Fabrication of Innovative Silk/Alginate Microcarriers for Mesenchymal Stem Cell Delivery and Tissue Regeneration
Abstract
:1. Introduction
2. Results
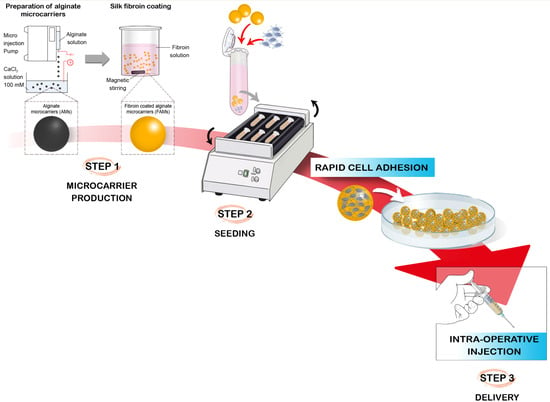
2.1. Production Process
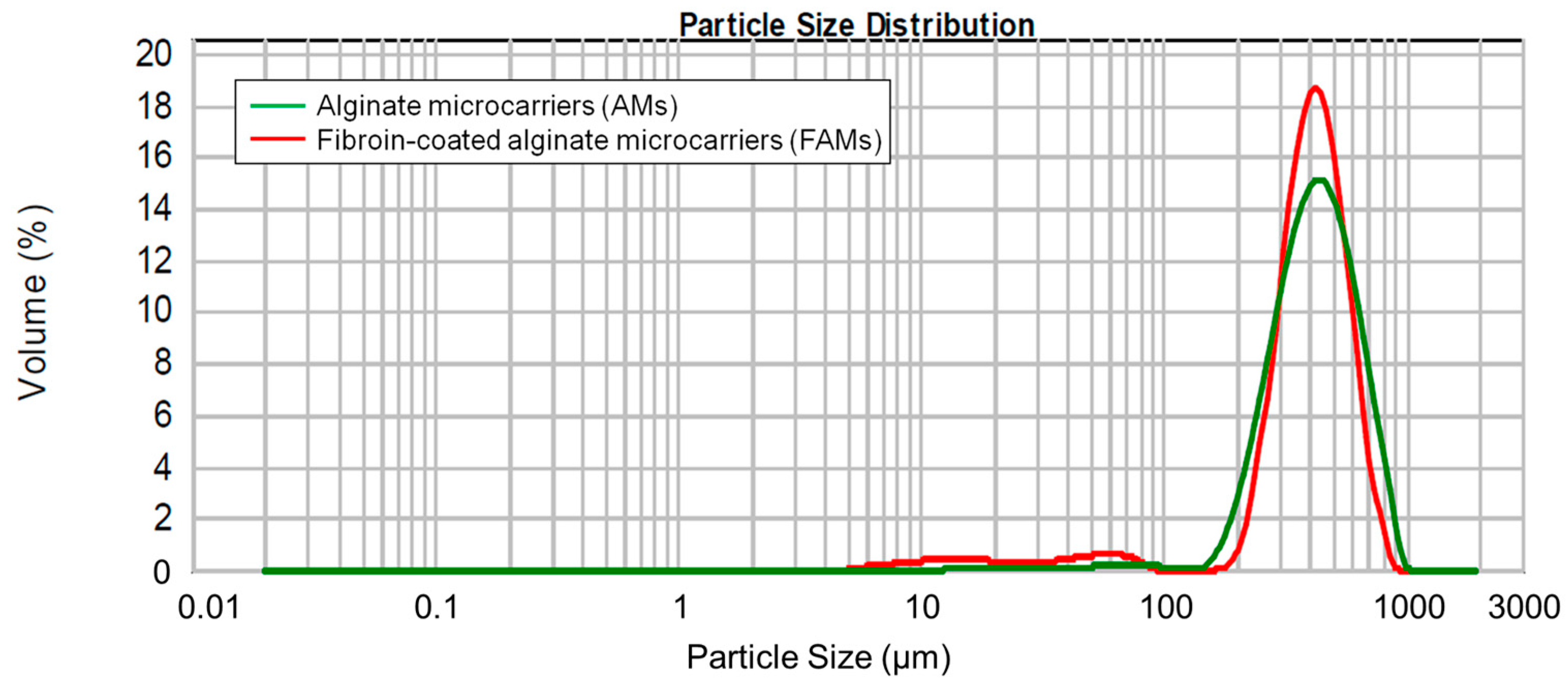
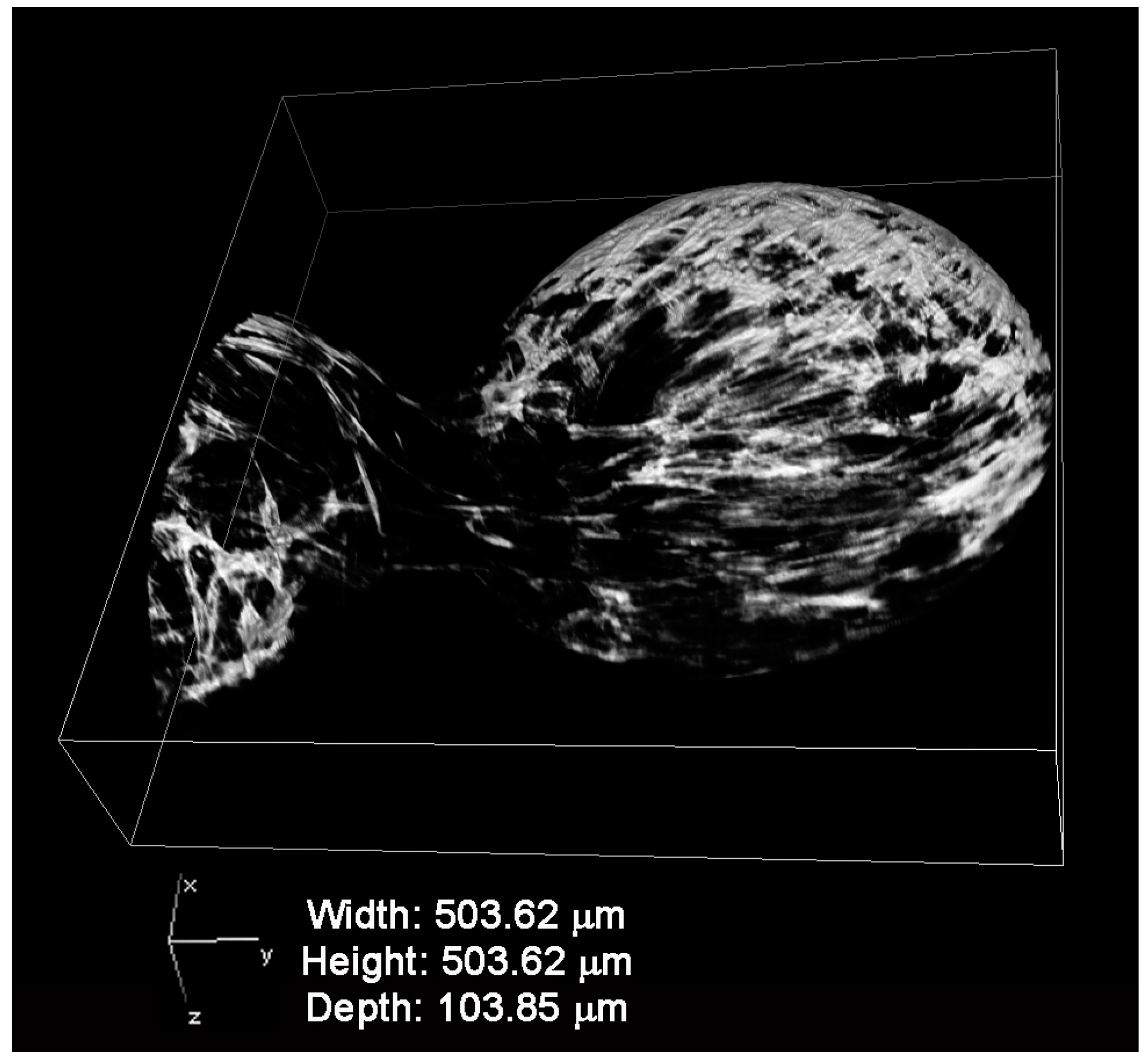
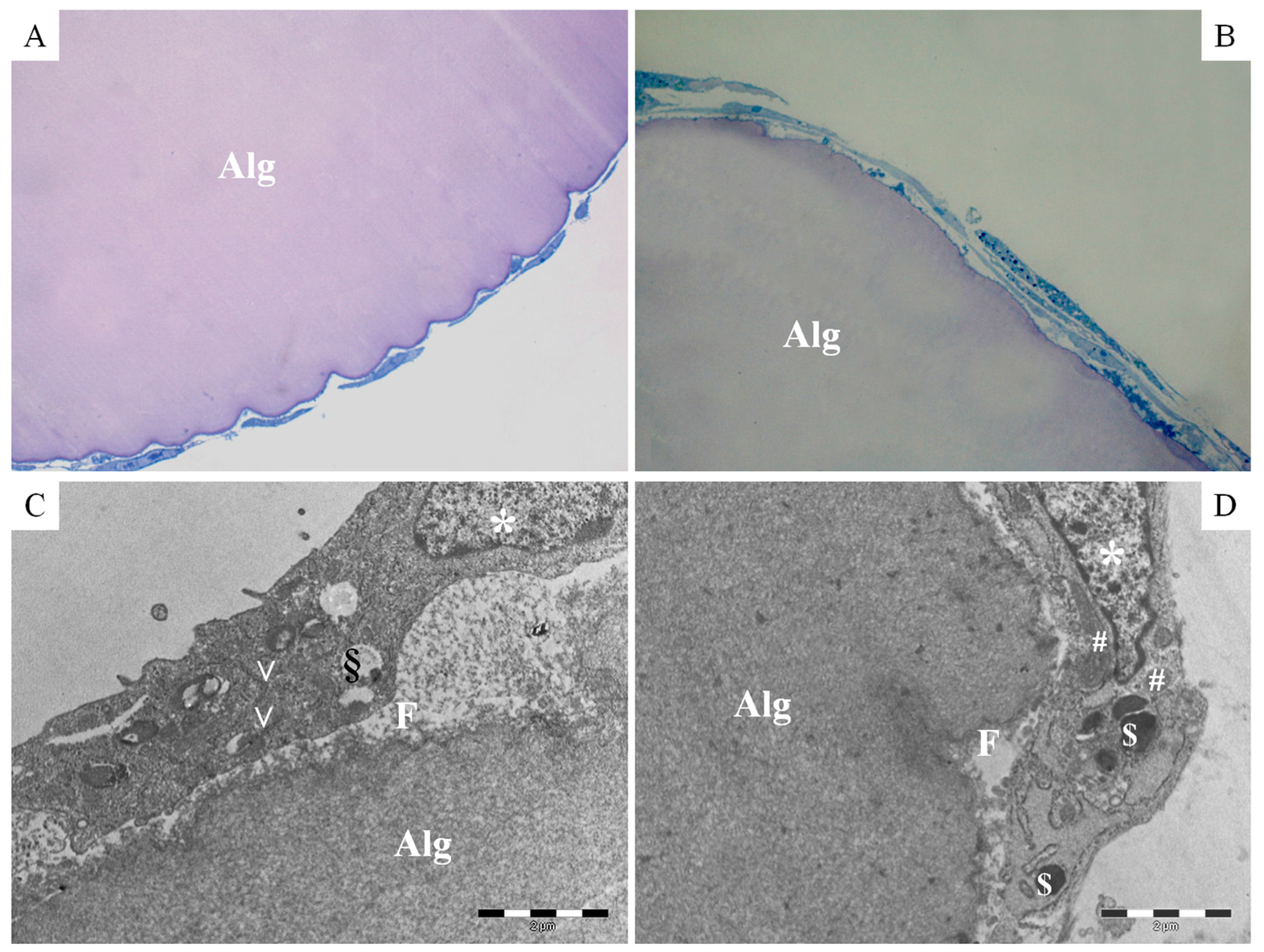
2.2. Physico-Chemical and Morphological Characterization of Microcarriers
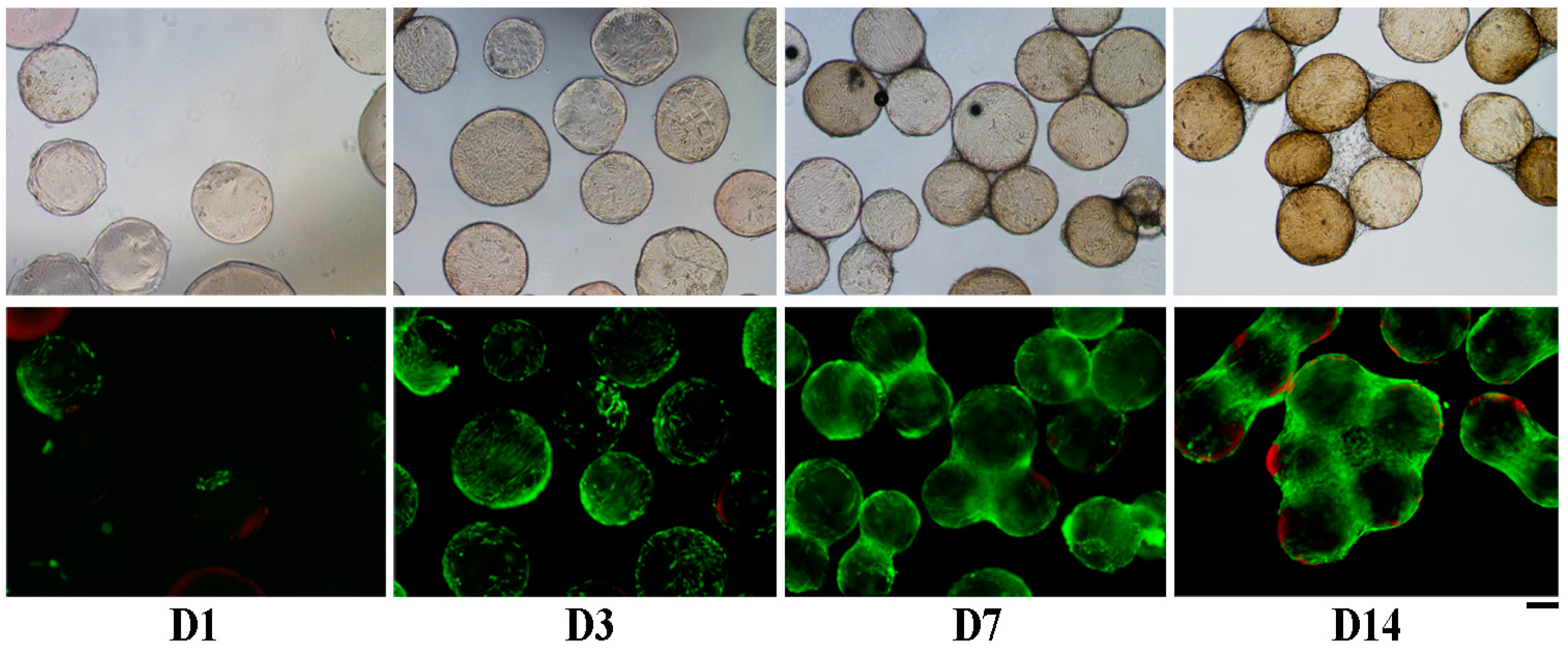
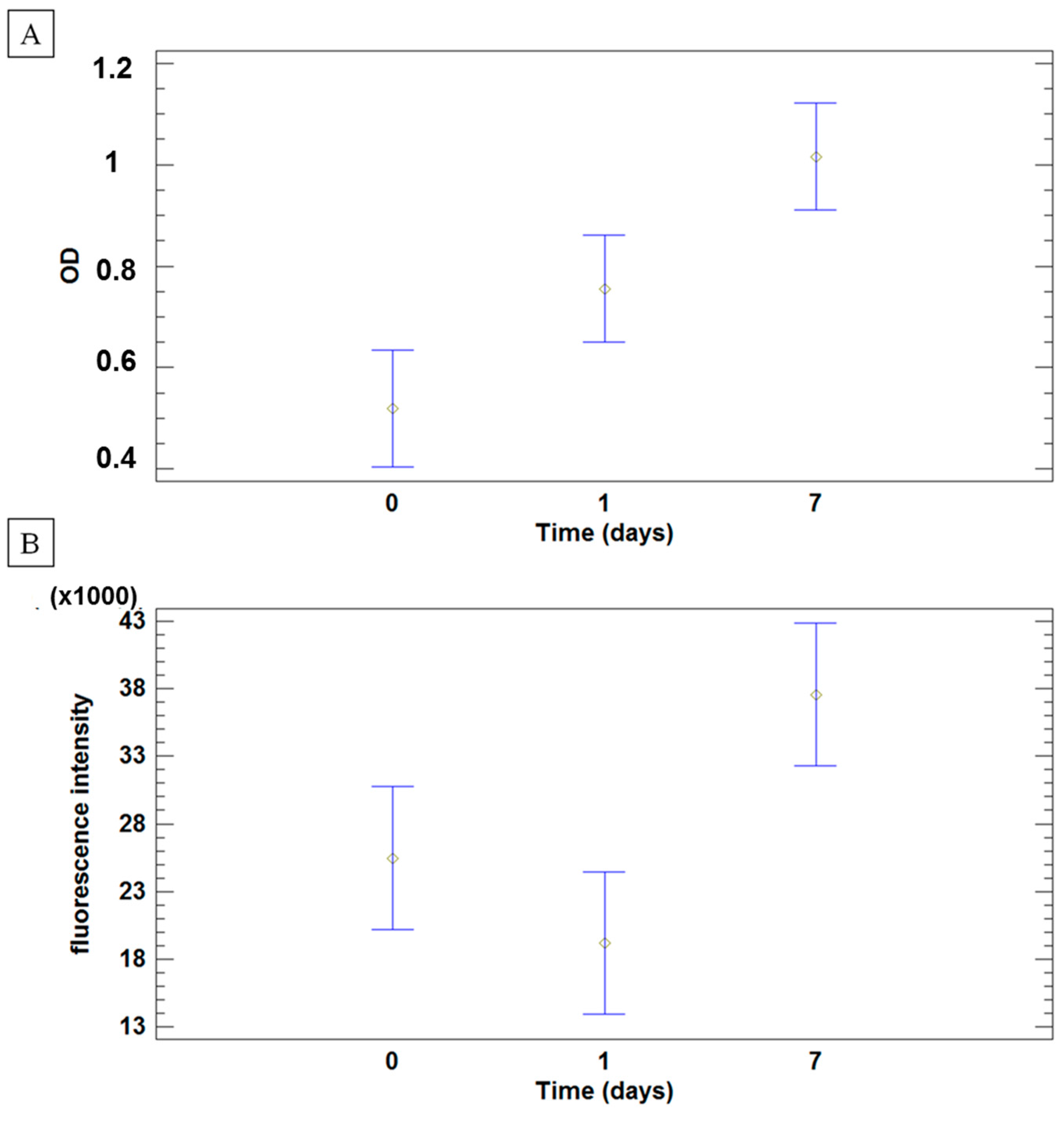
2.3. Human Adipose Derived Stem Cells (hASCs) Viability and Proliferation
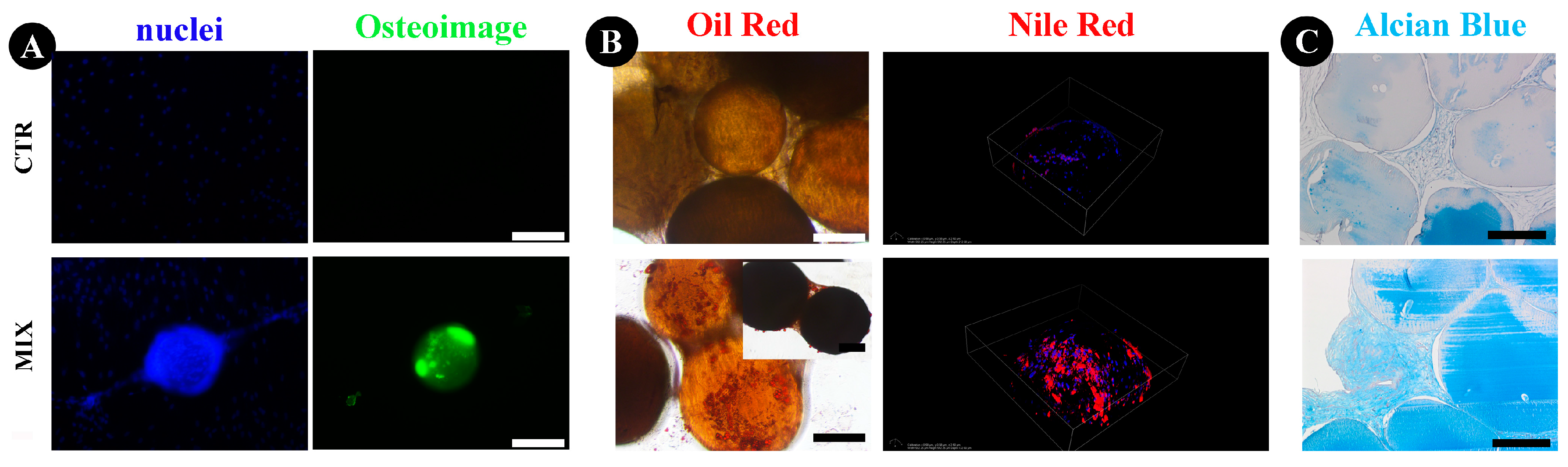
2.4. Human ASCs Multi-Differentiative Potential
3. Discussion
4. Materials and Methods
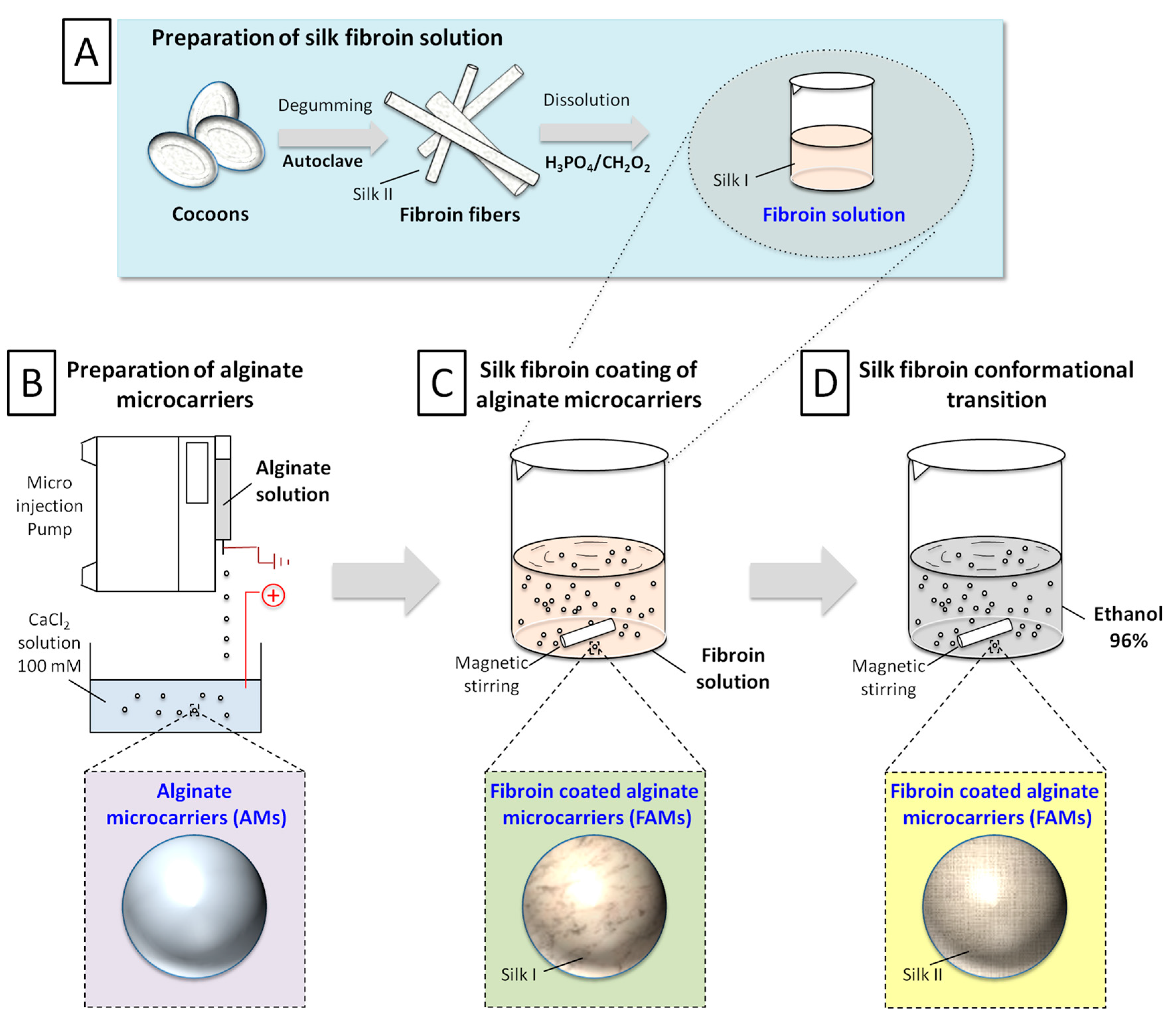
4.1. Preparation of Microcarriers
4.2. Characterization of FAMs
4.2.1. Particle Size
4.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
4.2.3. Scanning Electron Microscopy (SEM)–Energy Dispersive X-ray (EDX) Analysis
4.2.4. Confocal Laser Scanning Microscopy (CLSM)
4.3. Cytocompatibility of FAMs, Cell Proliferation and Cell Differentiation Potential
4.3.1. Isolation and Monolayer Culture of Human Stromal Cells (hASCs)
4.3.2. Cell Seeding on FAMs
4.3.3. Cell Viability and Adhesion
4.3.4. Metabolic Assays
4.3.5. Immunofluorescent Staining for Cytoskeletal Actin
4.3.6. Transmission Electron Microscopy (TEM)
4.3.7. Multilineage Differentiation of hASCs
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AM | Alginate microcarrier |
| ASC | Adipose mesenchymal Stem Cell |
| EDX | Energy dispersive X-ray |
| FAM | Fibroin-coated alginate microcarrier |
| FTIR | Fourier transform infrared spectroscopy |
| MSC | Mesenchymal stem cell |
References
- Van Wezel, A.L. Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature 1967, 216, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.K.-L.; Reuveny, S.; Oh, S.K.W. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: Achievements and future direction. Biotechnol. Adv. 2013, 31, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Frondoza, C.G. Microcarriers in the engineering of cartilage and bone. Trends Biotechnol. 2006, 24, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Markvicheva, E.; Grandfils, C. Microcarriers for animal cell culture. In Fundamentals of Cell Immobilisation Biotechnology; Nedović, V., Willaert, R., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 141–161. [Google Scholar]
