A Murine Model of Persistent Inflammation, Immune Suppression, and Catabolism Syndrome
Abstract
:1. Introduction
2. Results
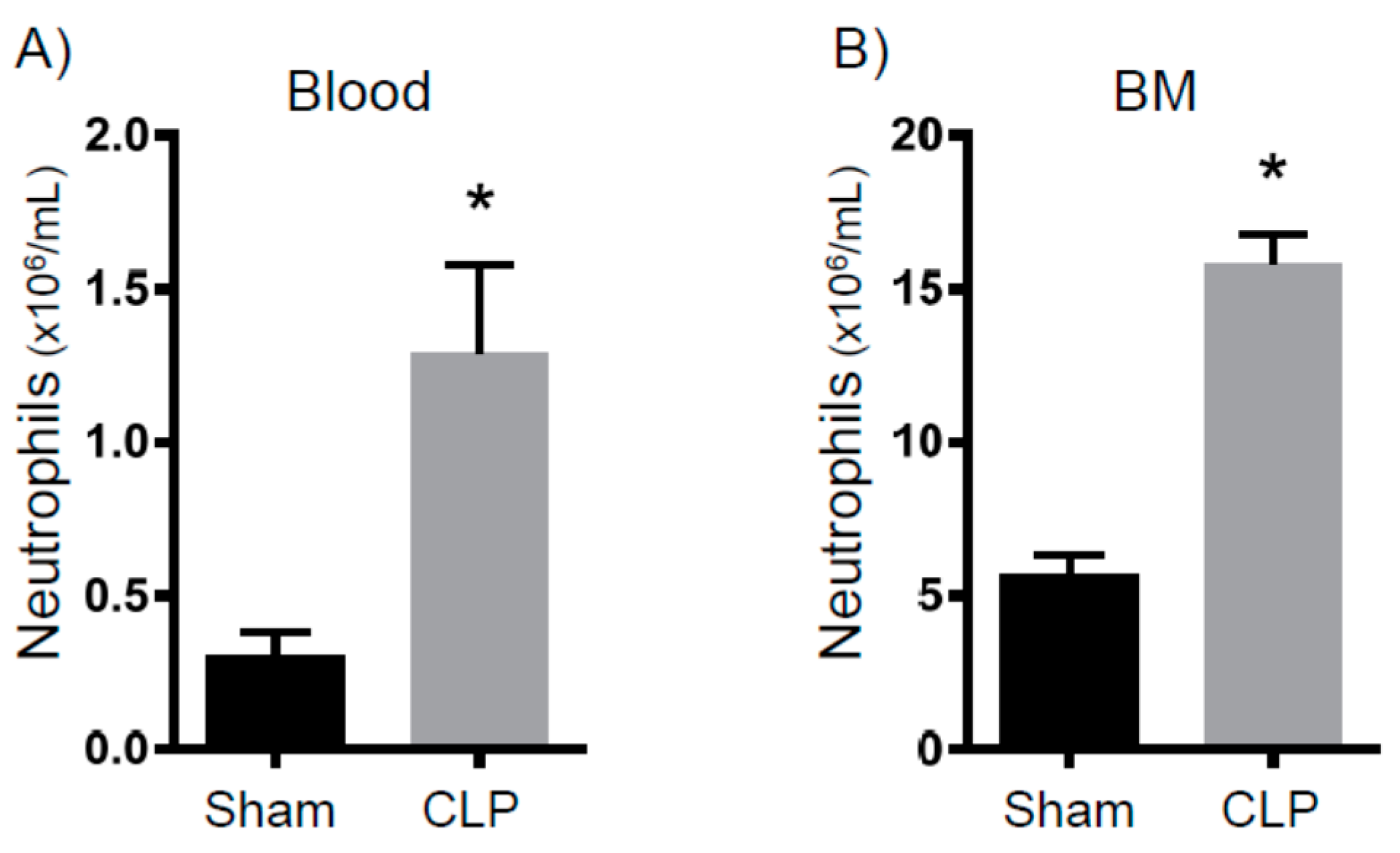
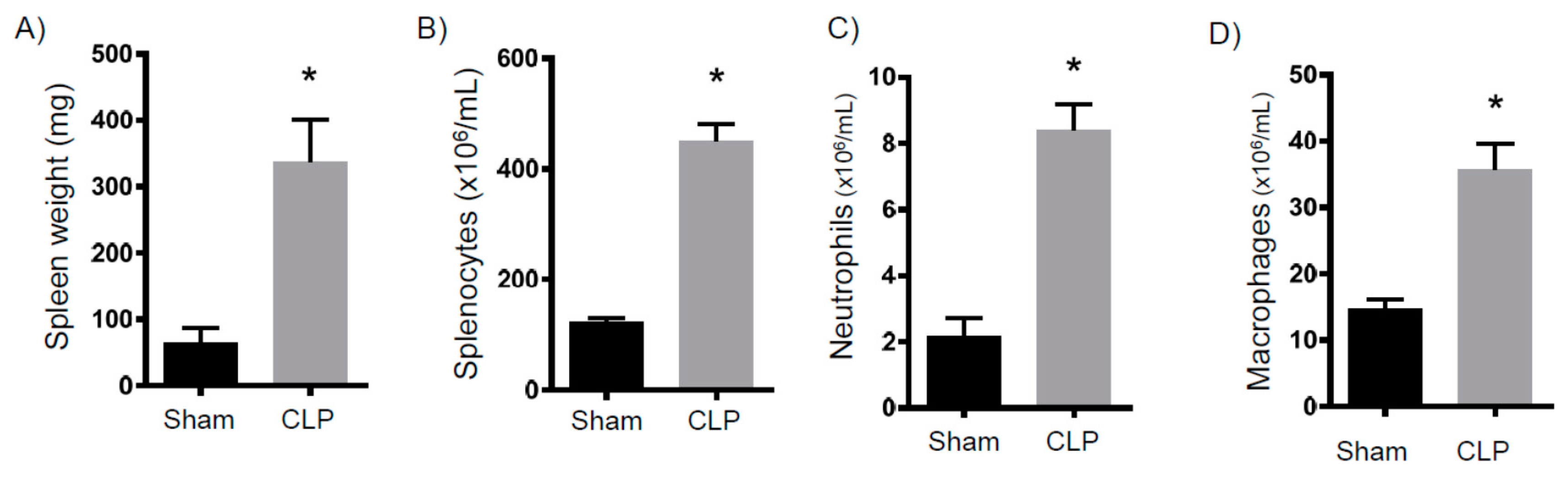
2.1. Persistent Inflammation
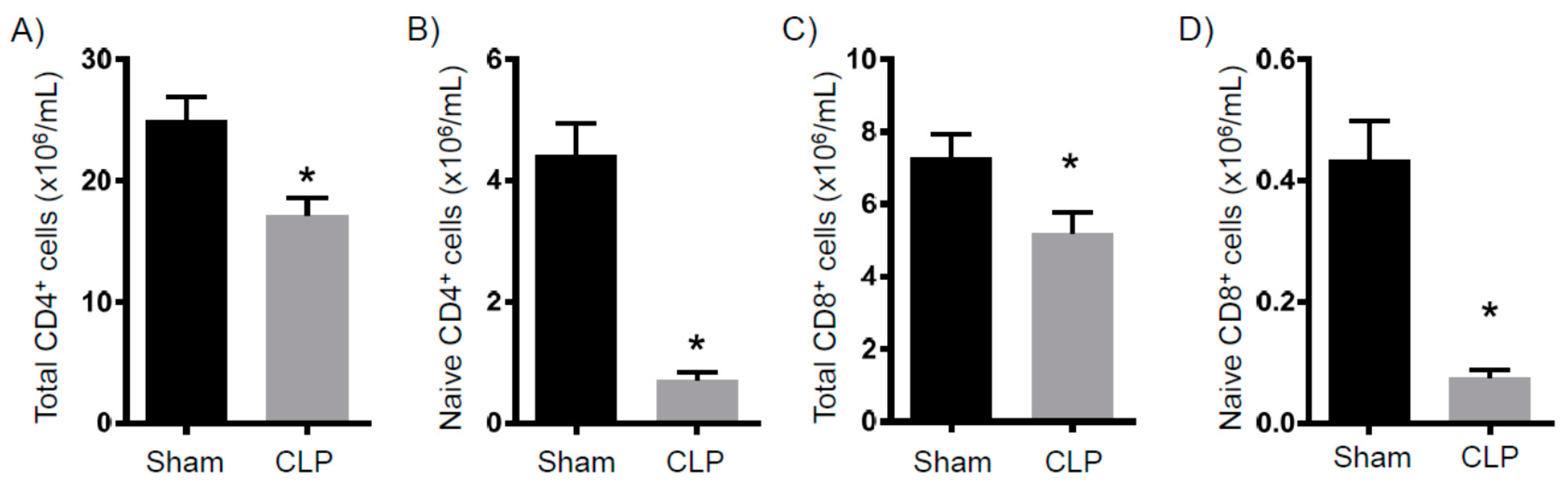
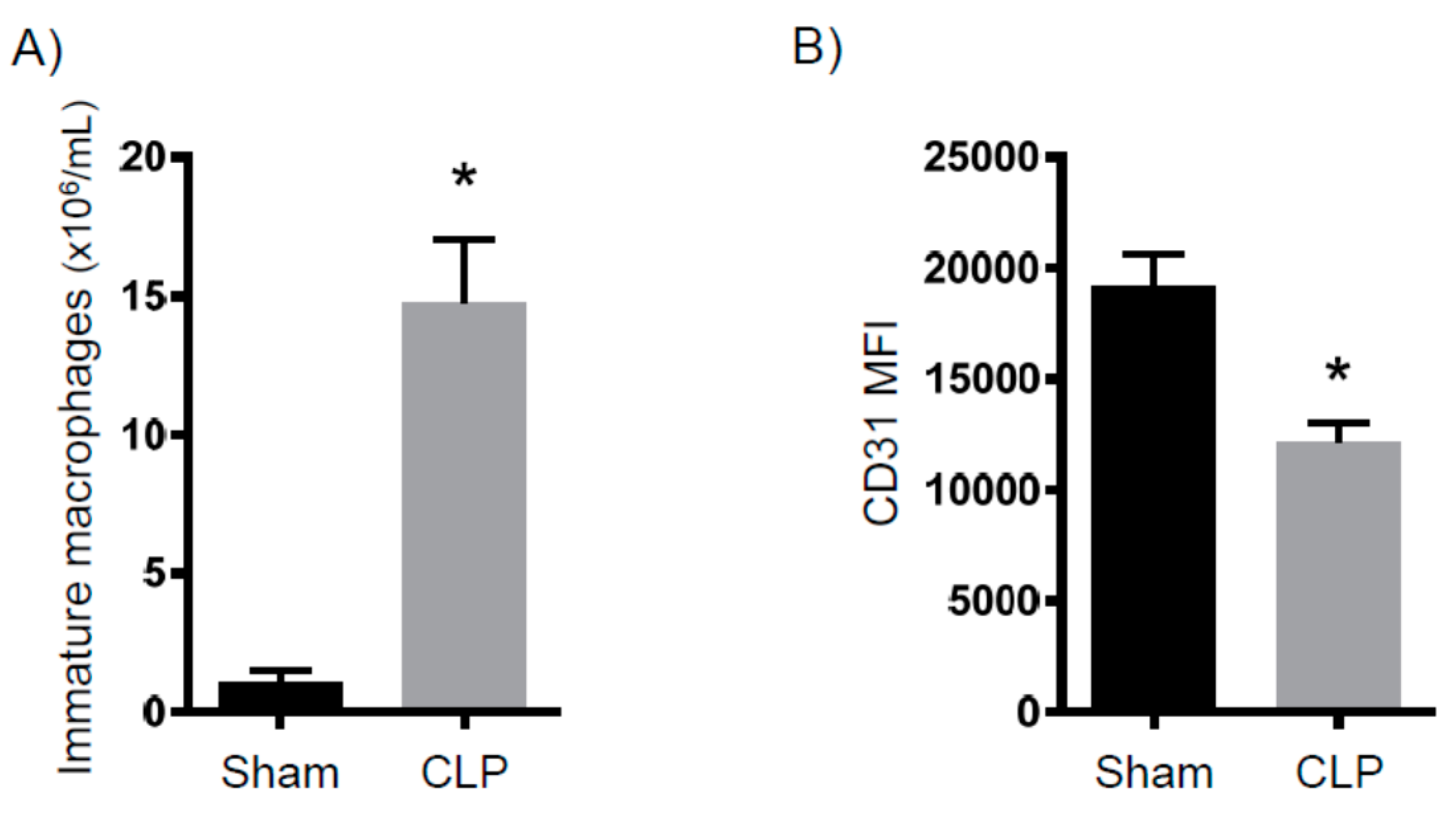
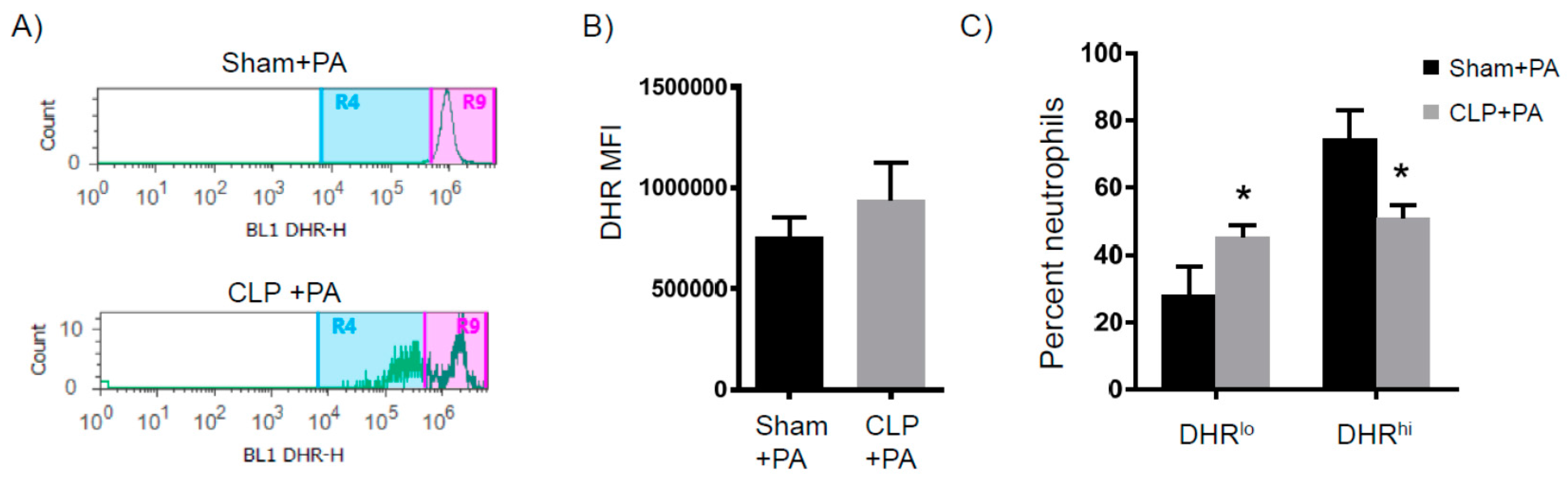
2.2. Immune Suppression
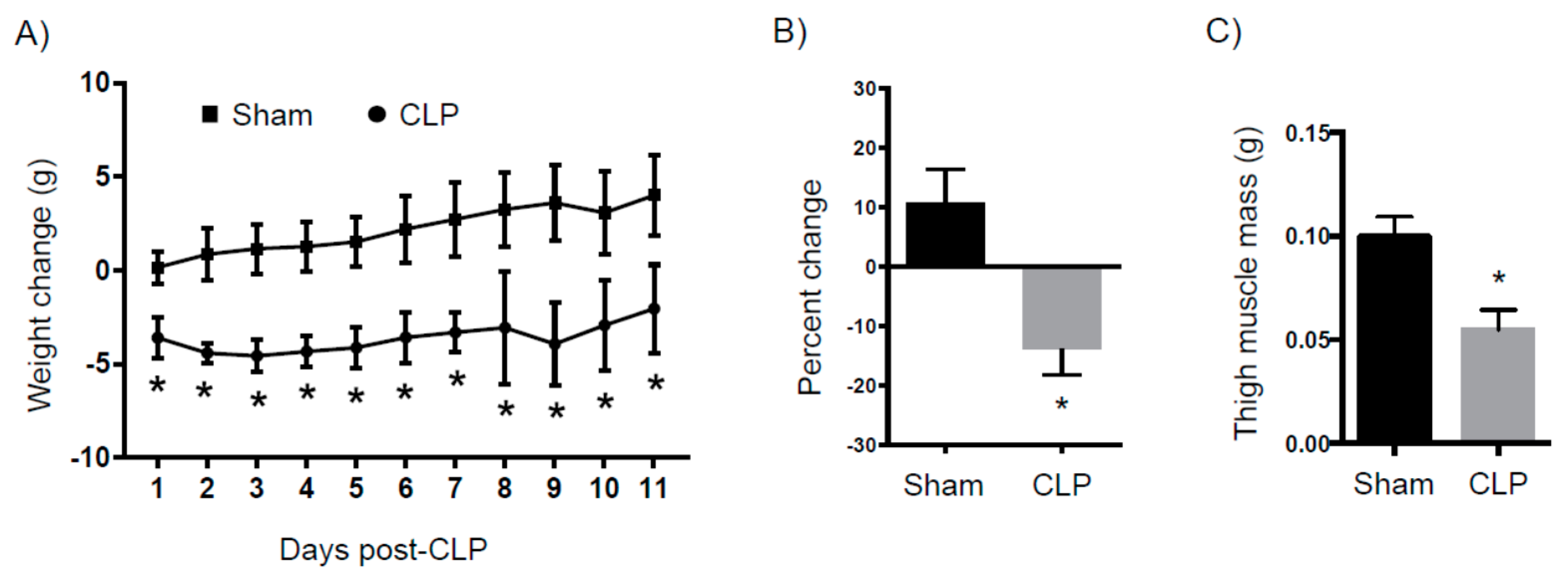
2.3. Catabolism
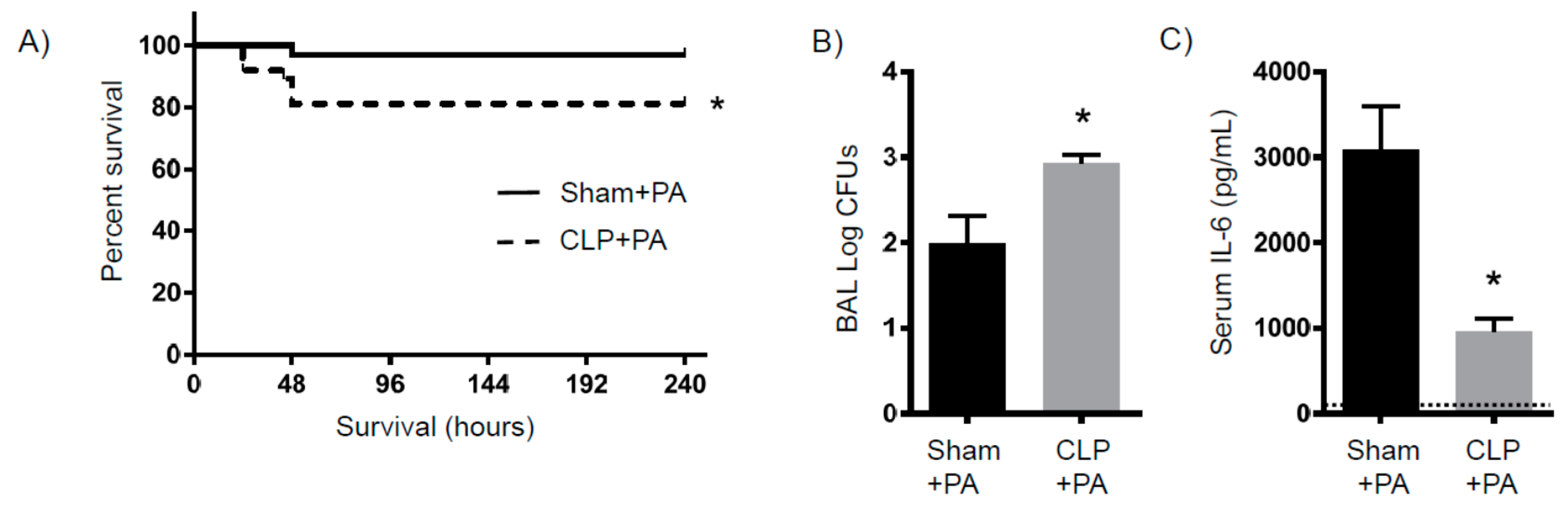
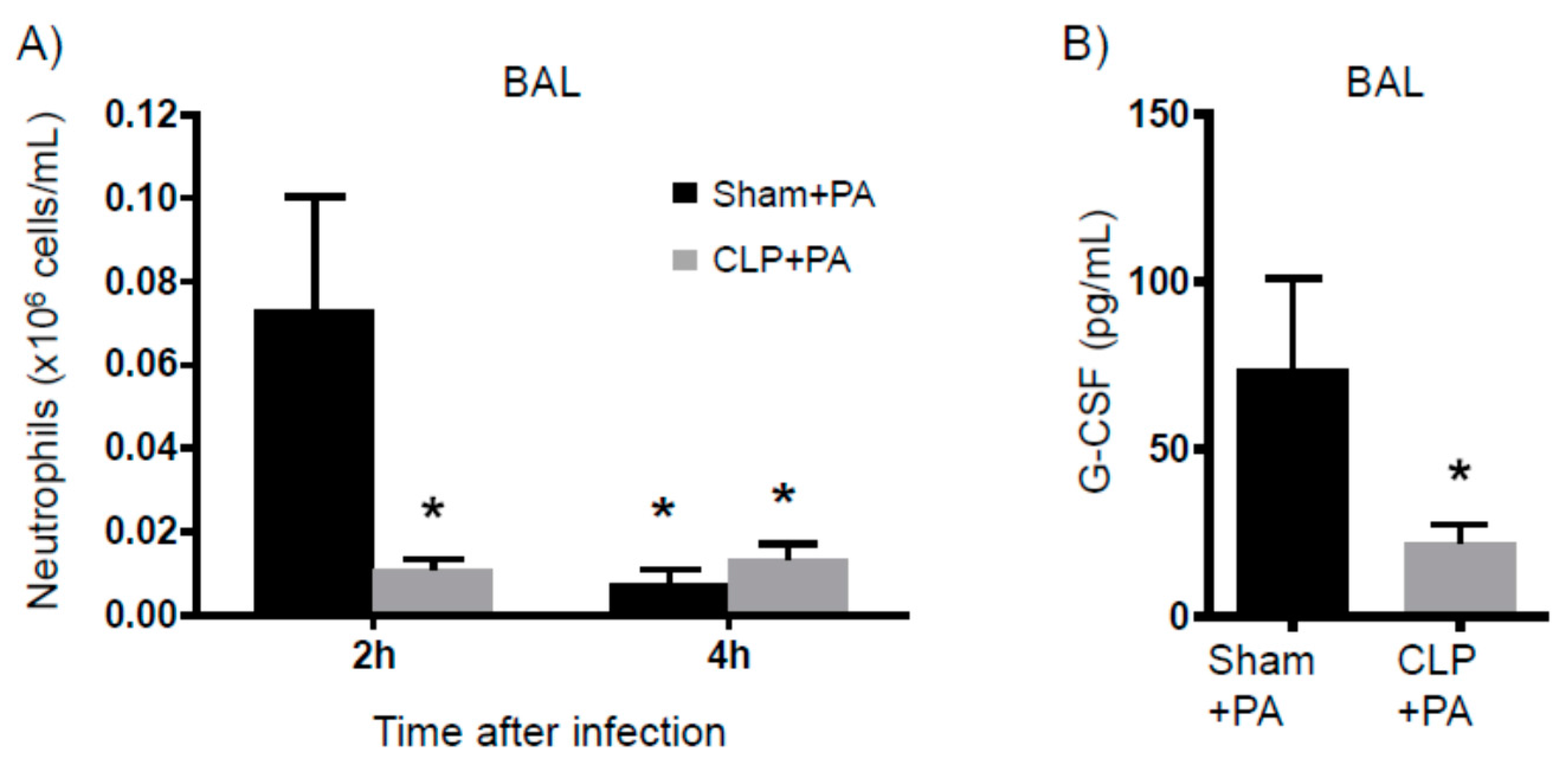
2.4. Susceptibility to Infection
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cecal Ligation and Puncture
4.3. Bacterial Preparation
4.4. Bronchoalveolar Lavage (BAL)
4.5. Bacterial Counts
4.6. Flow Cytometry
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lamas, D. Chronic critical illness. N. Engl. J. Med. 2014, 370, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.S.; Bach, P.B. The epidemiology and costs of chronic critical illness. Crit. Care Clin. 2002, 18, 461–476. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.D.; Moore, F.A. Persistent inflammation, immunosuppression, and catabolism: Evolution of multiple organ dysfunction. Surg. Infect. (Larchmt) 2016, 17, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.C.; Brakenridge, S.C.; Moldawer, L.L.; Moore, F.A. Persistent inflammation, immunosuppression and catabolism syndrome. Crit. Care Clin. 2017, 33, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Vanzant, E.L.; Lopez, C.M.; Ozrazgat-Baslanti, T.; Ungaro, R.; Davis, R.; Cuenca, A.G.; Gentile, L.F.; Nacionales, D.C.; Cuenca, A.L.; Bihorac, A.; et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J. Trauma Acute Care Surg. 2014, 76, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Schmieg, R.E., Jr.; Hui, J.J.; Chang, K.C.; Osborne, D.F.; Freeman, B.D.; Cobb, J.P.; Buchman, T.G.; et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001, 166, 6952–6963. [Google Scholar] [CrossRef] [PubMed]
- Kasten, K.R.; Tschop, J.; Goetzman, H.S.; England, L.G.; Dattilo, J.R.; Cave, C.M.; Seitz, A.P.; Hildeman, D.A.; Caldwell, C.C. T-cell activation differentially mediates the host response to sepsis. Shock 2010, 34, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Tschop, J.; Martignoni, A.; Goetzman, H.S.; Choi, L.G.; Wang, Q.; Noel, J.G.; Ogle, C.K.; Pritts, T.A.; Johannigman, J.A.; Lentsch, A.B.; et al. Gammadelta T cells mitigate the organ injury and mortality of sepsis. J. Leukoc. Biol. 2008, 83, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Moldawer, L.L. The origins of cachexia in acute and chronic inflammatory diseases. Nutr. Clin. Pract. 