Artemisia Iwayomogi Extract Attenuates High-Fat Diet-Induced Hypertriglyceridemia in Mice: Potential Involvement of the Adiponectin-AMPK Pathway and Very Low Density Lipoprotein Assembly in the Liver
Abstract
:1. Introduction
2. Results
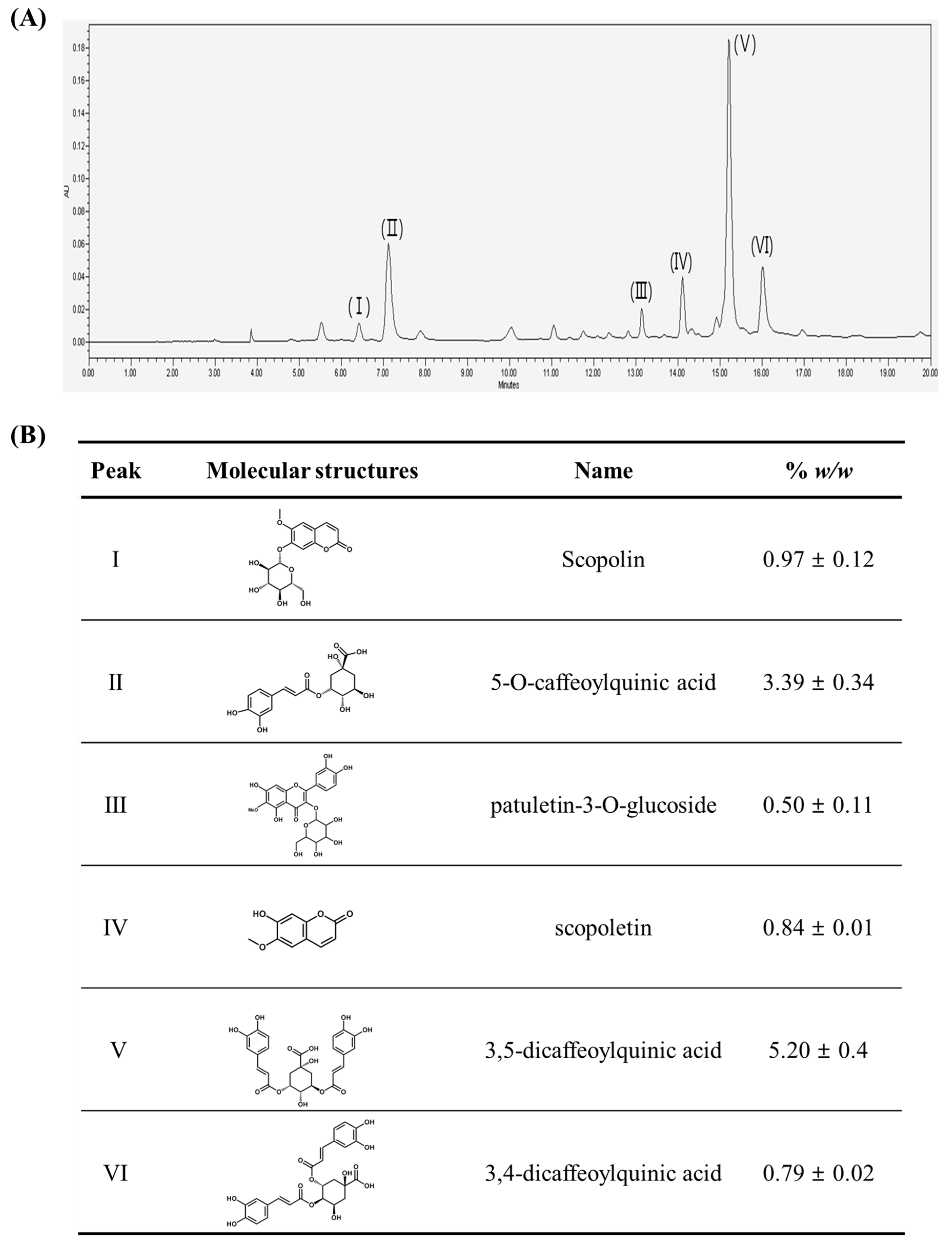
2.1. Chromatographic Analysis of A. iwayomogi Extract (AI)
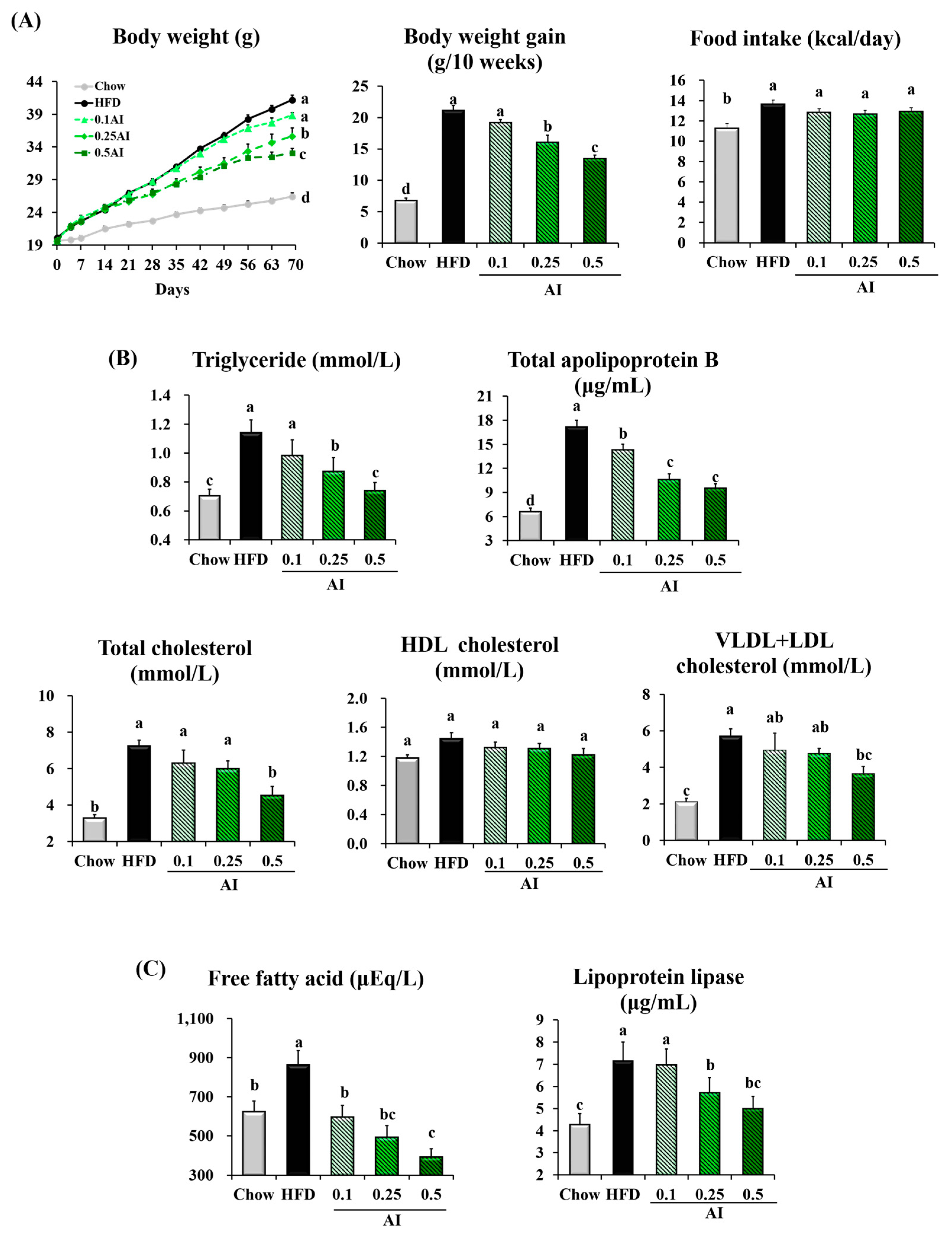
2.2. AI Attenuates Hypertriglyceridemia in Mice Fed a High-Fat Diet
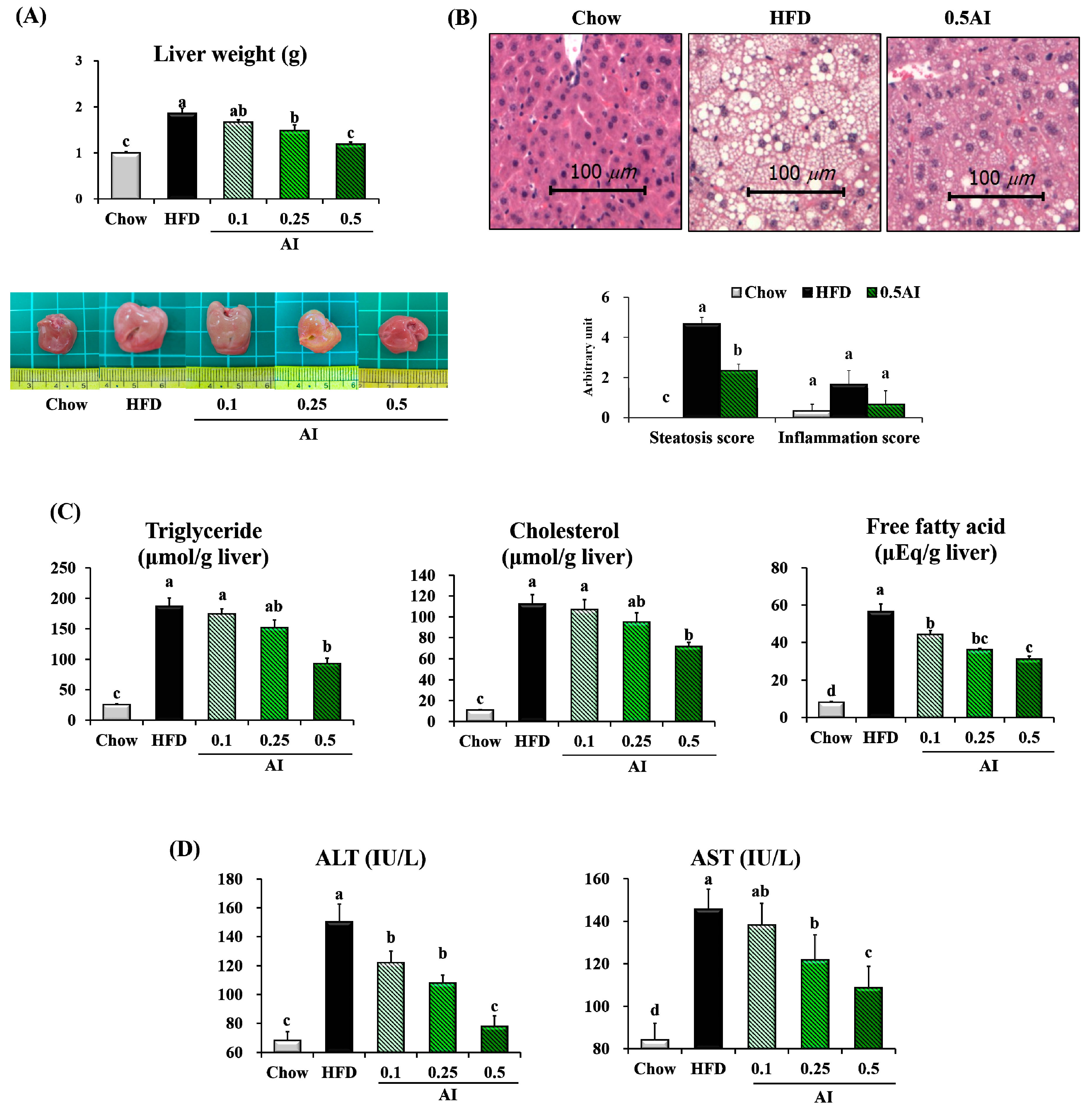
2.3. AI Alleviates High-Fat Diet-Induced Hepatic Lipid Accumulation
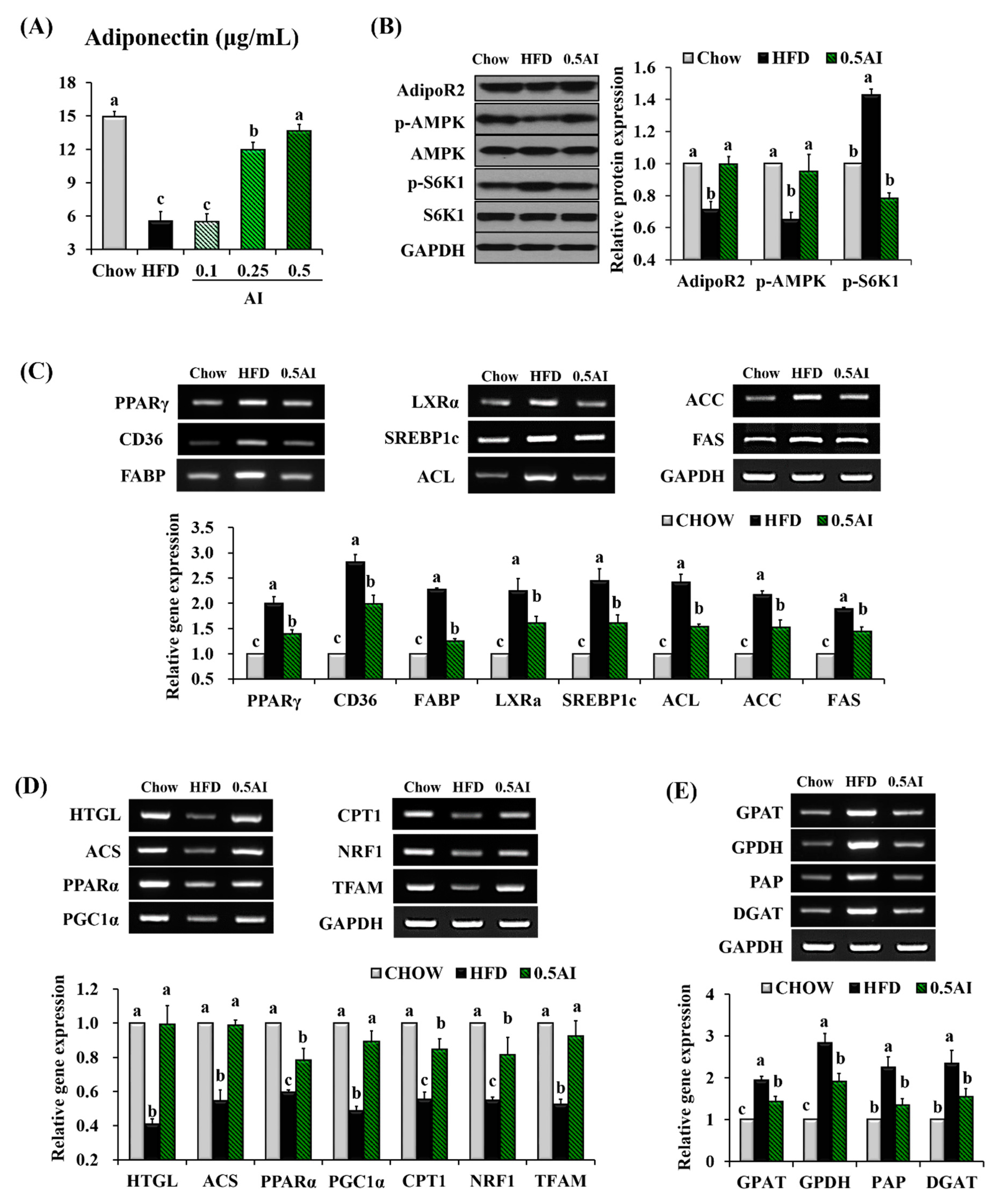
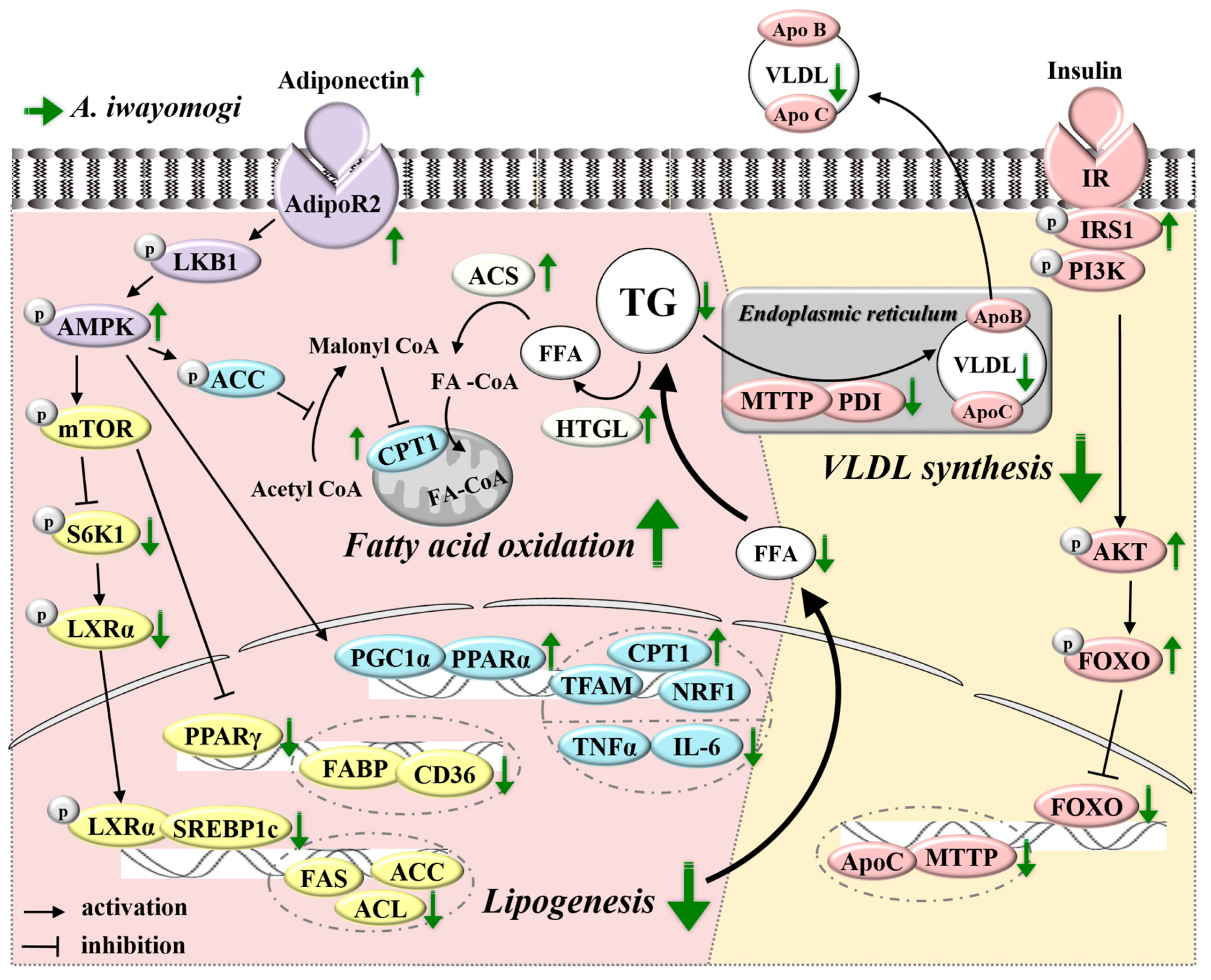
2.4. AI Activates Adiponectin-Adenosine Monophosphate-activated Protein Kinase (AMPK) Mediated Signaling Pathways in the Liver
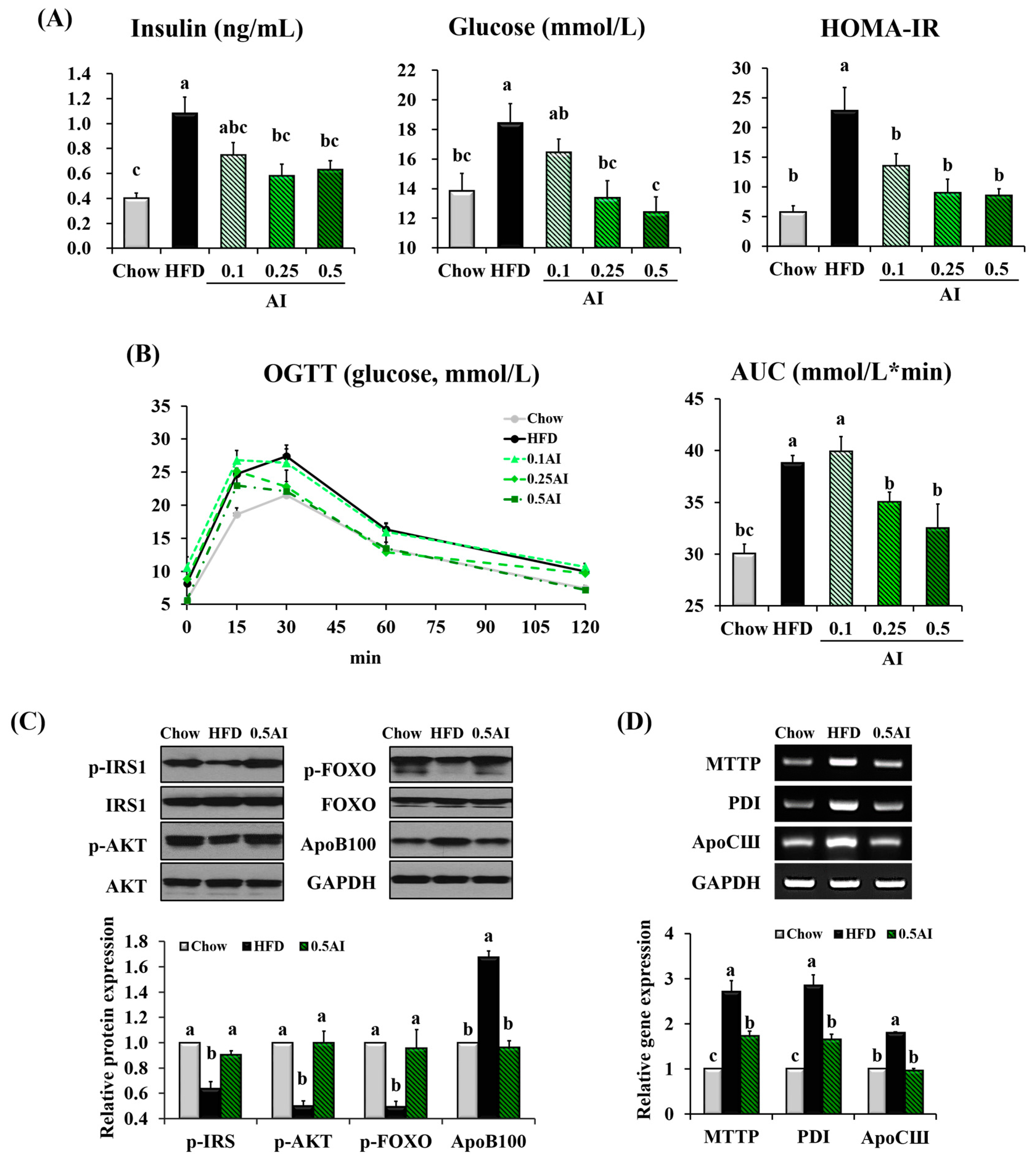
2.5. AI Improves Insulin Resistance and Downregulates Molecules Involved in Very Low Density Lipoprotein Assembly
3. Discussion
4. Materials and Methods
4.1. Preparation of AI and HPLC Analysis
4.2. Animals and Diets
4.3. Biochemical Analysis
4.4. Histological Examination
4.5. Semi-Quantitative RT-PCR Analysis
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylases |
| ACL | ATP citrate lyase |
| ACS | Acyl coa synthetase |
| AdipoR2 | Adiponectin receptor 2 |
| AI | Artemisia iwayomogi extract |
| AKT | Protein kinase B |
| ALT | Alanine aminotransferase |
| AMP | Adenosine monophosphate |
| AMPK | Adenosine monophosphate-activated protein kinase |
| ANOVA | Analysis of variance |
| ApoB | Apolipoprotein B |
| ApoC | Apolipoprotein C |
| AST | Aspartate aminotransferase |
| AUC | Area under the curve |
| CD36 | Cluster of differentiation 36 |
| CoA | Coenzyme A |
| CPT1 | Carnitine palmitoyltransferase 1 |
| DGAT | Diacylglycerol acyltransferase |
| ELISA | Enzyme-linked immunosorbent assay |