- Quittet, M.-S.; Touzani, O.; Sindji, L.; Cayon, J.; Fillesoye, F.; Toutain, J.; Divoux, D.; Marteau, L.; Lecocq, M.; Roussel, S.; et al. Effects of mesenchymal stem cell therapy, in association with pharmacologically active microcarriers releasing VEGF, in an ischaemic stroke model in the rat. Acta Biomater. 2015, 15, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgi, N.; van Blitterswijk, C.; Karperien, M. Mesenchymal stromal/stem cell-or chondrocyte-seeded microcarriers as building blocks for cartilage tissue engineering. Tissue Eng. Part A 2014, 20, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rossi, F.M.V.; Putnins, E.E. Ex vivo expansion of rat bone marrow mesenchymal stromal cells on microcarrier beads in spin culture. Biomaterials 2007, 28, 3110–3120. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, X.; Ye, Z.; Zhang, Y.; Zhou, Y.; Tan, W.-S. A modular approach to the engineering of a centimeter-sized bone tissue construct with human amniotic mesenchymal stem cells-laden microcarriers. Biomaterials 2011, 32, 7532–7542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, Z.; Zhang, H.; Lu, W.; Liu, S.; Huang, X.; Luo, H.; Jin, Y. Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng. Part A 2011, 17, 2981–2997. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-Y.; Hsieh, D.-K.; Syu, W.-S.; Li, Y.-S.; Chiu, H.-T.; Chiou, T.-W. Cell proliferation of human bone marrow mesenchymal stem cells on biodegradable microcarriers enhances in vitro differentiation potential. Cell Prolif. 2010, 43, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Goh, T.K.-P.; Zhang, Z.-Y.; Chen, A.K.-L.; Reuveny, S.; Choolani, M.; Chan, J.K.Y.; Oh, S.K.-W. Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores. Open Access 2013, 2, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Villani, S.; Marazzi, M.; Bucco, M.; Faustini, M.; Klinger, M.; Gaetani, P.; Crovato, F.; Vigo, D.; Caviggioli, F.; Torre, M.L. Statistical approach in alginate membrane formulation for cell encapsulation in a GMP-based cell factory. Acta Biomater. 