Modulatory Effects of Exogenously Applied Polyamines on Postharvest Physiology, Antioxidant System and Shelf Life of Fruits: A Review
Abstract
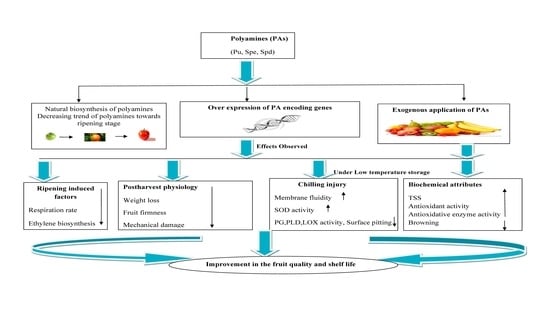
:1. Introduction
2. Biosynthetic Pathways of Important Polyamines
3. Effect of Polyamines on Postharvest Physiology, Antioxidant System and Biochemical Attributes of Some Fruits
3.1. Effect on Shelf Life and Fruit Quality
3.2. Effect on Postharvest Physiology
3.2.1. Effect on Weight Loss
3.2.2. Effect on Fruit Firmness
3.2.3. Effect on Mitigation of Mechanical Damage
3.3. Effect on Ripening Inducing Factors
3.3.1. Effect on Ethylene Biosynthesis
3.3.2. Effect on Respiration
3.4. Effect on Biochemical Attributes, Antioxidant Compounds and Antioxidant Enzymes
3.4.1. Effect on Total Soluble Solids
3.4.2. Effect on Browning
3.4.3. Effect on Antioxidant Compounds and Antioxidant Activity
3.4.4. Effect on Antioxidant Enzymes
3.5. Effect on Chilling Injury
4. Tomato Transgenics Showing Over-expression of Genes Encoding PAs for Maintaining Quality and Shelf Life
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| ADC | Arginine decarboxylase |
| AIH | Agmatimeimino hydrolase |
| AOS | Active oxygen species |
| APX | Ascorbate peroxidise |
| ATP | Adenosine triphosphate |
| CAT | Catalase |
| CI | Chilling injury |
| GR | Glutathione reductase |
| LOX | Lipoxygenase |
| MDA | Malondialdehyde |
| ODC | Ornithine decarboxylase |
| PAs | Polyamines |
| PE | Pectin esterase |
| PG | Polygalacturonase |
| PLD | Phospholipase D |
| PME | Pectin methyl esterase |
| POX | Peroxidise |
| PPO | Polyphenol oxidase |
| PUT | Putrescine |
| ROS | Reactive oxygen species |
| SAM | S-adenosyl methionine |
| SOD | Superoxide dismutase |
| SPD | Spermidine |
| SpdS | Spermidine synthase |
| SPE | Spermine |
| SpeS | Spermine synthase |
| TA | Titrable acidity |
| TBARS | Thio-barbituric acid-reactive substances |
| TPC | Total phenol content |
| TSS | Total soluble solids |
| ySAMdc | S-adenosylmethionine decarboxylase |
References
- Evans, P.T.; Malmberg, R.L. Do polyamines have roles in plant development. Ann. Rev. Plant Phys. Plant Mol. Biol. 1989, 40, 235–269. [Google Scholar] [CrossRef]
- Drolet, G.; Dumbroff, E.B.; Legge, R.L.; Thompson, J.E. Radical scavenging properties of polyamines. Phytochemistry 1986, 25, 367–371. [Google Scholar] [CrossRef]
- Pistocchi, R.; Bagni, N.; Creus, J.A. Polyamine uptake in carrot cell cultures. Plant Physiol. 1987, 84, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Leiting, V.A.; Wicker, L. Inorganic cations and polyamines moderate pectinesterase activity. J. Food Sci. 1997, 62, 253–255. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez-Madrid, M.C.; Martínez, G.; Riquelme, F.; Pretel, M.T.; Romojaro, F. Role of polyamines in chilling injury of fruit and vegetables. Food Sci. Technol. Int. 1996, 2, 195–199. [Google Scholar]
- Bouchereau, A.; Aziz, A.; Larher, F.; Martin-Tanguy, J. Polyamines and environmental challenges: Recent development. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]
- Kuznetsov, V.V.; Shevyakova, N.I. Polyamines and stress tolerance of plants. Plant Stress 2007, 1, 50–71. [Google Scholar]
- Rangan, P.; Subramani, R.; Kumar, R.; Singh, A.K.; Singh, R. Recent advances in polyamine metabolism and abiotic stress tolerance. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Romero, M.D.; Serrano, M. The role of polyamines in the improvement of shelf life of fruit. Trends Food Sci. Technol. 2002, 13, 228–234. [Google Scholar] [CrossRef]
- Mehta, R.A.; Cassol, T.; Li, N.; Ali, N.; Handa, A.K.; Mattoo, A.K. Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat. Biotechnol. 2002, 20, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.K.; Chung, S.H.; Goyal, R.K.; Fatima, T.; Solomos, T.; Srivastava, A.; Handa, A.K. Over accumulation of higher polyamines in ripening transgenic tomato fruit revives metabolic memory, upregulates anabolism-related genes, and positively impacts nutritional quality. J. AOAC Int. 2007, 90, 1456–1464. [Google Scholar] [PubMed]
