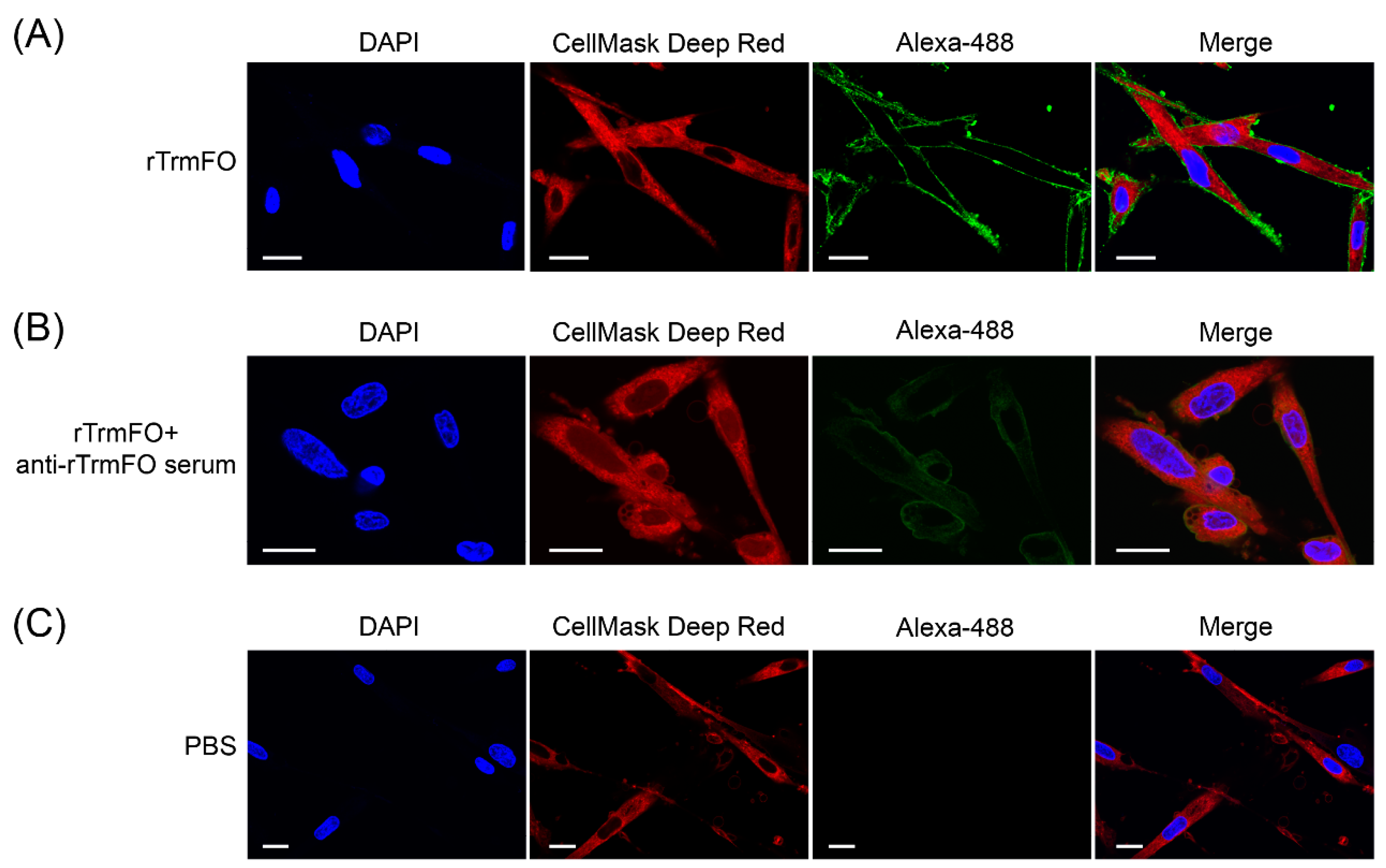
2.8. Direct Adhesion of rTrmFO to EBL Cells and Adhesion Inhibition
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Bioinformatics Analysis
4.3. Bacterial Strains, Cultivation Conditions and Cell Line
4.4. Cloning, Expression and Purification of rTrmFO
4.5. Preparation of Polyclonal Antibodies Against rTrmFO
4.6. Immunoblotting Analysis
4.7. Complement Dependent Mycoplasmacidal Assay
4.8. Subcellular Localization of M. bovis TrmFO
4.9. Fibronectin-Binding Assays
4.10. Adherence and Inhibition Assays
4.11. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pfutzner, H.; Sachse, K. Mycoplasma bovis as an agent of mastitis, pneumonia, arthritis and genital disorders in cattle. Rev. Sci. Tech. 1996, 15, 1477–1494. [Google Scholar] [CrossRef] [PubMed]
- Burki, S.; Frey, J.; Pilo, P. Virulence, persistence and dissemination of Mycoplasma bovis. Vet. Microbiol. 2015, 179, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, H.H.; Helmboldt, C.F.; Plastridge, W.N.; Stula, E.F. Bovine mastitis caused by a Mycoplasma species. Cornell Vet. 1962, 52, 582–591. [Google Scholar] [PubMed]
- Li, Y.; Zheng, H.; Liu, Y.; Jiang, Y.; Xin, J.; Chen, W.; Song, Z. The complete genome sequence of Mycoplasma bovis strain Hubei-1. PLoS ONE 2011, 6, e20999. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Guo, A.; Cui, P.; Chen, Y.; Mustafa, R.; Ba, X.; Hu, C.; Bai, Z.; Chen, X.; Shi, L.; et al. Comparative geno-plasticity analysis of Mycoplasma bovis HB0801 (Chinese isolate). PLoS ONE 2012, 7, e38239. [Google Scholar] [CrossRef] [PubMed]
- Razin, S.; Yogev, D.; Naot, Y. Molecular biology and pathogenicity of Mycoplasmas. Microbiol. Mol. Biol. Rev. 1998, 62, 1094–1156. [Google Scholar] [PubMed]
- Rottem, S. Interaction of Mycoplasmas with host cells. Physiol. Rev. 2003, 83, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Razin, S.; Jacobs, E. Mycoplasma adhesion. J. Gen. Microbiol. 1992, 138, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Sachse, K.; Grajetzki, C.; Rosengarten, R.; Hanel, I.; Heller, M.; Pfutzner, H. Mechanisms and factors involved in Mycoplasma bovis adhesion to host cells. Zent. Bakteriol. 1996, 284, 80–92. [Google Scholar] [CrossRef]
- Song, Z.; Li, Y.; Liu, Y.; Xin, J.; Zou, X.; Sun, W. α-Enolase, an adhesion-related factor of Mycoplasma bovis. PLoS ONE 2012, 7, e38836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, H.; Chen, X.; Zhu, X.; Guo, Y.; He, C.; Anwar Khan, F.; Chen, Y.; Hu, C.; Chen, H.; et al. Mycoplasma bovis NADH oxidase functions as both a NADH oxidizing and O2 reducing enzyme and an adhesin. Sci. Rep. 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Sachse, K.; Helbig, J.H.; Lysnyansky, I.; Grajetzki, C.; Muller, W.; Jacobs, E.; Yogev, D. Epitope mapping of immunogenic and adhesive structures in repetitive domains of Mycoplasma bovis variable surface lipoproteins. Infect. Immun. 2000, 68, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Li, Y.; Wang, Y.; Zhou, Y.; Liu, Y.; Xin, J. Molecular cloning and characterization of a surface-localized adhesion protein in Mycoplasma bovis Hubei-1 strain. PLoS ONE 2013, 8, e69644. [Google Scholar] [CrossRef] [PubMed]
- Joh, D.; Wann, E.R.; Kreikemeyer, B.; Speziale, P.; Hook, M. Role of fibronectin-binding MSCRAMMS in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999, 18, 211–223. [Google Scholar] [CrossRef]