| FABP | Liver fatty acid binding protein 1 |
| FAS | Fatty acid synthase |
| FOXO | Forkhead box O1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GPAT | Glycerol-3-phosphate acyltransferase |
| GPDH | Glycerol-3-phosphate dehydrogenase |
| HDL | High density lipoprotein |
| HFD | High-fat diet |
| HPLC | High performance liquid chromatography |
| HOMA-IR | Homeostasis model assessment of basal insulin resistance |
| HTGL | Hepatic triglyceride lipase |
| IL-6 | Interlukin-6 |
| IRS1 | Insulin receptor substrate 1 |
| LDL | Low density lipoprotein |
| LKB1 | Liver kinase B1 |
| LPL | Lipoprotein lipase |
| LXRα | Liver X receptor α |
| MTTP | Microsomal triglyceride transfer protein |
| NOAEL | No observed adverse effect level |
| NRF1 | Nuclear respiratory factor 1 |
| p | phospho- |
| PAP | Phosphatidate phosphohydrolase |
| PDI | Protein disulfide isomerase |
| PGC1α | Peroxisome proliferator-activated receptor-γ coactivator 1α |
| PPARα | Peroxisome proliferator-activated receptor α |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| RT-PCR | Reverse transcription polymerase chain reaction |
| S6K1 | S6 kinase 1 |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SEM | Standard error of the mean |
| SREBP1c | Sterol regulatory element binding protein 1c |
| TFAM | Mitochondrial transcription factor A |
| TG | Triglyceride |
| TNFα | Tumor necrosis factor α |
| VLDL | Very low density lipoprotein |
References
- Hegele, R.A. Monogenic dyslipidemias: Window on determinants of plasma lipoprotein metabolism. Am. J. Hum. Genet. 2001, 69, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Mack, W.J.; Krauss, R.M.; Alaupovic, P. Pathophysiology of triglyceride-rich lipoproteins in atherothrombosis: Clinical aspects. Clin. Cardiol. 1999, 22, 15–20. [Google Scholar] [CrossRef]