2008, 4, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, P.; Torre, M.L.; Klinger, M.; Faustini, M.; Crovato, F.; Bucco, M.; Marazzi, M.; Chlapanidas, T.; Levi, D.; Tancioni, F.; et al. Adipose-derived stem cell therapy for intervertebral disc regeneration: An in vitro reconstructed tissue in alginate capsules. Tissue Eng. Part A 2008, 14, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, G.; Nguyen, B.-N.B.; Campardelli, R.; Reverchon, E.; Fisher, J.P. Synergistic effect of sustained release of growth factors and dynamic culture on osteoblastic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. A 2014, 1–11. [Google Scholar]
- Chang, T.M. Semipermeable microcapsules. Science 1964, 146, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Steward, A.J.; Liu, Y.; Wagner, D.R. Engineering cell attachments to scaffolds in cartilage tissue engineering. JOM 2011, 63, 74–82. [Google Scholar] [CrossRef]
- Schmidt, J.J.; Jeong, J.; Kong, H. The interplay between cell adhesion cues and curvature of cell adherent alginate microgels in multipotent stem cell culture. Tissue Eng. Part A 2011, 17, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Yucel, T.; Lovett, M.L.; Kaplan, D.L. Silk-based biomaterials for sustained drug delivery. J. Control. Release 2014, 190, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, Y.; Lin, Y.; Shen, J.; Wang, D.-A. A novel gellan gel-based microcarrier for anchorage-dependent cell delivery. Acta Biomater. 2008, 4, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Zichner, L.; Langer, R.; Kaplan, D.; Vunjak-Novakovic, G. Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnol. Bioeng. 2004, 88, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Uebersax, L.; Merkle, H.P.; Meinel, L. Insulin-like growth factor I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells. J. Control. Release 2008, 127, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Hagenmuller, H.; Koch, A.M.; Muller, R.; Vunjak-Novakovic, G.; Kaplan, D.L.; Merkle, H.P.; Meinel, L. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials 2007, 28, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Mauney, J.R.; Nguyen, T.; Gillen, K.; Kirker-Head, C.; Gimble, J.M.; Kaplan, D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 2007, 28, 5280–5290. [Google Scholar] [CrossRef] [PubMed]
