RNA Interference and BMP-2 Stimulation Allows Equine Chondrocytes Redifferentiation in 3D-Hypoxia Cell Culture Model: Application for Matrix-Induced Autologous Chondrocyte Implantation
Abstract
:1. Introduction
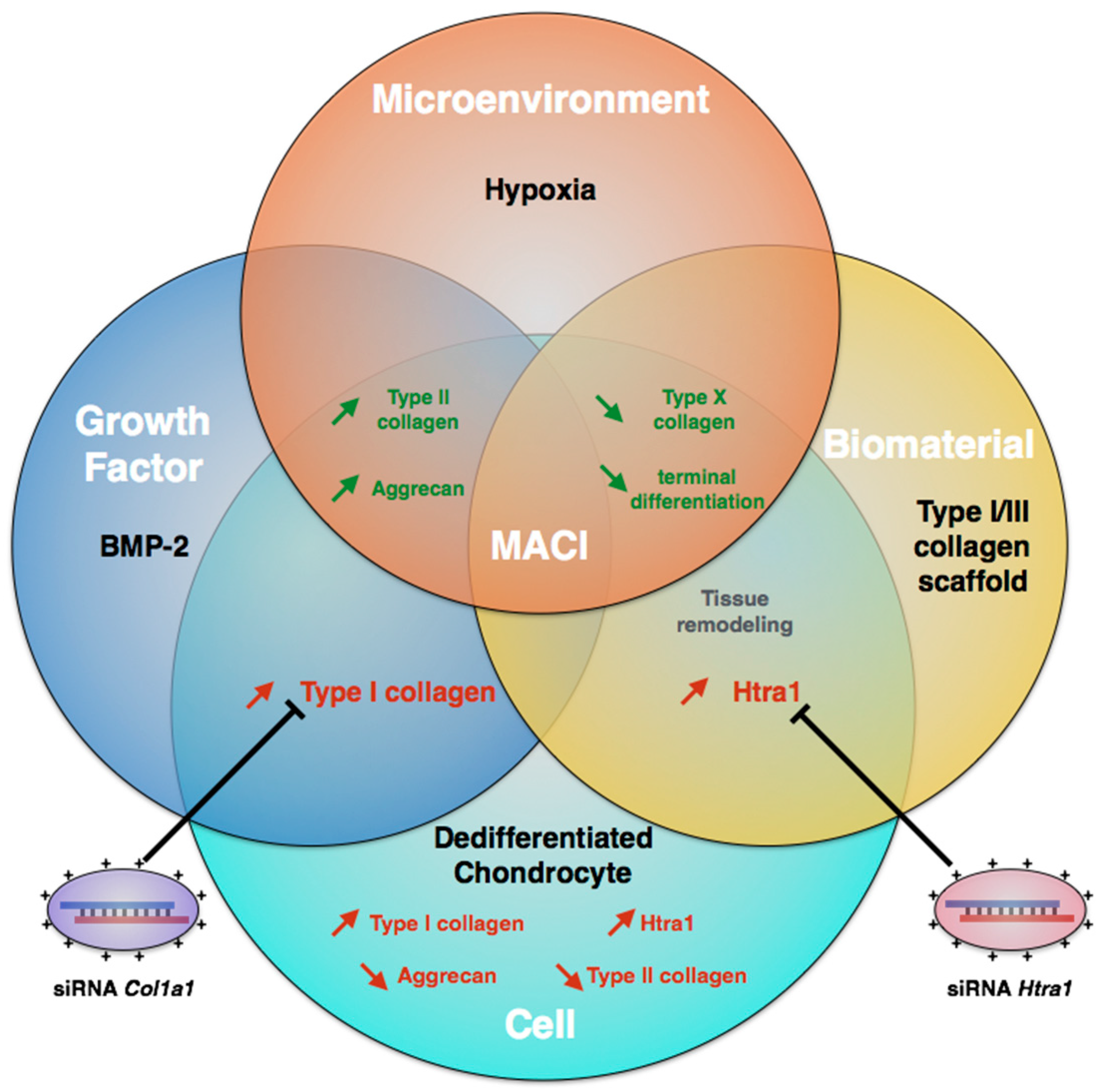
2. Results
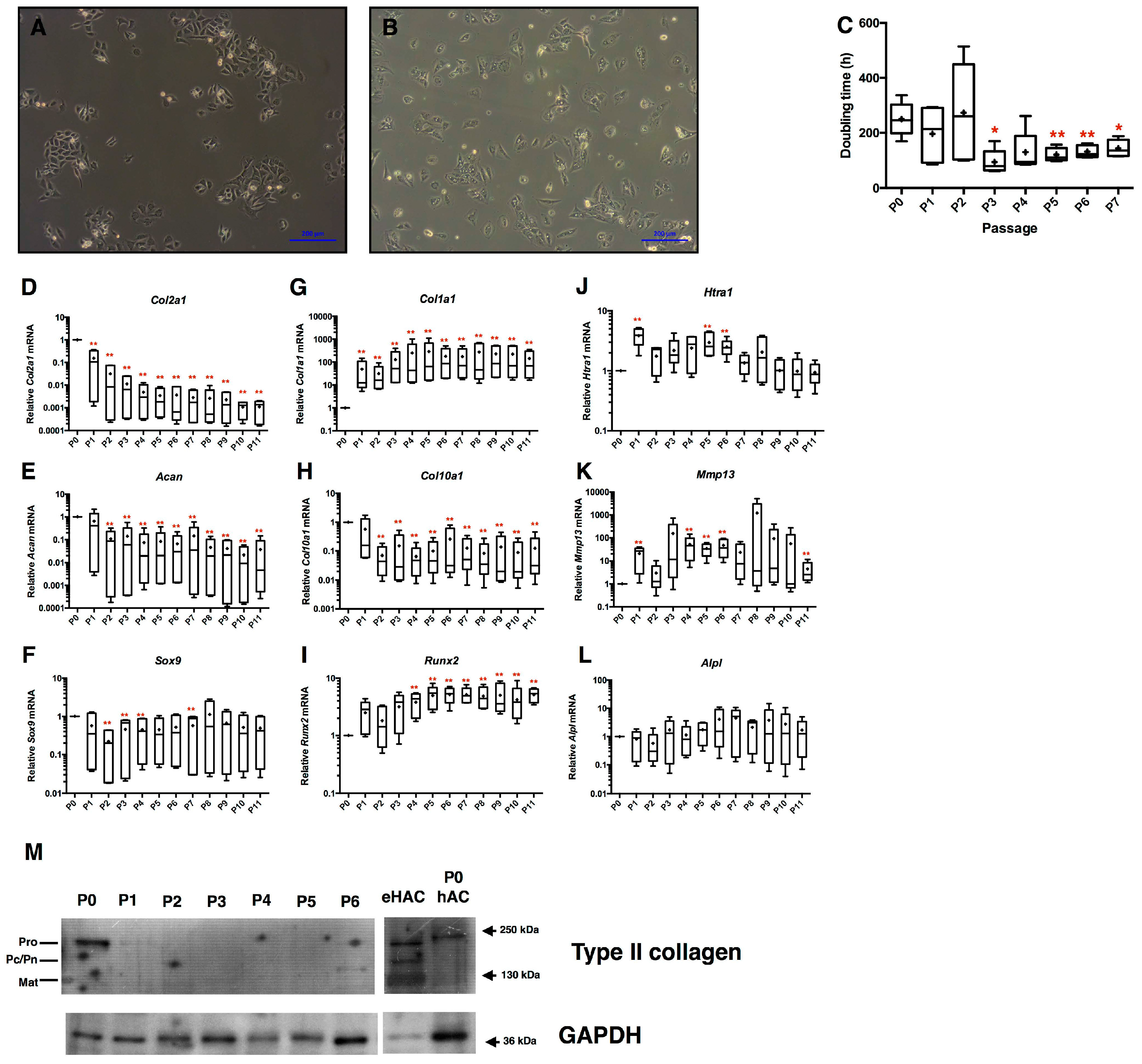
2.1. Loss of Equine Articular Chondrocyte Phenotype during Amplification
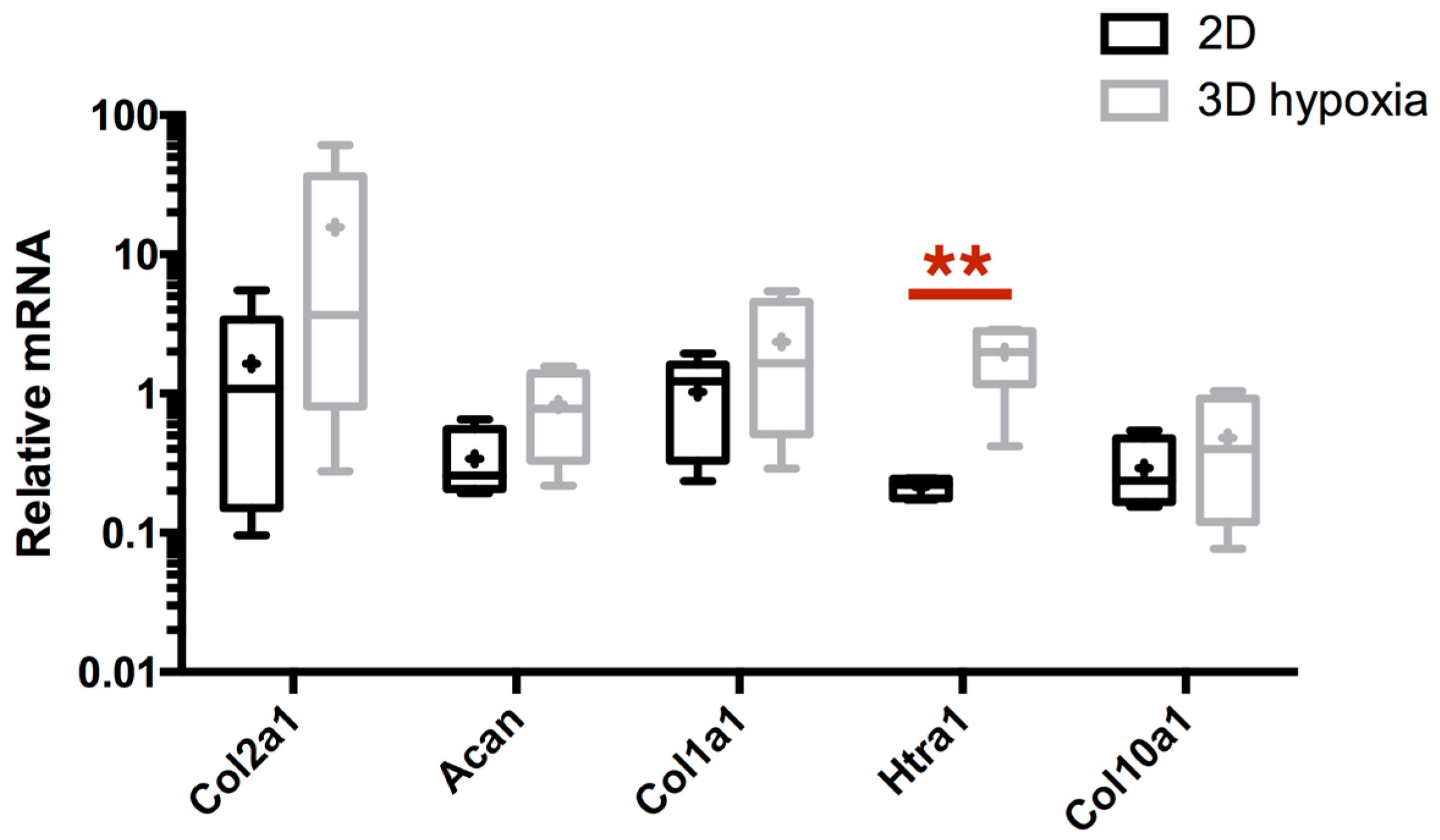
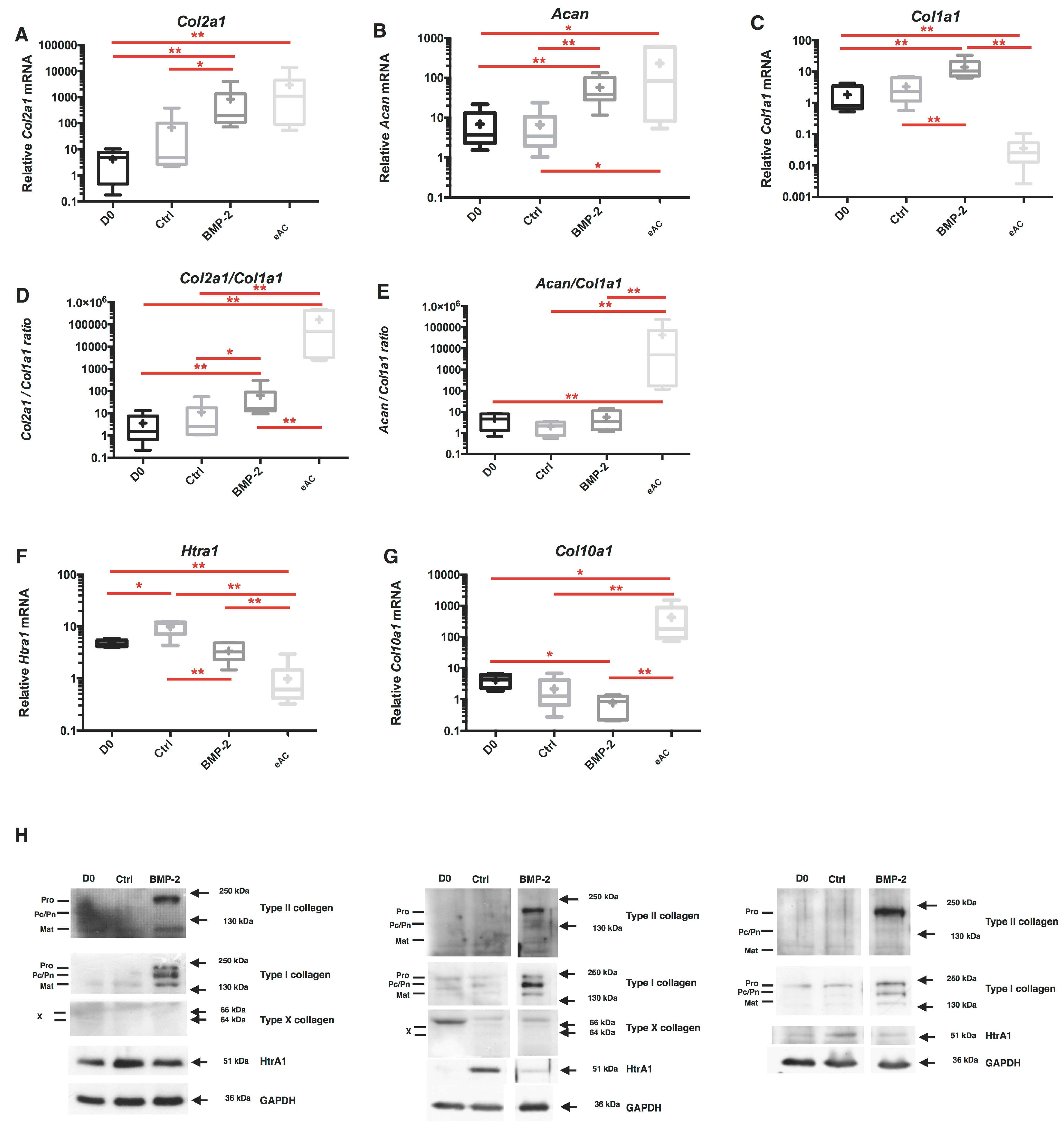
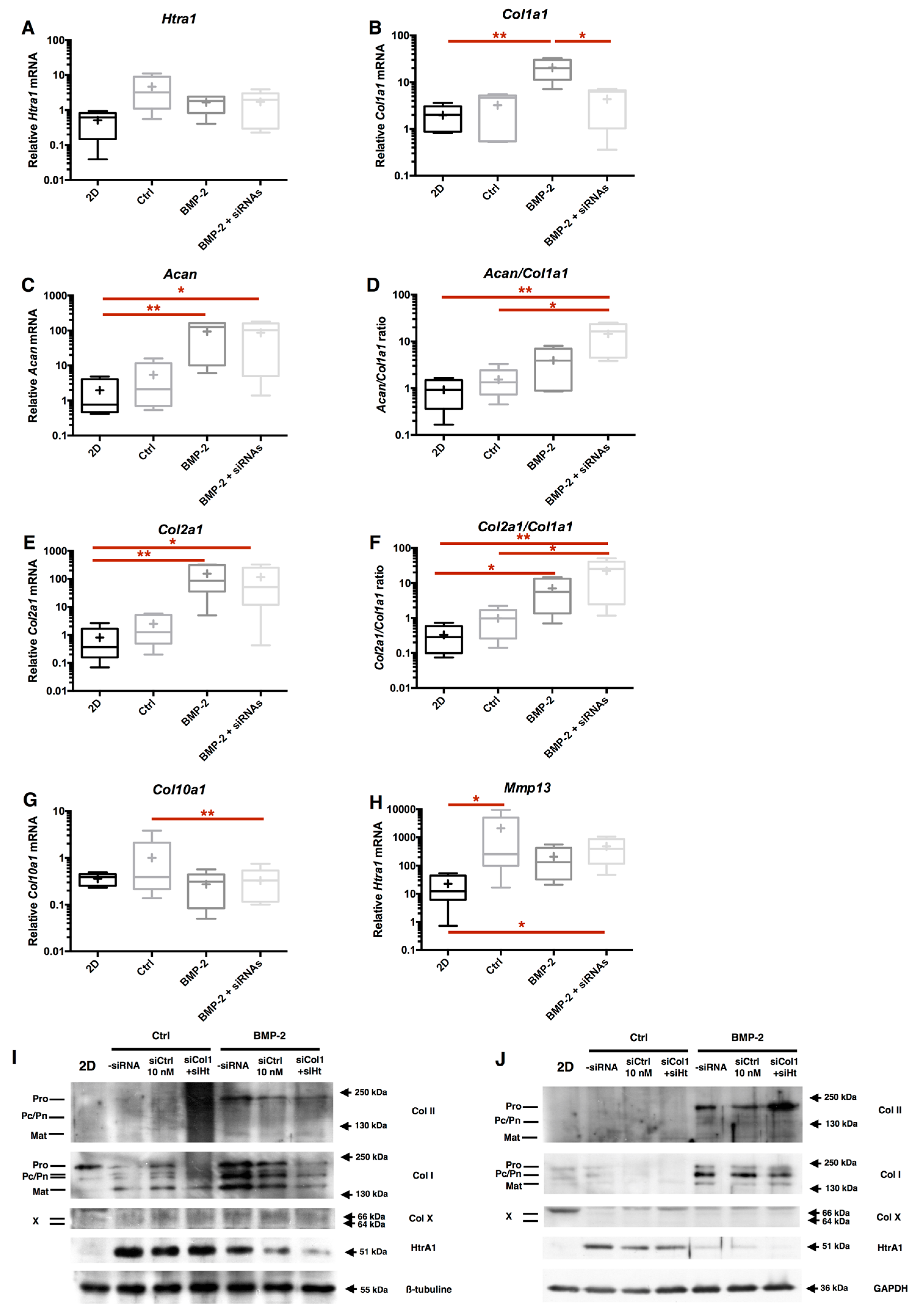
2.2. Combination of 3D-Cell Culture, Hypoxia, and BMP-2 Treatment Allows the Recovery of Cartilage Specific Marker Expression by eAC, but Does Not Abolish the Fibrotic and Catabolic Components
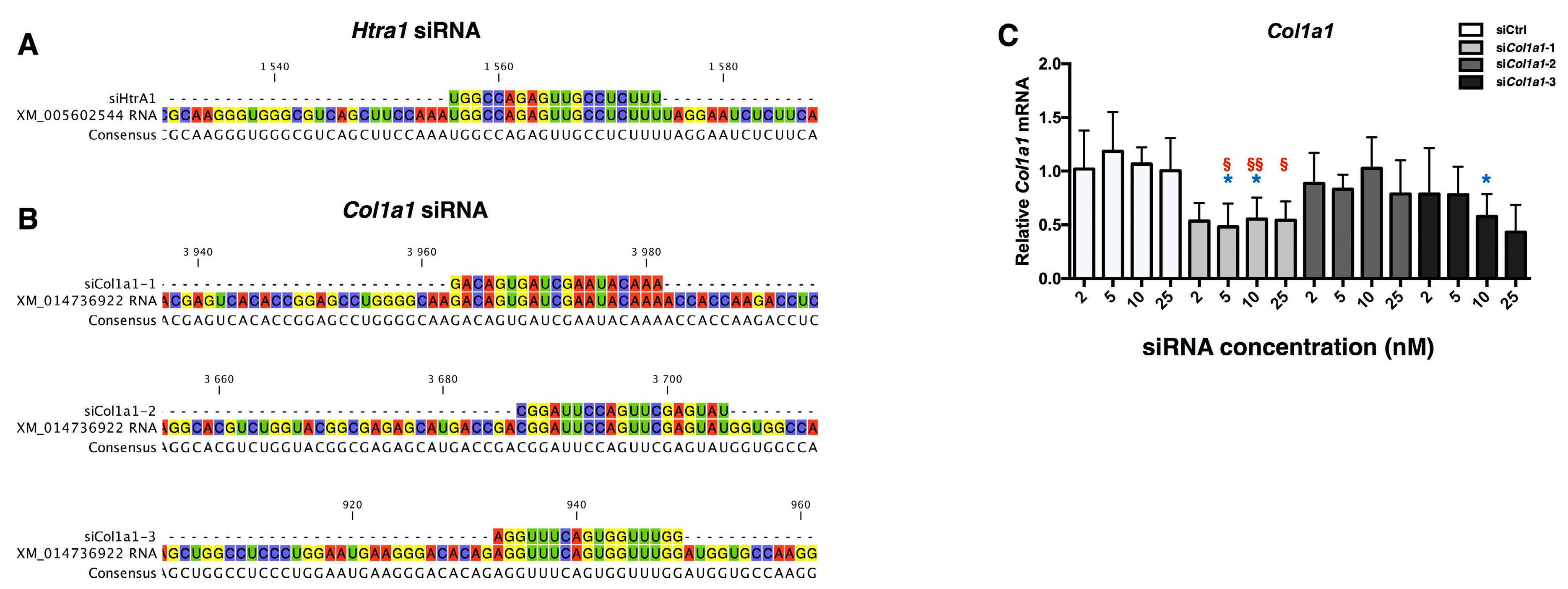
2.3. Design of siRNA
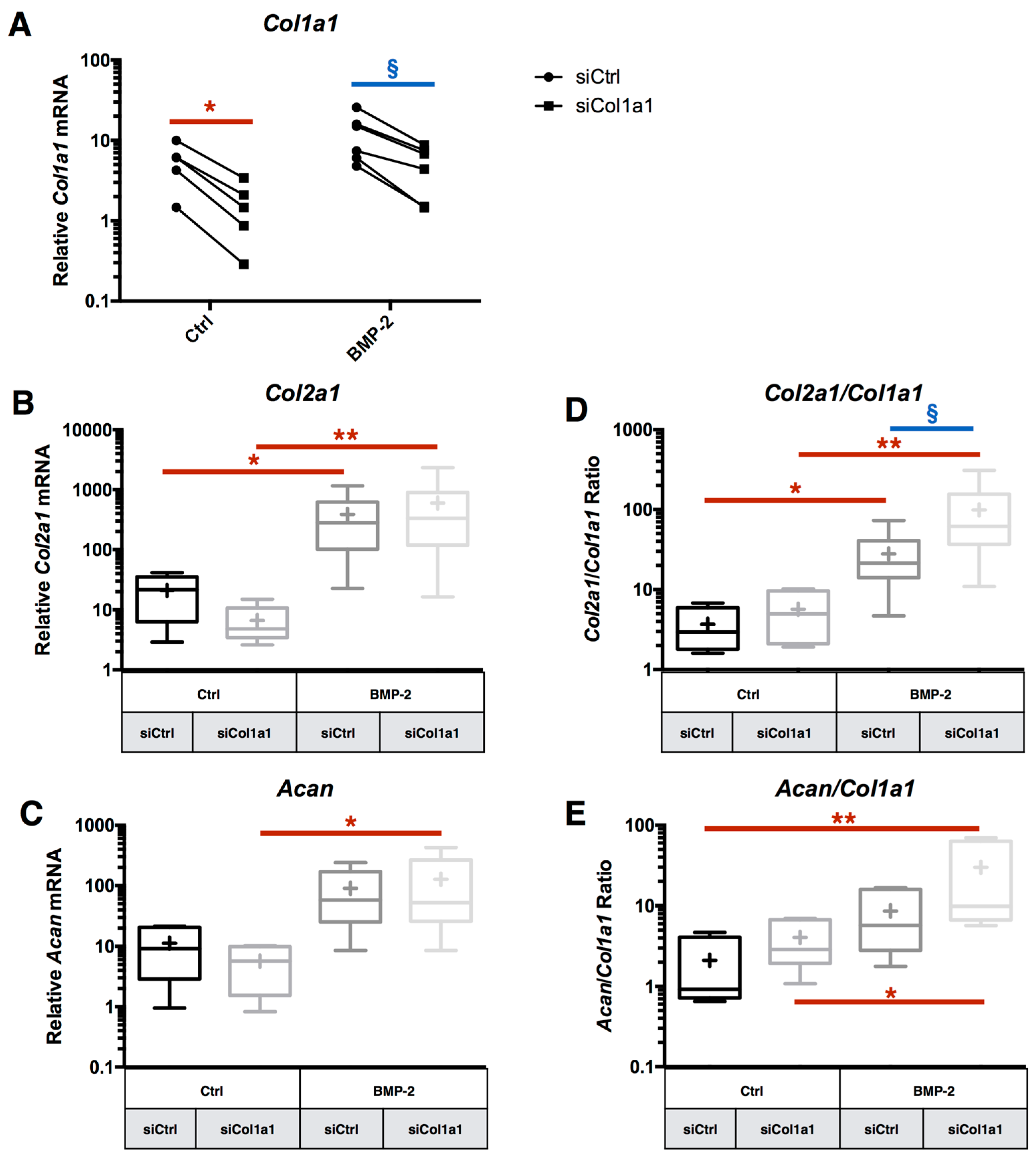
2.4. RNA Interference Targeting Col1a1 Improves eAC Specific Phenotypic Profile during the MACI Process
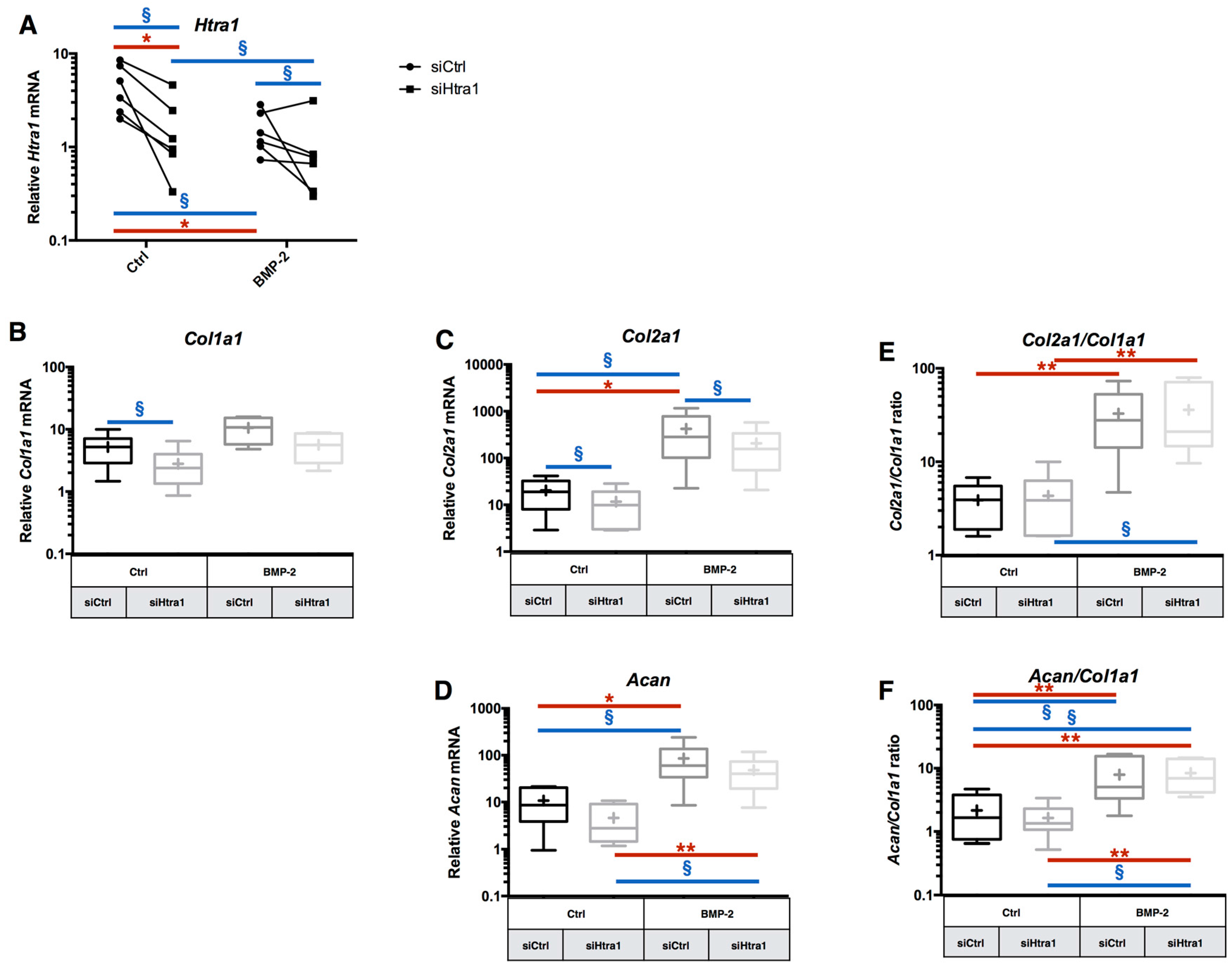
2.5. RNA Interference Targeting Htra1 Can Modulate the eAC Catabolic Process during Chondrocyte Redifferentiation
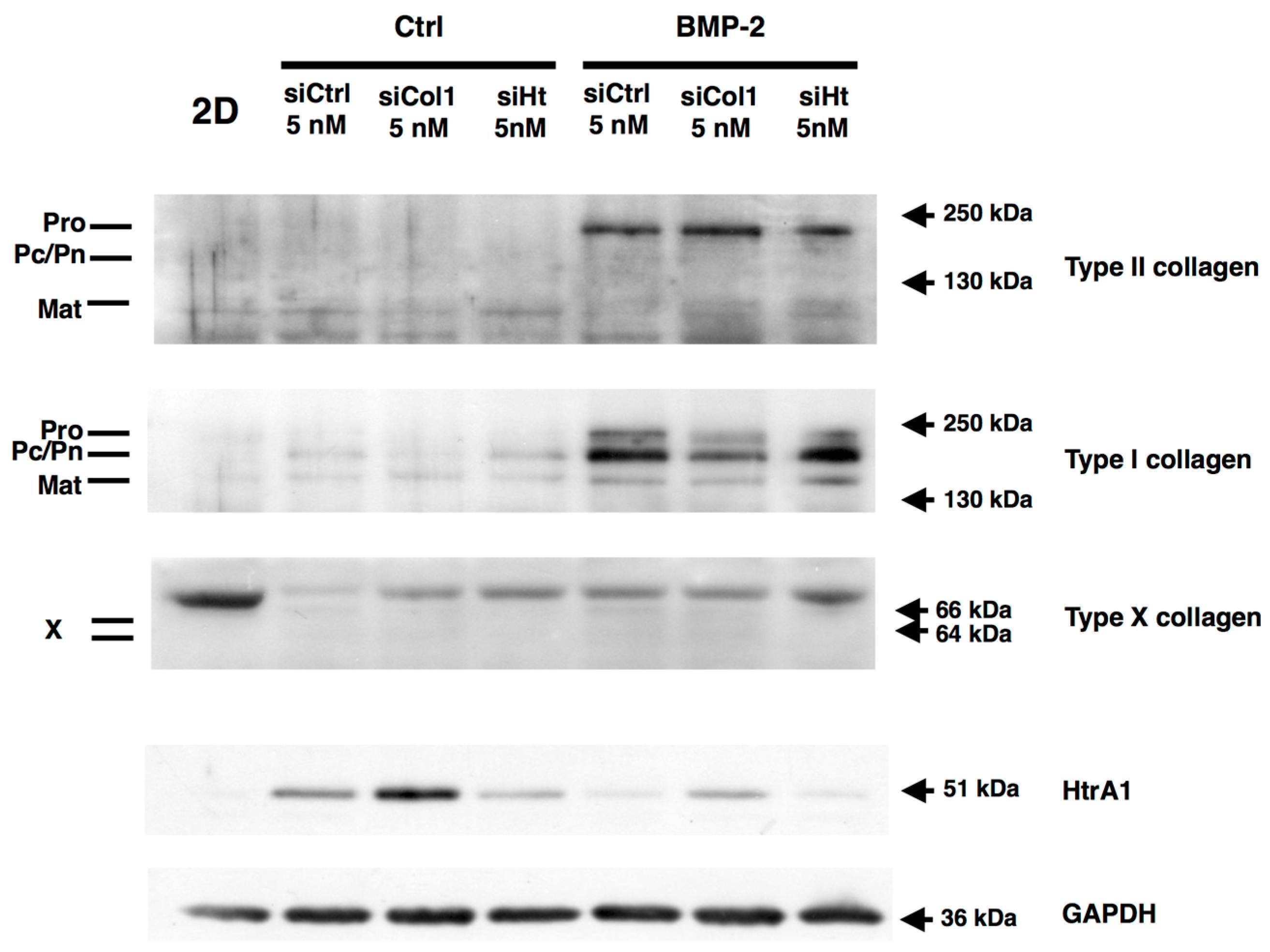
2.6. RNA Interference during the MACI Process Increases the Quality of the ECM Synthesized by eAC
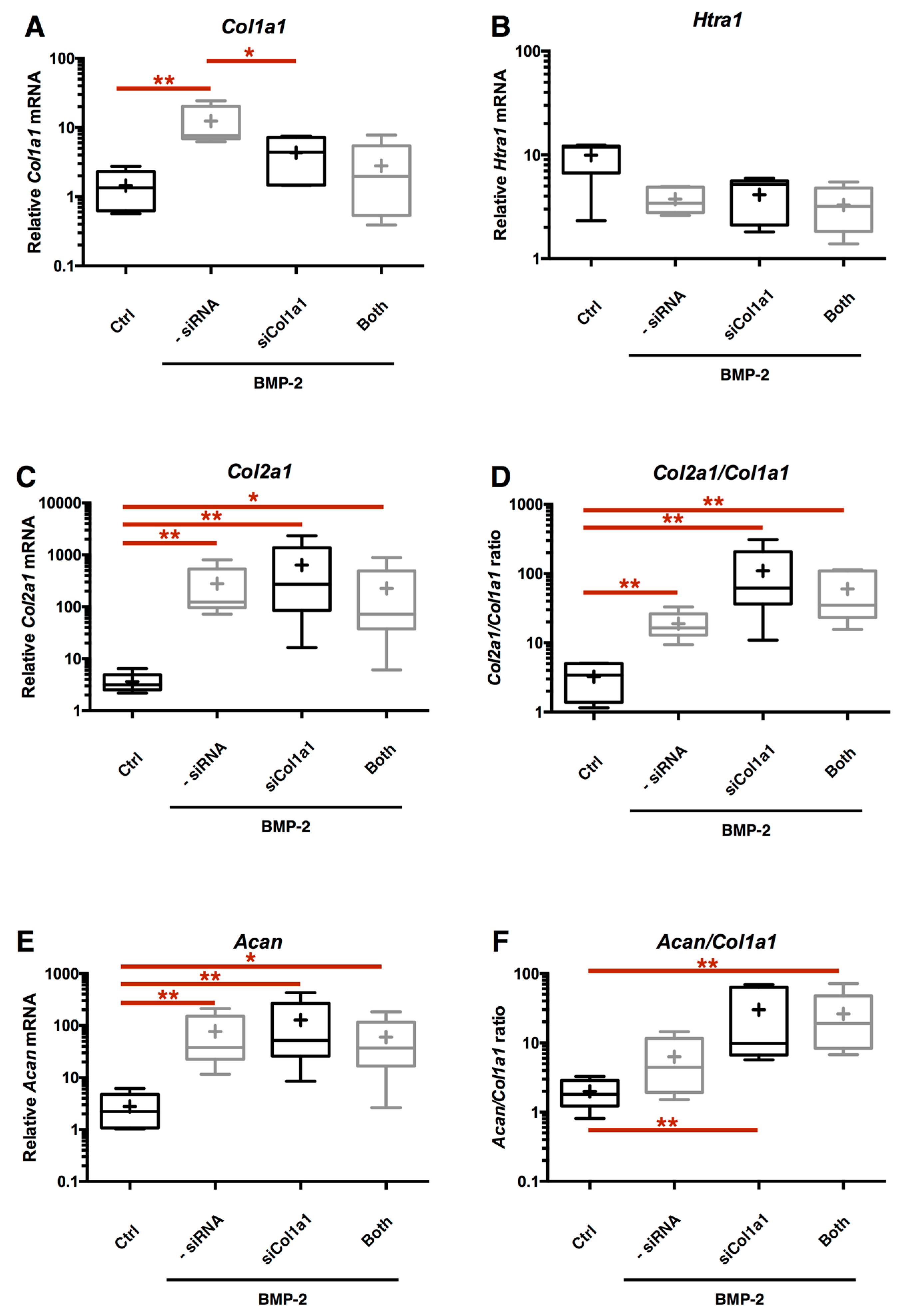
2.7. Chondrocyte Redifferentiation Mediated by BMP-2 Can Be Modulated by Both Col1a1 and Htra1 siRNAs
3. Discussion
4. Materials and Methods
4.1. eAC Isolation and Cell Culture
4.2. eAC Dedifferentiation
4.3. Cryopreservation
4.4. 3D Inclusion and Hypoxia Cell Culture
4.5. siRNA Transfection and In Silico Analysis
4.6. RNA Extraction and RT-qPCR Analysis
4.7. Protein Extraction and Western Blots
4.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Funding
References
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2012, 1, 297–309. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E.; Fuller, C.J.; Hurtig, M.; Cruz, A. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr. Cartil. 2010, 18, S93–S105. [Google Scholar] [CrossRef] [PubMed]
- Oke, S.L.; McIlwraith, C.W. Review of the economic impact of osteoarthritis and oral joint-health supplements in horses. In Proceedings of the 56th Annual Convention of the American Association of Equine Practitioners, Baltimore, MD, USA, 4–8 December 2010; pp. 12–16. [Google Scholar]