- Rizzo, M.; Berneis, K. Lipid triad or atherogenic lipoprotein phenotype: A role in cardiovascular prevention? J. Atheroscler. Thromb. 2005, 12, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Hokanson, J.E. Hypertriglyceridemia and risk of coronary heart disease. Curr. Cardiol. Rep. 2002, 4, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Despres, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Chew, G.T.; Watts, G.F. Recent advances in pharmacotherapy for hypertriglyceridemia. Prog. Lipid Res. 2014, 56, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Illustrated Natural Drugs Encyclopedia; Namsadang Publishers: Seoul, Korea, 1989; p. 249. [Google Scholar]
- Park, J. Korean Folk Medicine; Busan National University Publisher: Busan, Korea, 1999; pp. 184–185. [Google Scholar]
- Cho, K.; Park, S.-H.; Cha, A.; Park, T. Protective effect of artemisia iwayomogi water extract against alcoholic fatty liver. J. Clin. Biochem. Nutr. 2008, 43, 303–306. [Google Scholar]
- Wang, J.H.; Choi, M.K.; Shin, J.W.; Hwang, S.Y.; Son, C.G. Antifibrotic effects of Artemisia capillaris and artemisia iwayomogi in a carbon tetrachloride-induced chronic hepatic fibrosis animal model. J. Ethnopharmacol. 2012, 140, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Nan, J.X.; Kim, J.Y.; Kang, H.C.; Choi, J.H.; Lee, S.J.; Lee, B.H.; Kim, S.J.; Lee, J.H.; Kim, Y.C.; et al. The ethanol-soluble part of a hot-water extract from artemisia iwayomogi inhibits liver fibrosis induced by carbon tetrachloride in rats. J. Pharm. Pharmacol. 2000, 52, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Kim, H.G.; Choi, M.K.; Lee, J.S.; Park, H.J.; Wang, J.H.; Lee, J.S.; Son, S.W.; Hwang, S.Y.; Son, C.G. Aqueous extract of artemisia iwayomogi kitamura attenuates cholestatic liver fibrosis in a rat model of bile duct ligation. Food. Chem. Toxicol. 