- Altman, A.M.; Gupta, V.; Rios, C.N.; Alt, E.U.; Mathur, A.B. Adhesion, migration and mechanics of human adipose-tissue-derived stem cells on silk fibroin-chitosan matrix. Acta Biomater. 2010, 6, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Chlapanidas, T.; Farago, S.; Mingotto, F.; Crovato, F.; Tosca, M.C.; Antonioli, B.; Bucco, M.; Lucconi, G.; Scalise, A.; Vigo, D.; et al. Regenerated silk fibroin scaffold and infrapatellar adipose stromal vascular fraction as feeder-layer: A new product for cartilage advanced therapy. Tissue Eng. Part A 2011, 17, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Chlapanidas, T.; Perteghella, S.; Faragò, S.; Boschi, A.; Tripodo, G.; Vigani, B.; Crivelli, B.; Renzi, S.; Dotti, S.; Preda, S.; et al. Platelet lysate and adipose mesenchymal stromal cells on silk fibroin nonwoven mats for wound healing. J. Appl. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise Review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells Regen. Med. 2012, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Manferdini, C.; Maumus, M.; Gabusi, E.; Piacentini, A.; Filardo, G.; Peyrafitte, J.-A.; Jorgensen, C.; Bourin, P.; Fleury-Cappellesso, S.; Facchini, A.; et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013, 65, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, L.; Lucarelli, E.; Alessandri, G.; Avanzini, M.A.; Bernardo, M.E.; Biagi, E.; Brini, A.T.; D’Amico, G.; Fagioli, F.; Ferrero, I.; et al. Mesenchymal stem/stromal cells: A new “cells as drugs” paradigm. Efficacy and critical aspects in cell therapy. Curr. Pharm. Des. 2013, 19, 2459–2473. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Jorgensen, C.; Noel, D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 2013, 95, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.R.; Pollock, K.; Hubel, A.; McKenna, D. Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion 2014, 54, 1418–1437. [Google Scholar] [CrossRef] [PubMed]