2006, 21, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, W.; Yang, Y.; Wang, Y.; Peng, T.; Chang, J.; Caldwell, C.C.; Zingarelli, B.; Fan, G.C. Loss of duplexmir-223 (5p and 3p) aggravates myocardial depression and mortality in polymicrobial sepsis. Biochim. Biophys. Acta 2014, 1842, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Otto, G.P.; Sossdorf, M.; Claus, R.A.; Rodel, J.; Menge, K.; Reinhart, K.; Bauer, M.; Riedemann, N.C. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit. Care 2011, 15, R183. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Hotchkiss, R.S. Desperate times call for desperate measures: Self-cannibalism is protective during sepsis. Crit. Care Med. 2017, 45, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Chang, K.C.; Swanson, P.E.; Tinsley, K.W.; Hui, J.J.; Klender, P.; Xanthoudakis, S.; Roy, S.; Black, C.; Grimm, E.; et al. Caspase inhibitors improve survival in sepsis: A critical role of the lymphocyte. Nat. Immunol. 2000, 1, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Noel, J.G.; Osterburg, A.; Wang, Q.; Guo, X.; Byrum, D.; Schwemberger, S.; Goetzman, H.; Caldwell, C.C.; Ogle, C.K. Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock 2007, 28, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Boomer, J.S.; Green, J.M.; Hotchkiss, R.S. The changing immune system in sepsis: Is individualized immuno-modulatory therapy the answer? Virulence 2014, 5, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, A.G.; Delano, M.J.; Kelly-Scumpia, K.M.; Moreno, C.; Scumpia, P.O.; Laface, D.M.; Heyworth, P.G.; Efron, P.A.; Moldawer, L.L. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol. Med. 2011, 17, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Qin, C.; Shu, Q. Myeloid-derived suppressor cells in sepsis. Biomed. Res. Int. 2014, 2014, 598654. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, O.; Reid, M.B.; van den Berghe, G.; Vanhorebeek, I.; Hermans, G.; Rich, M.M.; Larsson, L. The sick and the weak: Neuropathies/myopathies in the critically ill. Physiol. Rev. 2015, 95, 1025–1109. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Rosenthal, M.D.; Moore, F.A. Persistent inflammatory, immunosuppressed, catabolic syndrome (PICS): A new phenotype of multiple organ failure. J. Adv. Nutr. Hum. Metab. 2015, 1. [Google Scholar] [CrossRef]