2012, 50, 3505–3513. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yanagawa, Y.; Kim, S.; Whang, W.K.; Park, T. Artemisia iwayomogi extract attenuates high-fat diet-induced obesity by decreasing the expression of genes associated with adipogenesis in mice. eCAM 2013, 2013, 915953. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. A thirteen week repeated oral dose toxicity test and a four week recovery test of artemisia iwayomogi extract in sprague-dawley rats. Dong A Pharmaceutical Company, 2015; Unpublished raw data. [Google Scholar]
- Kim, C.-D.; Kim, S.-M.; Im, M.-H.; Lee, J.-H.; Park, J.-H. A study on the reproductive toxicity of artemisia iwayomogi kitamura. Korean J. Herbol. 2003, 18, 157–173. [Google Scholar]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Kweon, M.H.; Hwang, H.J.; Sung, H.C. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis). J. Agric. Food Chem. 2001, 49, 4646–4655. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Patras, M.A.; Eravuchira, P.J.; Kuhnert, N. Profile and characterization of the chlorogenic acids in green robusta coffee beans by LC-MS(n): Identification of seven new classes of compounds. J. Agric. Food Chem. 2010, 58, 8722–8737. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Seki, E.; Aitani, M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC. Complement. Altern. Med. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, Y.G.; Lee, H.-J.; Lim, S.J.; Ahn, H.R.; Jung, S.H.; Nho, C.W. Youngia denticulata attenuates diet-induced obesity-related metabolic dysfunctions by activating amp-activated protein kinase and regulating lipid metabolism. J. Funct. Foods. 2015, 18, 714–726. [Google Scholar] [CrossRef]
- Randy, A.; Kim, M.; Nho, C.W. Ligularia fischeri and its constituent 3,4-dicaffeoylquinic acid improve obesity-induced nonalcoholic fatty liver disease by regulating lipid metabolism and activating AMPK. J. Funct. Foods 2016, 27, 1–16. [Google Scholar] [CrossRef]