- Aigner, T.; Cook, J.L.; Gerwin, N.; Glasson, S.S.; Laverty, S.; Little, C.B.; McIlwraith, W.; Kraus, V.B. Histopathology atlas of animal model systems—Overview of guiding principles. Osteoarthr. Cartil. 2010, 18, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Marlovits, S.; Zeller, P.; Singer, P.; Resinger, C.; Vécsei, V. Cartilage repair: Generations of autologous chondrocyte transplantation. Eur. J. Radiol. 2006, 57, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, W.; Skinner, J.A.; Gooding, C.R.; Carrington, R.W.J.; Flanagan, A.M.; Briggs, T.W.R.; Bentley, G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: A prospective, randomised study. J. Bone Jt. Surg. Br. 2005, 87, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M. Autologous chondrocyte implantation—Technique and long-term follow-up. Injury 2008, 39 (Suppl. S1), S40–S49. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.J.; Ramesh, A.; Brama, P.A.J.; O’Byrne, J.M.; O’Brien, F.J.; Levingstone, T.J. The benefits and limitations of animal models for translational research in cartilage repair. J. Exp. Orthop. 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Nixon, A.J.; Rickey, E.; Butler, T.J.; Scimeca, M.S.; Moran, N.; Matthews, G.L. A chondrocyte infiltrated collagen type I/III membrane (MACI® implant) improves cartilage healing in the equine patellofemoral joint model. Osteoarthr. Cartil. 2015, 23, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.J.; Bonnevie, E.D.; Lachowsky, D.J.; Hart, J.C.A.; Sparks, H.D.; Moran, N.; Matthews, G.; Nixon, A.J.; Cohen, I.; Bonassar, L.J. Mechanical characterization of matrix-induced autologous chondrocyte implantation (MACI®) grafts in an equine model at 53 weeks. J. Biomech. 2015, 48, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Ortved, K.F.; Nixon, A.J. Cell-based cartilage repair strategies in the horse. Vet. J. 2016, 208, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Benya, P.D.; Padilla, S.R.; Nimni, M.E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 1978, 15, 1313–1321. [Google Scholar] [CrossRef]
- Marlovits, S.; Hombauer, M.; Truppe, M.; Vécsei, V.; Schlegel, W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J. Bone Jt. Surg. Br. 2004, 86, 286–295. [Google Scholar] [CrossRef]