- Walden, R.; Cordeiro, A.; Tiburcio, A.F. Polyamines: Small molecules triggering pathways in plant growth and development. Plant Physiol. 1997, 113, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Altabella, T.; Borrell, A.; Masgrau, C. Polyamine metabolism and its regulation. Physiol. Plant 1997, 100, 664–674. [Google Scholar] [CrossRef]
- Galston, A.W.; Sawhney, R.K. Polyamines in plant physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Taylor, M.; Altabella, T.; Tiburcio, A.F. Recent advances in polyamine research. Trends Plant Sci. 1997, 2, 124–130. [Google Scholar] [CrossRef]
- D'Orazi, D.; Bagni, N. In vitro interactions between polyamines and pectic substances. Biochem. Biophys. Res. Commun. 1987, 148, 1259–1263. [Google Scholar] [CrossRef]
- Roberts, D.R.; Walker, M.A.; Thompson, J.E. The effects of inhibitors of polyamine and ethylene biosynthesis on senescence, ethylene production and polyamine levels in cut carnation flowers. Plant Cell Physiol. 1984, 25, 315–322. [Google Scholar]
- Martinez-Romero, D.; Serrano, M.; Carbonell, A.; Burgos, L.; Riquelme, F.; Valero, D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J. Food Sci. 2002, 67, 1706–1712. [Google Scholar] [CrossRef]
- KousheshSaba, M.; Arzani, K.; Barzegar, M. Postharvest polyamine application alleviates chilling injury and affects apricot storage ability. J. Agric. Food Chem. 2012, 60, 8947–8953. [Google Scholar] [CrossRef] [PubMed]
- Davarynejad, G.; Zarei, M.; Ardakani, E.; Nasrabadi, M.E. Influence of putrescine application on storability, postharvest quality and antioxidant activity of two Iranian apricot (Prunus armeniaca L.) cultivars. Not. Sci. Biol. 2013, 5, 212–219. [Google Scholar]
- Champa Harindra, W.A.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Postharvest treatment of polyamines maintains quality and extends shelf life of table grapes (Vitis vinifera L.) cv. Flame Seedless. Postharv. Biol. Technol. 2014, 91, 57–63. [Google Scholar] [CrossRef]
- Petkou, I.T.; Pritsa, T.S.; Sfakiotakis, E.M. Effects of polyamines on ethylene production, respiration and ripening of kiwifruit. J. Hort. Sci. 2004, 79, 977–980. [Google Scholar] [CrossRef]
- Jhalegar, M.J.; Sharma, R.R.; Pal, R.K.; Rana, V. Effect of postharvest treatments with polyamines on physiological and biochemical attributes of kiwifruit (Actinidia deliciosa) cv. Allison. Fruits 2012, 67, 13–22. [Google Scholar] [CrossRef]
- Malik, A.U.; Singh, Z. Improved fruit retention, yield and fruit quality in mango with exogenous application of polyamines. Sci. Hort. 2006, 110, 167–174. [Google Scholar] [CrossRef]
- Jawandha, S.K.; Gill, M.S.; Singh, N.; Gill, P.P.S.; Singh, N. Effect of postharvest treatments of putrescine on storage of mango cv. Langra. Afr. J. Agric. Res. 2012, 7, 6432–6436. [Google Scholar]
- Razzaq, K.; Khan, A.S.; Malik, A.U.; Shahid, M.; Ullah, S. Role of putrescine in regulating fruit softening and antioxidative enzyme systems in ‘Samar Bahisht Chaunsa’ mango. Postharv. Biol. Technol. 2014, 96, 23–32. [Google Scholar] [CrossRef]
- Perez-Vicente, A.; Martı́nez-Romero, D.; Carbonell, A.; Serrano, M.; Riquelme, F.; Guillen, F.; Valero, D. Role of polyamines in extending shelf life and the reduction of mechanical damage during plum (Prunus salicina Lindl.) storage. Postharv. Biol. Technol. 2002, 25, 25–32. [Google Scholar] [CrossRef]
- Serrano, M.; Romero, D.M.; Guillen, F.; Valero, D. Effects of exogenous putrescine on improving shelf life of four plum cultivars. Postharv. Biol. Technol. 2003, 30, 259–271. [Google Scholar] [CrossRef]