- Henderson, B.; Nair, S.; Pallas, J.; Williams, M.A. Fibronectin: A multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol. Rev. 2011, 35, 147–200. [Google Scholar] [CrossRef] [PubMed]
- Schwarz-Linek, U.; Hook, M.; Potts, J.R. The molecular basis of fibronectin-mediated bacterial adherence to host cells. Mol. Microbiol. 2004, 52, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Dallo, S.F.; Kannan, T.R.; Blaylock, M.W.; Baseman, J.B. Elongation factor Tu and E1 beta subunit of pyruvate dehydrogenase complex act as fibronectin binding proteins in Mycoplasma pneumoniae. Mol. Microbiol. 2002, 46, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Grundel, A.; Jacobs, E.; Dumke, R. Interactions of surface-displayed glycolytic enzymes of Mycoplasma pneumoniae with components of the human extracellular matrix. Int. J. Med. Microbiol. 2016, 306, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.M.; Jenkins, C.; Deutscher, A.T.; Raymond, B.B.; Padula, M.P.; Tacchi, J.L.; Bogema, D.R.; Eamens, G.J.; Woolley, L.K.; Dixon, N.E.; et al. Mhp182 (P102) binds fibronectin and contributes to the recruitment of plasmin(ogen) to the Mycoplasma hyopneumoniae cell surface. Cell. Microbiol. 2012, 14, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Deutscher, A.T.; Jenkins, C.; Minion, F.C.; Seymour, L.M.; Padula, M.P.; Dixon, N.E.; Walker, M.J.; Djordjevic, S.P. Repeat regions R1 and R2 in the P97 paralogue Mhp271 of Mycoplasma hyopneumoniae bind heparin, fibronectin and porcine cilia. Mol. Microbiol. 2010, 78, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Guo, X.; Yu, S.; Ding, J.; Tan, L.; Zhang, F.; Sun, Y.; Qiu, X.; Chen, G.; Ding, C. Mycoplasma synoviae enolase is a plasminogen/fibronectin binding protein. BMC Vet. Res. 2014, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Papazisi, L.; Gorton, T.S.; Geary, S.J. Identification of fibronectin-binding proteins in Mycoplasma gallisepticum strain R. Infect. Immun. 2006, 74, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Peterhans, E.; Frey, J. RGD motif of lipoprotein T, involved in adhesion of Mycoplasma conjunctivae to lamb synovial tissue cells. J. Bacteriol. 2010, 192, 3773–3779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, X.; Chen, Y.; Mustafa, R.; Qi, J.; Chen, X.; Hu, C.; Chen, H.; Guo, A. Attenuated Mycoplasma bovis strains provide protection against virulent infection in calves. Vaccine 2014, 32, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Urbonavicius, J.; Skouloubris, S.; Myllykallio, H.; Grosjean, H. Identification of a novel gene encoding a flavin-dependent tRNA:m5U methyltransferase in bacteria—Evolutionary implications. Nucleic Acids Res. 2005, 33, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Robino, P.; Alberti, A.; Pittau, M.; Chessa, B.; Miciletta, M.; Nebbia, P.; Le Grand, D.; Rosati, S. Genetic and antigenic characterization of the surface lipoprotein P48 of Mycoplasma bovis. Vet. Microbiol. 2005, 109, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fu, P.; Wei, K.; Zhang, H.; Zhang, Y.; Xu, J.; Jiang, F.; Liu, X.; Xu, W.; Wu, W. Identification of novel immunogenic proteins from Mycoplasma bovis and establishment of an indirect ELISA based on recombinant E1 beta subunit of the pyruvate dehydrogenase complex. PLoS ONE 2014, 9, e88328. [Google Scholar] [CrossRef] [PubMed]