- Miyamae, Y.; Kurisu, M.; Han, J.; Isoda, H.; Shigemori, H. Structure activity relationship of caffeoylquinic acids on the accelerating activity on ATP production. Chem. Pharm. Bull. 2011, 59, 502–507. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Hornick, A.; Langer, T.; Stuppner, H.; Prast, H. Acetylcholinesterase inhibitory activity of scopolin and scopoletin discovered by virtual screening of natural products. J. Med. Chem. 2004, 47, 6248–6254. [Google Scholar] [CrossRef] [PubMed]
- Yoo, A.; Narayan, V.P.; Hong, E.Y.; Whang, W.K.; Park, T. Scopolin ameliorates high-fat diet induced hepatic steatosis in mice: Potential involvement of sirt1-mediated signaling cascades in the liver. Sci. Rep. 2017, 7, 2251. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Dai, Y.; Gao, X.; Xia, Y. Scopolin isolated from erycibe obtusifolia benth stems suppresses adjuvant-induced rat arthritis by inhibiting inflammation and angiogenesis. Int. Immunopharmacol. 2009, 9, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Yun, K.W.; Seo, K.I.; Kim, M.J.; Lee, M.K. Scopoletin prevents alcohol-induced hepatic lipid accumulation by modulating the AMPK-SREBP pathway in diet-induced obese mice. Metabolism 2014, 63, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Geng, C.A.; Ma, Y.B.; Huang, X.Y.; Chen, H.; Cao, T.W.; He, K.; Wang, H.; Zhang, X.M.; Chen, J.J. UFLC/MS-IT-TOF guided isolation of anti-HBV active chlorogenic acid analogues from Artemisia capillaris as a traditional Chinese herb for the treatment of hepatitis. J. Ethnopharmacol. 