- Khan, A.S.; Singh, Z.; Abbasi, N.A. Pre-storage putrescine application suppresses ethylene biosynthesis and retards fruit softening during low temperature storage in ‘Angelino’ plum. Postharv. Biol. Technol. 2007, 46, 36–46. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M.; Castillo, S.; Martínez-Romero, D.; Serrano, M.; Valero, D. Pre-storage application of polyamines by pressure or immersion improves shelf-life of pomegranate stored at chilling temperature by increasing endogenous polyamine levels. Postharv. Biol. Technol. 2007, 44, 26–33. [Google Scholar] [CrossRef]
- Ramezanian, A.; Rahemi, M.; Maftoun, M.; Bahman, K.; Eshghi, S.; Safizadeh, M.R.; Tavallali, V. The ameliorative effects of spermidine and calcium chloride on chilling injury in pomegranate fruits after long-term storage. Fruits 2010, 65, 169–178. [Google Scholar] [CrossRef]
- Barman, K.; Asrey, R.; Pal, R.K. Putrescine and carnauba wax pre-treatments alleviate chilling injury, enhance shelf life and preserve pomegranate fruit quality during cold storage. Sci. Hort. 2011, 130, 795–800. [Google Scholar] [CrossRef]
- Khosroshahi, M.R.Z.; Esna-Ashari, M.; Ershadi, A. Effect of exogenous putrescine on postharvest life of strawberry (Fragaria ananassa Duch.) fruit, cultivar Selva. Sci. Hort. 2007, 114, 27–32. [Google Scholar] [CrossRef]
- Palma, F.; Carvajal, F.; Ramos, J.M.; Jamilena, M.; Garrido, D. Effect of putrescine application on maintenance of zucchini fruit quality during cold storage: Contribution of GABA shunt and other related nitrogen metabolites. Postharv. Biol. Technol. 2015, 99, 131–140. [Google Scholar] [CrossRef]
- Champa Harindra, W.A.; Gill, M.I.S.; Mahajan, B.V.C.; Bedi, S. Exogenous treatment of spermine to maintain quality and extend postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless under low temperature storage. LWT Food Sci. Technol. 2015, 60, 412–419. [Google Scholar] [CrossRef]
- Malik, A.U.; Singh, Z.; Tan, S.C. Exogenous application of polyamines improves shelf life and fruit quality of mango. Acta Hortic. 2005, 699, 291–296. [Google Scholar] [CrossRef]
- Fischer, R.L.; Bennett, A.B. Role of cell wall hydrolases in fruit ripening. Ann. Rev. Plant Biol. 1991, 42, 675–703. [Google Scholar] [CrossRef]
- Walheim, L. Citrus: Complete Guide to Selecting and Growing More Than 100 Varieties for California, Arizona, Texas, The Gulf Coast and Florida; Ironwood Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Bregoli, A.M.; Scaramagli, S.; Costa, G.; Sabatini, E.; Ziosi, V.; Biondi, S.; Torrigiani, P. Peach (Prunus persica) fruit ripening: Aminoethoxyvinylglycine (AVG) and exogenous polyamines affect ethylene emission and flesh firmness. Physiol. Plant. 2002, 114, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Bal, E. Effects of exogenous polyamine and ultrasound treatment to improve peach storability. Chil. J. Agric. Res. 2013, 73, 435–440. [Google Scholar] [CrossRef]
- Pandey, S.; Ranade, S.A.; Nagar, P.K.; Kumar, N. Role of polyamines and ethylene as modulators of plant senescence. J. Biol. Sci. 2000, 25, 291–299. [Google Scholar] [CrossRef]
- Torrigiani, P.; Bregoli, A.M.; Ziosi, V.; Scaramagli, S.; Ciriaci, T.; Rasori, A.; Biondi, S.; Costa, G. Pre-harvest polyamine and aminoethoxyvinylglycine (AVG) applications modulate fruit ripening in Stark Red Gold nectarines (Prunus persica L. Batsch). Postharv. Biol. Technol. 2004, 33, 293–308. [Google Scholar] [CrossRef]
- Purvis, A.C. Interaction of waxes and temperature in retarding moisture loss from and chilling injury of cucumber fruit during storage. Proc. Fla. State Hort. Soc. 1994, 107, 257. [Google Scholar]
- Zokaee-Khosroshahi, M.R.; Esna-Ashari, M. Effect of putrescine application on postharvest life and physiology of strawberry, apricot, peach and sweet cherry fruits. J. Sci. Tech. Agric. Nat. Res. 2008, 12, 219–230. [Google Scholar]