- Claus, S.; Mayer, N.; Aubert-Foucher, E.; Chajra, H.; Perrier-Groult, E.; Lafont, J.; Piperno, M.; Damour, O.; Mallein-Gerin, F. Cartilage-Characteristic matrix reconstruction by sequential addition of soluble factors during expansion of human articular chondrocytes and their cultivation in collagen sponges. Tissue Eng. Part C Methods 2012, 18, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, M.; Marlovits, S.; Eckhoff, G.; Fichtel, I.; Gotzen, L.; Vécsei, V.; Schlegel, J. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthr. Cartil. 2002, 10, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fitzgerald, J.B.; Xu, J.; Willers, C.; Wood, D.; Grodzinsky, A.J.; Zheng, M.H. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 2008, 26, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Blaise, R.; Mahjoub, M.; Salvat, C.; Barbe, U.; Brou, C.; Corvol, M.-T.; Savouret, J.-F.; Rannou, F.; Berenbaum, F.; Bausero, P. Involvement of the Notch pathway in the regulation of matrix metalloproteinase 13 and the dedifferentiation of articular chondrocytes in murine cartilage. Arthritis Rheum. 2009, 60, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Pelttari, K.; Lorenz, H.; Boeuf, S.; Templin, M.F.; Bischel, O.; Goetzke, K.; Hsu, H.-Y.; Steck, E.; Richter, W. Secretion of matrix metalloproteinase 3 by expanded articular chondrocytes as a predictor of ectopic cartilage formation capacity in vivo. Arthritis Rheum. 2008, 58, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.-M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta 2014, 1840, 2414–2440. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.J.; Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Engineering biomechanically functional neocartilage derived from expanded articular chondrocytes through the manipulation of cell-seeding density and dexamethasone concentration. J. Tissue Eng. Regen. Med. 2016, 11, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Mrugala, D.; Jorgensen, C.; Guicheux, J.; Noël, D. Cartilage engineering: A crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009, 27, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Duval, E.; Leclercq, S.; Elissalde, J.-M.; Demoor, M.; Galera, P.; Boumédiene, K. Hypoxia-inducible factor 1alpha inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: Hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1alpha-dependent redifferentiation of chondrocytes. Arthritis Rheum. 2009, 60, 3038–3048. [Google Scholar] [PubMed]