- Perez-Casal, J.; Prysliak, T. Detection of antibodies against the Mycoplasma bovis glyceraldehyde-3-phosphate dehydrogenase protein in beef cattle. Microb. Pathog. 2007, 43, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Faisal, M.; Chao, J.; Liu, K.; Chen, X.; Zhao, G.; Menghwar, H.; Zhang, H.; Zhu, X.; Rasheed, M.A.; et al. Immunoproteomic identification of MbovP579, a promising diagnostic biomarker for serological detection of Mycoplasma bovis infection. Oncotarget 2016, 7, 39376–39395. [Google Scholar] [CrossRef] [PubMed]
- Scherm, B.; Gerlach, G.F.; Runge, M. Analysis of heat shock protein 60 encoding genes of Mycoplasmas and investigations concerning their role in immunity and infection. Vet. Microbiol. 2002, 89, 141–150. [Google Scholar] [CrossRef]
- Wawegama, N.K.; Browning, G.F.; Kanci, A.; Marenda, M.S.; Markham, P.F. Development of a recombinant protein-based enzyme-linked immunosorbent assay for diagnosis of Mycoplasma bovis infection in cattle. Clin. Vaccine Immunol. 2014, 21, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Prysliak, T.; van der Merwe, J.; Perez-Casal, J. Vaccination with recombinant Mycoplasma bovis GAPDH results in a strong humoral immune response but does not protect feedlot cattle from an experimental challenge with M. bovis. Microb. Pathog. 2013, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, V.; Korhonen, T.K. Dancing to another tune-adhesive moonlighting proteins in bacteria. Biology 2014, 3, 178–204. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Martin, A. Bacterial virulence in the moonlight: Multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect. Immun. 2011, 79, 3476–3491. [Google Scholar] [CrossRef] [PubMed]
- Rahi, A.; Matta, S.K.; Dhiman, A.; Garhyan, J.; Gopalani, M.; Chandra, S.; Bhatnagar, R. Enolase of Mycobacterium tuberculosis is a surface exposed plasminogen binding protein. Biochim. Biophys. Acta 2017, 1861, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Rohde, M.; Chhatwal, G.S.; Hammerschmidt, S. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 2001, 40, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Kulshreshtha, P.; Bambah Mukku, D.; Bhatnagar, R. α-Enolase binds to human plasminogen on the surface of Bacillus anthracis. Biochim. Biophys. Acta 2008, 1784, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, S.; Shen, X.; Chen, D.; Qiu, X.; Song, C.; Ding, C. The Mycoplasma gallisepticum α-enolase is cell surface-exposed and mediates adherence by binding to chicken plasminogen. Microb. Pathog. 2011, 51, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, S.A.; Sokoli, A.; Felder, K.M.; Wittenbrink, M.M.; Schwarzenbach, S.; Guhl, B.; Hoelzle, K.; Hoelzle, L.E. The surface-localised α-enolase of Mycoplasma suis is an adhesion protein. Vet. Microbiol. 2012, 156, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavlovich, A.; Rechnitzer, H.; Rottem, S. α-Enolase resides on the cell surface of Mycoplasma fermentans and binds plasminogen. Infect. Immun. 2007, 75, 5716–5719. [Google Scholar] [CrossRef] [PubMed]