- Miller, A.R. Physiology, biochemistry and detection of bruising (mechanical stress) in fruits and vegetables. Postharv. News Inf. 1992, 3, 53–58. [Google Scholar]
- Mohammadrezakhani, S.; Pakkish, Z. Chilling injury induces lipid peroxidation and alters the hydrogen peroxide content in peel and pulp of “Valencia” orange fruit under low temperature storage conditions. Act. Adv. Agric. Sci. 2014, 2, 10–26. [Google Scholar]
- Ishaq, S.; Rathore, H.A.; Majeed, S.; Awan, S.; Shah, S.Z.A. The studies on the physico-chemical and organoleptic characteristics of apricot (Prunus armeniaca L.) produced in Rawalakot, Azad Jammu and Kashmir during storage. Pakistan J. Nutr. 2009, 8, 856–860. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Shiri, M.A. Effect of chitosan coatings on some quality indices of apricot (Prunus armeniaca L.) during cold storage. Casp. J. Env. Sci. 2010, 8, 25–33. [Google Scholar]
- Bartosz, G. Oxidative stress in plants. Acta Phys. Plant. 1997, 19, 47–64. [Google Scholar] [CrossRef]
- Shewfelt, R.L.; Del Rosario, B.A. The role of lipid peroxidation in storage disorders of fresh fruits and vegetables. Hort. Sci. 2000, 35, 575–579. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Hamilton, G. Chemical models and mechanisms for oxygenases. In Molecular Mechanism of Oxygen Activation; Hayashi, O., Ed.; Academic Press: New York, NY, USA, 1974; Volume 1, pp. 405–451. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Mechanisms for scavenging reactive molecules generated in chloroplasts under light stress. Photoinh. Photo. Mol. Mech. Field. 1994, 129–142. [Google Scholar]
- Liso, B.R.; Calabrese, G.; Bitonti, M.B.; Arrigoni, O. Relationship between AsA and cell division. Exp. Cell Res. 1984, 150, 314–320. [Google Scholar] [CrossRef]
- Liso, B.R.; Innocenti, A.M.; Bitonti, M.B.; Arrigoni, O. AsA-induced progression of quiescent centre cells from G1 to S phase. New Phytol. 1988, 110, 469–471. [Google Scholar] [CrossRef]
- Clowes, F.A.L. Regeneration of the discrete root epidermis of Pistia stratiotes L. after perturbation of the meristem. New Phytol. 1992, 120, 209–213. [Google Scholar] [CrossRef]
- Kerk, N.M.; Feldman, N.J. A biochemical model for the initiation and maintenance of the quiescent center: Implications for organization of root meristems. Development 1995, 121, 2825–2833. [Google Scholar]
- Takahama, U.; Oniki, T. Effects of ascorbate on the oxidation of derivatives of hydroxycinnamic acid and the mechanism of oxidation of sinapic acid by cell wall-bound peroxidases. Plant Cell Physiol. 1994, 35, 593–600. [Google Scholar] [CrossRef]
- Joy, R.W.; Patel, K.R.; Thorpe, T.A. Ascorbic acid enhancement of organogenesis in tobacco callus. Plant Cell Tissue Organ Cult. 1988, 13, 219–228. [Google Scholar] [CrossRef]
- Jimenez, A.; Creissen, G.; Kular, B.; Firmin, J.; Robinson, S.; Verhoeyen, M.; Mullineaux, P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 2002, 214, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Ritenour, M.A.; Dou, H.; McCollum, G.T. Chilling Injury of Grapefruit and Its Control; Bulletin No. HS935; Fla. Coop. Ext. Serv.: Washington, DC, USA, 2003. [Google Scholar]
- Shah, K.; Kumar, R.G.; Verma, S.; Dubey, R.S. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001, 161, 1135–1144. [Google Scholar] [CrossRef]
- Amin, H.; Rahemi, M. Investigation of effect of polyamines on chilling control in lemon. In Proceedings of the 5th Congress of Iranian for Horticultural Science, Shiraz, Iran, 3–6 September 2007. [Google Scholar]
- Raeisi, M.; Samani, R.B.; Honarvar, M. Application of exogenous spermidine treatment for reducing of chilling on fruit quality and quantity of Valencia orange var. Olinda. Int. J. Farm. Allied Sci. 2013, 2, 1292–1297. [Google Scholar]