- Marmotti, A.; de Girolamo, L.; Bonasia, D.E.; Bruzzone, M.; Mattia, S.; Rossi, R.; Montaruli, A.; Dettoni, F.; Castoldi, F.; Peretti, G. Bone marrow derived stem cells in joint and bone diseases: A concise review. Int. Orthop. 2014, 38, 1787–1801. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells. Tissues. Organs 2003, 174, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Lott, K.E.; Awad, H.A.; Cao, Q.; Hicok, K.C.; Fermor, B.; Gimble, J.M. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J. Cell. Physiol. 2006, 206, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Gao, T.; Yang, Z.-L.; Zhang, S.-C.; Ren, M.-L.; Wang, Z.-G.; Zhang, B. Adipose-derived stem cells induced dendritic cells undergo tolerance and inhibit Th1 polarization. Cell. Immunol. 2012, 278, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Jo, T.T.; Kim, S.G.; Kwon, K.J.; Kweon, H.Y.; Chae, W.S.; Yang, W.G.; Lee, E.Y.; Seok, H. Silk fibroin-alginate-hydroxyapatite composite particles in bone tissue engineering applications in vivo. Int. J. Mol. Sci. 2017, 18, 858. [Google Scholar] [CrossRef] [PubMed]
- Farago, S.; Lucconi, G.; Perteghella, S.; Vigani, B.; Tripodo, G.; Sorrenti, M.; Catenacci, L.; Boschi, A.; Faustini, M.; Vigo, D.; et al. A dry powder formulation from silk fibroin microspheres as a topical auto-gelling device. Pharm. Dev. Technol. 2016, 21, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Perteghella, S.; Crivelli, B.; Catenacci, L.; Sorrenti, M.; Bruni, G.; Necchi, V.; Vigani, B.; Sorlini, M.; Torre, M.L.; Chlapanidas, T. Stem cell-extracellula vesicles as drug delivery systems: New frontiers for silk/curcumin nanoparticles. Int. J. Pharm. 2017, 520, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Chlapanidas, T.; Farago, S.; Lucconi, G.; Perteghella, S.; Galuzzi, M.; Mantelli, M.; Avanzini, M.A.; Tosca, M.C.; Marazzi, M.; Vigo, D.; et al. Sericins exhibit ROS-scavenging, anti-tyrosinase, anti-elastase, and in vitro immunomodulatory activities. Int. J. Biol. Macromol. 2013, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Lee, J.; Lim, J.F.Y.; Choolani, M.; Chan, J.K.Y.; Reuveny, S.; Oh, S.K.W. Critical attributes of human early mesenchymal stromal cell-laden microcarrier constructs for improved chondrogenic differentiation. Stem Cell Res. Ther. 2017, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Olmos, E.; Balandras, F.; Tran, N.; Chevalot, I.; Guedon, E.; Marc, A. Investigation of growth conditions for the expansion of porcine mesenchymal stem cells on microcarriers in stirred cultures. Appl. Biochem. Biotechnol. 