- Lee, H.-H.; Chang, C.-C.; Shieh, M.-J.; Wang, J.-P.; Chen, Y.-T.; Young, T.-H.; Hung, S.-C. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci. Rep. 2013, 3, 2683. [Google Scholar] [CrossRef] [PubMed]
- Lafont, J.E.; Poujade, F.A.; Pasdeloup, M.; Neyret, P.; Mallein-Gerin, F. Hypoxia potentiates the BMP-2 driven COL2A1 stimulation in human articular chondrocytes via p38 MAPK. Osteoarthr. Cartil. 2016, 24, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Hautier, A.; Salentey, V.; Aubert-Foucher, E.; Bougault, C.; Beauchef, G.; Ronzière, M.-C.; de Sobarnitsky, S.; Paumier, A.; Galera, P.; Piperno, M.; et al. Bone morphogenetic protein-2 stimulates chondrogenic expression in human nasal chondrocytes expanded in vitro. Growth Factors 2008, 26, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.; Aubert-Foucher, E.; Demoor, M.; Camuzeaux, B.; Paumier, A.; Piperno, M.; Damour, O.; Duterque-Coquillaud, M.; Galera, P.; Mallein-Gerin, F. Chronic exposure of bone morphogenetic protein-2 favors chondrogenic expression in human articular chondrocytes amplified in monolayer cultures. J. Cell. Biochem. 2010, 111, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.; López-Ruiz, E.; Kwiatkowski, W.; Montañez, E.; Arrebola, F.; Carrillo, E.; Gray, P.C.; Izpisua Belmonte, J.C.; Choe, S.; Perán, M.; et al. Activin A/BMP2 chimera AB235 drives efficient redifferentiation of long term cultured autologous chondrocytes. Sci. Rep. 2015, 5, 16400. [Google Scholar] [CrossRef] [PubMed]
- Ollitrault, D.; Legendre, F.; Drougard, C.; Briand, M.; Bénateau, H.; Goux, D.; Chajra, H.; Poulain, L.; Hartmann, D.; Vivien, D.; et al. BMP-2, hypoxia, and COL1A1/HtrA1 siRNAs favor neo-cartilage hyaline matrix formation in chondrocytes. Tissue Eng. Part C Methods 2015, 21, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Launay, S.; Maubert, E.; Lebeurrier, N.; Tennstaedt, A.; Campioni, M.; Docagne, F.; Gabriel, C.; Dauphinot, L.; Potier, M.C.; Ehrmann, M.; et al. HtrA1-dependent proteolysis of TGF-β controls both neuronal maturation and developmental survival. Cell Death Differ. 