- Shindo, Y.; Fuchs, A.G.; Davis, C.G.; Eitas, T.; Unsinger, J.; Burnham, C.D.; Green, J.M.; Morre, M.; Bochicchio, G.V.; Hotchkiss, R.S. Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia. J. Leukoc. Biol. 2017, 101, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Alves-Filho, J.C.; de Freitas, A.; Spiller, F.; Souto, F.O.; Cunha, F.Q. The role of neutrophils in severe sepsis. Shock 2008, 30, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chiswick, E.L.; Mella, J.R.; Bernardo, J.; Remick, D.G. Acute-phase deaths from murine polymicrobial sepsis are characterized by innate immune suppression rather than exhaustion. J. Immunol. 2015, 195, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Kasten, K.R.; Prakash, P.S.; Unsinger, J.; Goetzman, H.S.; England, L.G.; Cave, C.M.; Seitz, A.P.; Mazuski, C.N.; Zhou, T.T.; Morre, M.; et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect. Immun. 2010, 78, 4714–4722. [Google Scholar] [CrossRef] [PubMed]
- Unsinger, J.; McGlynn, M.; Kasten, K.R.; Hoekzema, A.S.; Watanabe, E.; Muenzer, J.T.; McDonough, J.S.; Tschoep, J.; Ferguson, T.A.; McDunn, J.E.; et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J. Immunol. 2010, 184, 3768–3779. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Davis, R.; Sadat, E.L.; Collins, J.; Sternweis, P.C.; Yuan, D.; Jiang, L.I. Distinct roles of adenylyl cyclase vii in regulating the immune responses in mice. J. Immunol. 2010, 185, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argiles, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by special interest groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, D.W. Metabolic response to severe surgical illness: Overview. World J. Surg. 2000, 24, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Klaude, M.; Mori, M.; Tjader, I.; Gustafsson, T.; Wernerman, J.; Rooyackers, O. Protein metabolism and gene expression in skeletal muscle of critically ill patients with sepsis. Clin. Sci. (Lond) 2012, 122, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.; Gabrielli, A.; Moore, F. The evolution of nutritional support in long term icu patients: From multisystem organ failure to persistent inflammation immunosuppression catabolism syndrome. Minerva Anestesiol. 2016, 82, 84–96. [Google Scholar] [PubMed]
- Baker, C.C.; Chaudry, I.H.; Gaines, H.O.; Baue, A.E. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery 1983, 94, 331–335. [Google Scholar] [PubMed]
- Piersma, F.E.; Daemen, M.A.; Bogaard, A.E.; Buurman, W.A. Interference of pain control employing opioids in in vivo immunological experiments. Lab Anim. 1999, 33, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.C.; Seitz, A.P.; Edwards, M.J.; Gulbins, E.; Caldwell, C.C. Frontline science: Sphingosine rescues burn-injured mice from pulmonary Pseudomonas aeruginosa infection. J. Leukoc. Biol. 2016, 100, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, A.; Tschop, J.; Goetzman, H.S.; Choi, L.G.; Reid, M.D.; Johannigman, J.A.; Lentsch, A.B.; Caldwell, C.C. CD4-expressing cells are early mediators of the innate immune system during sepsis. Shock 2008, 29, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.L., 3rd; Rice, T.C.; Xia, B.T.; Boone, K.I.; Green, E.A.; Gulbins, E.; Caldwell, C.C. Amitriptyline usage exacerbates the immune suppression following burn injury. Shock 2016, 46, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.C.; Armocida, S.M.; Kuethe, J.W.; Midura, E.F.; Jain, A.; Hildeman, D.A.; Healy, D.P.; Gulbins, E.; Caldwell, C.C. Burn injury influences the T cell homeostasis in a butyrate-acid sphingomyelinase dependent manner. Cell Immunol. 