- Lasanajak, Y.; Minocha, R.; Minocha, S.C.; Goyal, R.; Fatima, T.; Handa, A.K. Enhanced flux of substrates into polyamine biosynthesis but not ethylene in tomato fruit engineered with yeast S-adenosyl methionine decarboxylase gene. Amino Acids 2013, 46, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Chung, S.H.; Fatima, T.; Datsenka, T.; Handa, A.K.; Mattoo, A.K. Polyamines as anabolic growth regulators revealed by transcriptome analysis and metabolite profiles of tomato fruits engineered to accumulate spermidine and spermine. Plant Biotechnol. 2007, 24, 57–70. [Google Scholar] [CrossRef]
- Cheng, L.; Zou, Y.; Ding, S.; Zhang, J.; Yu, X.; Cao, J.; Lu, G. Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J. Int. Plant Biol. 2009, 51, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Nambeesan, S.; Datsenka, T.; Ferruzzi, M.G.; Malladi, A.; Mattoo, A.K.; Handa, A.K. Over-expression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato. Plant J. 2010, 63, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.P.; Neelam, A.; Fatima, T.; Shukla, V.; Handa, A.K.; Mattoo, A.K. Genetic introgression of ethylene-suppressed transgenic tomatoes with higher-polyamines trait overcomes many unintended effects due to reduced ethylene on the primary metabolome. Front. Plant Sci. 2014, 5, 632. [Google Scholar] [CrossRef] [PubMed]
- Neily, M.H.; Matsukura, C.; Maucourt, M.; Bernillon, S.; Deborde, C.; Moing, A. Enhanced polyamine accumulation alters carotenoid metabolism at the transcriptional level in tomato fruit over-expressing spermidine synthase. J. Plant Physiol. 2011, 168, 242–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhulatha, P.; Gupta, A.; Gupta, S.; Kumar, A.; Pal, R.K.; Rajam, M.V. Fruit specific over-expression of human S-adenosyl methionine decarboxylase gene results in polyamine accumulation and affects diverse aspects of tomato fruit development and quality. J. Plant Biochem. Biotechnol. 2014, 23, 151–156. [Google Scholar] [CrossRef]

 ); Increasing trend (
); Increasing trend (  ).
).
 ); Increasing trend (
); Increasing trend (  ).
).


| Fruit | Polyamine | Doses | Effects | Reference |
|---|---|---|---|---|
| Apricot | PUT | 1 mM | Increased shelf life, reduced respiration rate, weight loss, ethylene biosynthesis, lowered cellular juice leakage and browning | [18] |
| PUT | 4 mM | Reduced weight loss | [48] | |
| PUT or SPD | 1 mM | Tolerance to CI at 1°C for 21 days, reduction in ethylene release, stabilisation in colour and firmness of fruits | [19] | |
| PUT | 4 mM | Reduction in weight loss, maintain firmness, delay ripening | [20] | |
| Grapes cv.Flame Seedless | PUT or SPD | 0.5 mM | Maintained berry firmness, peel colour and anthocyanins, reduced electrolyte leakage, PME, decay incidence, degradation of TSS and TA and enhanced shelf life and low temperature storability | [21] |
| Kiwifruit | PUT | 1 mM | Reduction in ethylene evolution | [22] |
| SPM&SPD | 1.5, 2.0 mM | Low rate of respiration, diminished PGand LPX activities, enhanced shelf life | [23] | |
| Mango | SPM | 0.5 mM | Reduction in weight loss, decrease in softness, decrease in respiration rate | [36] |
| SPM and PUT | 0.5, 1 mM | Reduced weight loss, reduced fruit colour | [24] | |
| PUT | 2.0 mM | Reduced physiological weight loss, improved blend of TSS, acidity and palatability rate | [25] | |
| PUT | 2.0 mM | Inhibition of ethylene release, respiration rate, fruit softening, suppression of cell wall enzymes endo, exo-PG and EGase, modulation of antioxidant enzymes: SOD CAT, POX, overall fruit quality maintained | [26] | |
| Plum | PUT | 1 mM | Increased firmness peel and flesh firmness, decreased ethylene and CO2 evolution. SPD acted as physiological marker against mechanical damage | [27] |