2008, 15, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.R.; Chamberland, A.; Lin, Q.; Li, X.J.; Dai, D.; Zeng, W.; Ryan, M.S.; Rivera-Bermúdez, M.A.; Flannery, C.R.; Yang, Z. Serine protease HTRA1 antagonizes transforming growth factor-β signaling by cleaving its receptors and loss of HTRA1 in vivo enhances bone formation. PLoS ONE 2013, 8, e74094. [Google Scholar] [CrossRef] [PubMed]
- Oka, C.; Tsujimoto, R.; Kajikawa, M.; Koshiba-Takeuchi, K.; Ina, J.; Yano, M.; Tsuchiya, A.; Ueta, Y.; Soma, A.; Kanda, H.; et al. HtrA1 serine protease inhibits signaling mediated by TGF-β family proteins. Development 2004, 131, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Tiaden, A.N.; Breiden, M.; Mirsaidi, A.; Weber, F.A.; Bahrenberg, G.; Glanz, S.; Cinelli, P.; Ehrmann, M.; Richards, P.J. Human serine protease HTRA1 positively regulates osteogenesis of human bone marrow-derived mesenchymal stem cells and mineralization of differentiating bone-forming cells through the modulation of extracellular matrix protein. Stem Cells 2012, 30, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Tiaden, A.N.; Bahrenberg, G.; Mirsaidi, A.; Glanz, S.; Blüher, M.; Richards, P.J. Novel Function of Serine Protease HTRA1 in Inhibiting Adipogenic Differentiation of Human Mesenchymal Stem Cells via MAP Kinase-Mediated MMP Upregulation. Stem Cells 2016, 34, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Richards, P.J.; Kerr, B.; Hughes, C.; Caterson, B.; Williams, A.S.; Junker, U.; Jones, S.A.; Clausen, T.; Ehrmann, M. The role of human HtrA1 in arthritic disease. J. Biol. Chem. 2006, 281, 6124–6129. [Google Scholar] [CrossRef] [PubMed]
- Polur, I.; Lee, P.L.; Servais, J.M.; Xu, L.; Li, Y. Role of HTRA1, a serine protease, in the progression of articular cartilage degeneration. Histol. Histopathol. 2010, 25, 599–608. [Google Scholar] [PubMed]