2014, 156, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Li, Y.; Kim, B.; Zhang, H.; Hwangbo, K.; Piao, D.G.; Chi, M.J.; Woo, M.H.; Choi, J.S.; Lee, J.H.; et al. High-performance liquid chromatographic analysis for quantitation of marker compounds of Artemisia capillaris thunb. Arch. Pharm. Res. 2012, 35, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.J.; Towler, M.J. Changes in key constituents of clonally propagated Artemisia annua L. During preparation of compressed leaf tablets for possible therapeutic use. Ind. Crops Prod. 2014, 62, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Guo, H.; Yuan, Z.; Wang, T.; Zhang, L.; Jiang, Z. Emodin alleviates hepatic steatosis by inhibiting sterol regulatory element binding protein 1 activity by way of the calcium/calmodulin-dependent kinase kinase-AMP-activated protein kinase-mechanistic target of rapamycin-p70 ribosomal S6 kinase signaling pathway. Hepatol. Res. 2017, 47, 683–701. [Google Scholar]
- Hwahng, S.H.; Ki, S.H.; Bae, E.J.; Kim, H.E.; Kim, S.G. Role of adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway in repression of liver X receptor-α-dependent lipogenic gene induction and hepatic steatosis by a novel class of dithiolethiones. Hepatology 2009, 49, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Wang, C.; Zhao, H.; Zhao, C.; Chen, Y.; Wang, Y.; McClain, C.; Feng, W. Enhanced AMPK phosphorylation contributes to the beneficial effects of lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J. Nutr. Biochem. 2015, 26, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J. Storing up trouble: Does accumulation of intramyocellular triglyceride protect skeletal muscle from insulin resistance? Clin. Exp. Pharmacol. Physiol. 2009, 36, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Morita, A.; Mori, N.; Miura, S. The role of glycerol-3-phosphate dehydrogenase 1 in the progression of fatty liver after acute ethanol administration in mice. Biochem. Biophys. Res. Commun. 