2017, 313, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.L., 3rd; Midura, E.F.; Prakash, P.S.; Rice, T.C.; Kunz, N.; Kalies, K.; Caldwell, C.C. Neutrophil derived microparticles increase mortality and the counter-inflammatory response in a murine model of sepsis. Biochim. Biophys. Acta 2017. [Google Scholar] [CrossRef] [PubMed]
- Kuethe, J.W.; Midura, E.F.; Rice, T.C.; Caldwell, C.C. Peritoneal wash contents used to predict mortality in a murine sepsis model. J. Surg. Res. 2015, 199, 211–219. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugh, A.M.; Auteri, N.J.; Goetzman, H.S.; Caldwell, C.C.; Nomellini, V. A Murine Model of Persistent Inflammation, Immune Suppression, and Catabolism Syndrome. Int. J. Mol. Sci. 2017, 18, 1741. https://doi.org/10.3390/ijms18081741
Pugh AM, Auteri NJ, Goetzman HS, Caldwell CC, Nomellini V. A Murine Model of Persistent Inflammation, Immune Suppression, and Catabolism Syndrome. International Journal of Molecular Sciences. 2017; 18(8):1741. https://doi.org/10.3390/ijms18081741
Chicago/Turabian StylePugh, Amanda M., Nicholas J. Auteri, Holly S. Goetzman, Charles C. Caldwell, and Vanessa Nomellini. 2017. "A Murine Model of Persistent Inflammation, Immune Suppression, and Catabolism Syndrome" International Journal of Molecular Sciences 18, no. 8: 1741. https://doi.org/10.3390/ijms18081741