- Vonk, L.A.; Kragten, A.H.M.; Dhert, W.J.A.; Saris, D.B.F.; Creemers, L.B. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes. Osteoarthr. Cartil. 2014, 22, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.W.; Lou, S.Q.; Zhang, K. Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-specific siRNA in vitro. Rheumatology 2004, 43, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Lianxu, C.; Hongti, J.; Changlong, Y. NF-κBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1β-induced and TNF-α-induced chondrocytes. Osteoarthr. Cartil. 2006, 14, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Galera, P.; Ollitrault, D.; Legendre, F.; Demoor, M.; Mallein-Gerin, F.; Boumediene, K.; Herbage, B.; Duterque-Coquillaud, M.; Damour, O. Method for Obtaining Differentiated Articular Chondrocytes In Vitro or Ex Vivo, and Uses of Same. WO2012038668 A1, 29 March 2012. [Google Scholar]
- Fermor, B.; Christensen, S.E.; Youn, I.; Cernanec, J.M.; Davies, C.M.; Weinberg, J.B. Oxygen, nitric oxide and articular cartilage. Eur. Cell Mater. 2007, 13, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.; Southan, C.; Ehrmann, M. The HtrA family of proteases: Implications for protein composition and cell fate. Mol. Cell 2002, 10, 443–455. [Google Scholar] [CrossRef]
- Ringe, J.; Burmester, G.R.; Sittinger, M. Regenerative medicine in rheumatic disease-progress in tissue engineering. Nat. Rev. Rheumatol. 2012, 8, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Leduc, T.; Hervieu, M.; Legendre, F.; Bouyoucef, M.; Gruchy, N.; Poulain, L.; de Vienne, C.; Herlicoviez, M.; Demoor, M.; Galera, P. Chondrogenic commitment of human umbilical cord blood-derived mesenchymal stem cells in collagen matrices for cartilage engineering. Sci. Rep. 2016, 6, 32786. [Google Scholar] [CrossRef] [PubMed]
- Legendre, F.; Ollitrault, D.; Hervieu, M.; Baugé, C.; Maneix, L.; Goux, D.; Chajra, H.; Mallein-Gerin, F.; Boumédiene, K.; Galera, P.; et al. Enhanced hyaline cartilage matrix synthesis in collagen sponge scaffolds by using siRNA to stabilize chondrocytes phenotype cultured with bone morphogenetic protein-2 under hypoxia. Tissue Eng. Part C Methods 2013, 19, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.M.; Giulotto, E.; Sigurdsson, S.; Zoli, M.; Gnerre, S.; Imsland, F.; Lear, T.L.; Adelson, D.L.; Bailey, E.; Bellone, R.R.; et al. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science 2009, 326, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Tran-Khanh, N.; Hoemann, C.D.; McKee, M.D.; Henderson, J.E.; Buschmann, M.D. Aged bovine chondrocytes display a diminished capacity to produce a collagen-rich, mechanically functional cartilage extracellular matrix. J. Orthop. Res. 2005, 23, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Sumita, Y.; Honda, M.J.; Ohara, T.; Tsuchiya, S.; Sagara, H.; Kagami, H.; Ueda, M. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials 2006, 27, 3238–3248. [Google Scholar] [CrossRef] [PubMed]
- Nakase, Y.; Hagiwara, A.; Nakamura, T.; Kin, S.; Nakashima, S.; Yoshikawa, T.; Fukuda, K.-I.; Kuriu, Y.; Miyagawa, K.; Sakakura, C.; et al. Tissue engineering of small intestinal tissue using collagen sponge scaffolds seeded with smooth muscle cells. Tissue Eng. 2006, 12, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kawai, K.; Ashoori, F.; Morimoto, N.; Nishimura, Y.; Ikada, Y. Long-term follow-up study of artificial dermis composed of outer silicone layer and inner collagen sponge. Br. J. Plast. Surg. 2000, 53, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Russlies, M.; Behrens, P.; Wünsch, L.; Gille, J.; Ehlers, E.M. A cell-seeded biocomposite for cartilage repair. Ann. Anat. 2002, 184, 317–323. [Google Scholar] [CrossRef]
- Cho, Y.-G.; Cho, M.-L.; Min, S.-Y.; Kim, H.-Y. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun. Rev. 2007, 7, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.-P.; Chadjichristos, C.; Legendre, F.; Baugé, C.; Beauchef, G.; Andriamanalijaona, R.; Galera, P.; Boumédiene, K. Interleukin-1 and transforming growth factor-β 1 as crucial factors in osteoarthritic cartilage metabolism. Connect. Tissue Res. 2008, 49, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Leake, D.; Boese, Q.; Scaringe, S.; Marshall, W.S.; Khvorova, A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004, 22, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chan, C.Y.; Maliyekkel, A.; Lawrence, C.E.; Roninson, I.B.; Ding, Y. Effect of target secondary structure on RNAi efficiency. RNA 2007, 13, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.; Bernhart, S.H.; Höner Zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Gotkin, M.G.; Ripley, C.R.; Lamande, S.R.; Bateman, J.F.; Bienkowski, R.S. Intracellular