| PUT | 1 mM | Reduction in ethylene release and soluble solids, increased fruit and flesh firmness, and extended storability | [28] | |
| Pomegranate | PUT or SPD | 1 mM | Maintained fruit firmness, enhanced shelf life, reduced husk scald, prevented skin browning | [31] |
| PUT or SPD | 1 mM | Heat treatment induced PA induction, reduction in fruit softening, CI mitigation | [30] * | |
| PUT + Carnauba wax | 2 mM Ratio (1:10) | Low respiration rate, ethylene release and CI, no discoloration of fruit peel, fruit firmness maintained, mitigation in pitting surface | [32] | |
| Strawberry cv. Selva | PUT | 1 mM 2 mM | Reduced weight loss and ethylene release and maintained firmness | [33] |
| “Valencia” oranges | PUT + Methyl jasmonate | 5 mM + 10 µmol | Lowered enzymatic activity and chilling injury | [46] |
| SPD | 1, 1.5 mM | Maintained TSS, tritatable acidity, flavour index | [65] |
| Tomato Gene Constructs Encoding Polyamines with Promoters | Observations | Reference |
|---|---|---|
| ySAMdc-E8 | Levels of SPE and SPD increased and accumulated; ySAMdc resulted in expedited conversion of PUT to the higher PAs; lycopene increased; vine life prolonged; improved juice quality from fruits. | [10] |
| ySAMdc(Ripening targeted) | Increased levels of SPE and SPD; differential gene expression showed that majority of genes were up-regulated in the fruits and possessed higher levels of PAs; endogenously produced PAs acted as anabolic growth regulators. | [67] |
| ySAMdc-E8 | Inverse relationship between PAs and ethylene during ripening of fruit; expression of ySAMdc modulated the inverse relationship between higher PAs and ethylene; decline in SPE and SPD could be mitigated without any alteration in ethylene biosynthetic pathway. | [66] |
| E8-ySAMdc | Accumulation of PAs specifically SPE and SPD; fruit cells perceived SPE and SPD as organic nitrogenous signalling metabolites which facilitated synthesis of organic acids, asparagine and glutamine in red coloured fruits; led to sequestration of carbon, enhanced ratio of acid to sugar, fruit flavour; synthesis of essential amine chlorine important for biological functioning of brain; inferred PAs as anti-apoptotic in nature. | [11] |
| ySpdSyn expressed with constitutive promoter CaMV35S and ripening specific promoter E8 | Higher lycopene accumulation; reduction in postharvest senescence; reduction in spoilage | [69] |
| E8-ySpdSyn | Accumulation of SPD during ripening of fruit | [69] |
| SPDS1 | The over expression of spermidine synthase a gene encoding SPE in the transgenic tomato led to the synthesis of lycopene followed by its accumulation in fruit. | [71] |
| Human SAMdc and promoter specific to fruits (2A11) | Accumulation of higher PAs such as SPE and SPD to increased levels; reduction in the evolution of ethylene gas by 50%; increase in the amount of vitamin C, TSS and lycopene. | [72] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Pareek, S.; Sagar, N.A.; Valero, D.; Serrano, M. Modulatory Effects of Exogenously Applied Polyamines on Postharvest Physiology, Antioxidant System and Shelf Life of Fruits: A Review. Int. J. Mol. Sci. 2017, 18, 1789. https://doi.org/10.3390/ijms18081789
Sharma S, Pareek S, Sagar NA, Valero D, Serrano M. Modulatory Effects of Exogenously Applied Polyamines on Postharvest Physiology, Antioxidant System and Shelf Life of Fruits: A Review. International Journal of Molecular Sciences. 2017; 18(8):1789. https://doi.org/10.3390/ijms18081789
Chicago/Turabian StyleSharma, Sunil, Sunil Pareek, Narashans Alok Sagar, Daniel Valero, and Maria Serrano. 2017. "Modulatory Effects of Exogenously Applied Polyamines on Postharvest Physiology, Antioxidant System and Shelf Life of Fruits: A Review" International Journal of Molecular Sciences 18, no. 8: 1789. https://doi.org/10.3390/ijms18081789