2014, 444, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Ji, C.; Han, X.; Zhao, X.; Li, X.; Mao, Y.; Wong, L.L.; Bartlam, M.; Rao, Z. Crystal structures of human glycerol 3-phosphate dehydrogenase 1 (GPD1). J. Mol. Biol. 2006, 357, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W.; Weiss, S.B.; Kennedy, E.P. The enzymatic dephosphorylation of phosphatidic acids. J. Biol. Chem. 1957, 228, 915–922. [Google Scholar] [PubMed]
- Lewis, G.F.; Uffelman, K.D.; Szeto, L.W.; Weller, B.; Steiner, G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J. Clin. Investig. 1995, 95, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Den Boer, M.A.; Voshol, P.J.; Kuipers, F.; Romijn, J.A.; Havekes, L.M. Hepatic glucose production is more sensitive to insulin-mediated inhibition than hepatic VLDL-triglyceride production. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1360–E1364. [Google Scholar] [CrossRef] [PubMed]
- Tietge, U.J.; Bakillah, A.; Maugeais, C.; Tsukamoto, K.; Hussain, M.; Rader, D.J. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J. Lipid Res. 1999, 40, 2134–2139. [Google Scholar] [PubMed]
- Jamil, H.; Chu, C.H.; Dickson, J.K., Jr.; Chen, Y.; Yan, M.; Biller, S.A.; Gregg, R.E.; Wetterau, J.R.; Gordon, D.A. Evidence that microsomal triglyceride transfer protein is limiting in the production of apolipoprotein B-containing lipoproteins in hepatic cells. J. Lipid Res. 1998, 39, 1448–1454. [Google Scholar] [PubMed]
- Hussain, M.M.; Shi, J.; Dreizen, P. Microsomal triglyceride transfer protein and its role in ApoB-lipoprotein assembly. J. Lipid Res. 2003, 44, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, R.; Packard, C.J.; Caslake, M.; Bedford, D.; Stewart, P.; Yki-Jarvinen, H.; Shepherd, J.; Taskinen, M.R. Effects of insulin and acipimox on VLDL1 and VLDL2 apolipoprotein B production