2014, 172, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.P.; Bennett, J.M.; Doctor, J.S.; Tebbets, B.M.; Marra, K.G. Collagenous microbeads as a scaffold for tissue engineering with adipose-derived stem cells. Plast. Reconstr. Surg. 2007, 120, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Shin, Y.N.; Cho, M.H.; Kim, S.H.; Kim, S.K.; Cho, Y.H.; Khang, G.; Lee, I.W.; Lee, H.B. Adhesion behavior of human bone marrow stromal cells on differentially wettable polymer surfaces. Tissue Eng. 2007, 13, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, Y.; Wang, X.; Lin, T. Layer-by-layer assembly of silica nanoparticles on 3D fibrous scaffolds: Enhancement of osteoblast cell adhesion, proliferation, and differentiation. J. Biomed. Mater. Res. Part A 2014, 102, 3803–3812. [Google Scholar] [CrossRef] [PubMed]
- Dumbauld, D.W.; Shin, H.; Gallant, N.D.; Michael, K.E.; Radhakrishna, H.; Garcia, A.J. Contractility modulates cell adhesion strengthening through focal adhesion kinase and assembly of vinculin-containing focal adhesions. J. Cell. Physiol. 2010, 223, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-C.; Young, T.-H.; Wang, T.-M.; Peng, H.-W.; Hou, S.-M.; Yen, M.-L. Spontaneous osteogenesis of MSCs cultured on 3D microcarriers through alteration of cytoskeletal tension. Biomaterials 2012, 33, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.R.; Orellana, M.D.; Mizukami, A.; Fernandes, T.R.; Fontes, A.M.; Suazo, C.A.T.; Oliveira, V.C.; Covas, D.T.; Swiech, K. Growth and functional harvesting of human mesenchymal stromal cells cultured on a microcarrier-based system. Biotechnol. Prog. 2014, 30, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Heathman, T.R.J.; Nienow, A.W.; McCall, M.J.; Coopman, K.; Kara, B.; Hewitt, C.J. The translation of cell-based therapies: Clinical landscape and manufacturing challenges. Regen. Med. 2015, 10, 49–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Kallos, M.S.; Hunter, C.; Sen, A. Improved expansion of human bone marrow-derived mesenchymal stem cells in microcarrier-based suspension culture. J. Tissue Eng. Regen. Med. 2014, 8, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Enea, D.