- Muchnik, L.; Adawi, A.; Ohayon, A.; Dotan, S.; Malka, I.; Azriel, S.; Shagan, M.; Portnoi, M.; Kafka, D.; Nahmani, H.; et al. NADH oxidase functions as an adhesin in Streptococcus pneumoniae and elicits a protective immune response in mice. PLoS ONE 2013, 8, e61128. [Google Scholar] [CrossRef]
- Adamu, J.Y.; Wawegama, N.K.; Browning, G.F.; Markham, P.F. Membrane proteins of Mycoplasma bovis and their role in pathogenesis. Res. Vet. Sci. 2013, 95, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Giron, J.A.; Lange, M.; Baseman, J.B. Adherence, fibronectin binding, and induction of cytoskeleton reorganization in cultured human cells by Mycoplasma penetrans. Infect. Immun. 1996, 64, 197–208. [Google Scholar] [PubMed]
- Balasubramanian, S.; Kannan, T.R.; Baseman, J.B. The surface-exposed carboxyl region of Mycoplasma pneumoniae elongation factor Tu interacts with fibronectin. Infect. Immun. 2008, 76, 3116–3123. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.A.; Qi, J.; Zhu, X.; Chenfei, H.; Menghwar, H.; Khan, F.A.; Zhao, G.; Zubair, M.; Hu, C.; Chen, Y.; et al. Comparative genomics of Mycoplasma bovis strains reveals that decreased virulence with increasing passages might correlate with potential virulence-related factors. Front. Cell. Infect. Microbiol. 2017, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- BLASTP. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi/ (accessed on 2 March 2017).
- SignalP. Available online: http://www.cbs.dtu.dk/services/SignalP/ (accessed on 2 March 2017).
- TMHMM. Available online: http://www.cbs.dtu.dk/services/TMHMM/ (accessed on 2 March 2017).
- CELLO. Available online: http://cello.life.nctu.edu.tw/ (accessed on 2 March 2017).
- Alvarez, R.A.; Blaylock, M.W.; Baseman, J.B. Surface localized glyceraldehyde-3-phosphate dehydrogenase of Mycoplasma genitalium binds mucin. Mol. Microbiol. 2003, 48, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Wilton, J.L.; Minion, F.C.; Falconer, L.; Walker, M.J.; Djordjevic, S.P. Two domains within the Mycoplasma hyopneumoniae cilium adhesin bind heparin. Infect. Immun. 2006, 74, 481–487. [Google Scholar] [CrossRef] [PubMed]


| Species | Identity | NCBI Protein ID |
|---|---|---|
| Mycoplasma bovis Hubei-1 | 100% | AEI89864.1 |
| Mycoplasma bovis CQ-W70 | 100% | AIA33742.1 |
| Mycoplasma bovis PG45 | 98% | ADR24753.1 |
| Mycoplasma agalactiae PG2 | 80% | CAL58845.1 |
| Mycoplasma putrefaciens KS1 | 56% | AEM68895.1 |
| Mycoplasma sp. HU0214 | 55% | KNG79513.1 |
| Mycoplasma yeastsii GM274B | 54% | AJM71909.1 |
| Mycoplasma capricolum subsp. capricolum 14232 | 48% | KEZ18460.1 |
| Mycoplasma mycoides subsp. capri str. GM12 | 48% | ACU78480.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhu, H.; Wang, J.; Huang, J.; Khan, F.A.; Zhang, J.; Guo, A.; Chen, X. TrmFO, a Fibronectin-Binding Adhesin of Mycoplasma bovis. Int. J. Mol. Sci. 2017, 18, 1732. https://doi.org/10.3390/ijms18081732
Guo Y, Zhu H, Wang J, Huang J, Khan FA, Zhang J, Guo A, Chen X. TrmFO, a Fibronectin-Binding Adhesin of Mycoplasma bovis. International Journal of Molecular Sciences. 2017; 18(8):1732. https://doi.org/10.3390/ijms18081732
Chicago/Turabian StyleGuo, Yongpeng, Hongmei Zhu, Jiayao Wang, Jing Huang, Farhan Anwar Khan, Jingjing Zhang, Aizhen Guo, and Xi Chen. 2017. "TrmFO, a Fibronectin-Binding Adhesin of Mycoplasma bovis" International Journal of Molecular Sciences 18, no. 8: 1732. https://doi.org/10.3390/ijms18081732