in normal subjects. Diabetes 1998, 47, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, R.; Packard, C.J.; Watson, T.D.; Rannikko, S.; Caslake, M.; Bedford, D.; Stewart, P.; Yki-Jarvinen, H.; Shepherd, J.; Taskinen, M.R. Metabolic basis of hypotriglyceridemic effects of insulin in normal men. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Steiner, G. Acute effects of insulin in the control of VLDL production in humans: Implications for the insulin-resistant state. Diabetes Care 1996, 19, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.D.; Sparks, C.E. Insulin regulation of triacylglycerol-rich lipoprotein synthesis and secretion. Biochim. Biophys. Acta 1994, 1215, 9–32. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Narayan, V.P.; Hong, E.Y.; Whang, W.K.; Park, T. Artemisia Iwayomogi Extract Attenuates High-Fat Diet-Induced Hypertriglyceridemia in Mice: Potential Involvement of the Adiponectin-AMPK Pathway and Very Low Density Lipoprotein Assembly in the Liver. Int. J. Mol. Sci. 2017, 18, 1762. https://doi.org/10.3390/ijms18081762
Lee J, Narayan VP, Hong EY, Whang WK, Park T. Artemisia Iwayomogi Extract Attenuates High-Fat Diet-Induced Hypertriglyceridemia in Mice: Potential Involvement of the Adiponectin-AMPK Pathway and Very Low Density Lipoprotein Assembly in the Liver. International Journal of Molecular Sciences. 2017; 18(8):1762. https://doi.org/10.3390/ijms18081762
Chicago/Turabian StyleLee, Jinhui, Vikram P. Narayan, Eun Young Hong, Wan Kyunn Whang, and Taesun Park. 2017. "Artemisia Iwayomogi Extract Attenuates High-Fat Diet-Induced Hypertriglyceridemia in Mice: Potential Involvement of the Adiponectin-AMPK Pathway and Very Low Density Lipoprotein Assembly in the Liver" International Journal of Molecular Sciences 18, no. 8: 1762. https://doi.org/10.3390/ijms18081762