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Manzotti, S.; Gigante, A. One-step cartilage repair in the knee: Collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee 2015, 22, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Calcagno, S.; Cecconi, S.; Ramazzotti, D.; Manzotti, S.; Enea, D. Use of collagen scaffold and autologous bone marrow concentrate as a one-step cartilage repair in the knee: Histological results of second-look biopsies at 1 year follow-up. Int. J. Immunopathol. Pharmacol. 2011, 24, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Mastracci, L.; Grillo, F.; Perteghella, S.; Preda, S.; Crivelli, B.; Antonioli, B.; Galuzzi, M.; Tosca, M.C.; Marazzi, M.; et al. Local biological effects of adipose stromal vascular fraction delivery systems after subcutaneous implantation in a murine model. J. Bioact. Compat. Polym. 2016, 31, 600–612. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Perteghella, S.; Leoni, F.; Farago, S.; Marazzi, M.; Rossi, D.; Martino, E.; Gaggeri, R.; Collina, S. TNF-α blocker effect of naringenin-loaded sericin microparticles that are potentially useful in the treatment of psoriasis. Int. J. Mol. Sci. 2014, 15, 13624–13636. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, L.; Lopa, S.; Arrigoni, E.; Sartori, M.F.; Baruffaldi Preis, F.W.; Brini, A.T. Human adipose-derived stem cells isolated from young and elderly women: Their differentiation potential and scaffold interaction during in vitro osteoblastic differentiation. Cytotherapy 2009, 11, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.G.; Marano, F.; Rinella, L.; de Girolamo, L.; Bosco, O.; Fortunati, N.; Berta, L.; Frairia, R. Extracorporeal shockwaves (ESWs) enhance the osteogenic medium-induced differentiation of adipose-derived stem cells into osteoblast-like cells. J. Tissue Eng. Regen. Med. 2017, 11, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Faustini, M.; Bucco, M.; Chlapanidas, T.; Lucconi, G.; Marazzi, M.; Tosca, M.C.; Gaetani, P.; Klinger, M.; Villani, S.; Ferretti, V.V.; et al. Nonexpanded mesenchymal stem cells for regenerative medicine: Yield in stromal vascular fraction from adipose tissues. Tissue Eng. Part C Methods 2010, 16, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Schop, D.; van Dijkhuizen-Radersma, R.; Borgart, E.; Janssen, F.W.; Rozemuller, H.; Prins, H.-J.; de Bruijn, J.D. Expansion of human mesenchymal stromal cells on microcarriers: Growth and metabolism. J. Tissue Eng. Regen. Med. 2010, 4, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Pierini, M.; Di Bella, C.; Dozza, B.; Frisoni, T.; Martella, E.; Bellotti, C.; Remondini, D.; Lucarelli, E.; Giannini, S.; Donati, D. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J. Bone Jt. Surg. Am. 2013, 95, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.; Ploegert, S.; Heberer, M.; Martin, I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003, 48, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chong, M.; Chan, J.; Zhang, Z.; Teoh, S.H.; Thian, E.S. A scalable approach to obtain mesenchymal stem cells with osteogenic potency on apatite microcarriers. J. Biomater. Appl. 2013, 29, 93–103. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perteghella, S.; Martella, E.; De Girolamo, L.; Perucca Orfei, C.; Pierini, M.; Fumagalli, V.; Pintacuda, D.V.; Chlapanidas, T.; Viganò, M.; Faragò, S.; et al. Fabrication of Innovative Silk/Alginate Microcarriers for Mesenchymal Stem Cell Delivery and Tissue Regeneration. Int. J. Mol. Sci. 2017, 18, 1829. https://doi.org/10.3390/ijms18091829
Perteghella S, Martella E, De Girolamo L, Perucca Orfei C, Pierini M, Fumagalli V, Pintacuda DV, Chlapanidas T, Viganò M, Faragò S, et al. Fabrication of Innovative Silk/Alginate Microcarriers for Mesenchymal Stem Cell Delivery and Tissue Regeneration. International Journal of Molecular Sciences. 2017; 18(9):1829. https://doi.org/10.3390/ijms18091829
Chicago/Turabian StylePerteghella, Sara, Elisa Martella, Laura De Girolamo, Carlotta Perucca Orfei, Michela Pierini, Valentina Fumagalli, Domenica Valeria Pintacuda, Theodora Chlapanidas, Marco Viganò, Silvio Faragò, and et al. 2017. "Fabrication of Innovative Silk/Alginate Microcarriers for Mesenchymal Stem Cell Delivery and Tissue Regeneration" International Journal of Molecular Sciences 18, no. 9: 1829. https://doi.org/10.3390/ijms18091829