trafficking and degradation of unassociated proα2 chains of collagen type I. Exp. Cell Res. 2004, 296, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Jónsson, Z.O.; Dutta, A. Small RNAs with imperfect match to endogenous mRNA repress translation. Implications for off-target activity of small inhibitory RNA in mammalian cells. J. Biol. Chem. 2003, 278, 44312–44319. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Golshirazian, I.; Asbury, B.J.; Li, Y. Induction of high temperature requirement A1, a serine protease, by TGF-β1 in articular chondrocytes of mouse models of OA. Histol. Histopathol. 2014, 29, 609–618. [Google Scholar] [PubMed]
- Wu, X.; Chim, S.M.; Kuek, V.; Lim, B.S.; Chow, S.T.; Zhao, J.; Yang, S.; Rosen, V.; Tickner, J.; Xu, J. HtrA1 is upregulated during RANKL-induced osteoclastogenesis, and negatively regulates osteoblast differentiation and BMP2-induced Smad1/5/8, ERK and p38 phosphorylation. FEBS Lett. 2014, 588, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Branly, T.; Bertoni, L.; Contentin, R.; Rakic, R.; Gomez-Leduc, T.; Desancé, M.; Hervieu, M.; Legendre, F.; Jacquet, S.; Audigié, F.; et al. Characterization and use of equine bone marrow mesenchymal stem cells in equine cartilage engineering. Study of their hyaline cartilage forming potential when cultured under hypoxia within a biomaterial in the presence of BMP-2 and TGF-β1. Stem Cell Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.E.; Pelz, O.; Buhlmann, S.; Kerr, G.; Horn, T.; Boutros, M. GenomeRNAi: A database for cell-based and in vivo RNAi phenotypes, 2013 update. Nucleic Acids Res. 2013, 41, D1021–D1026. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Dodge, M.; Gundapaneni, D.; Michnoff, C.; Roth, M.; Lum, L. A genome-wide RNAi screen for Wnt/beta-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 9697–9702. [Google Scholar] [CrossRef] [PubMed]
- Day, T.F.; Guo, X.; Garrett-Beal, L.; Yang, Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 2005, 8, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S. Cellular signaling in developmental chondrogenesis: N-cadherin, Wnts, and BMP-2. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. S2), 137–141. [Google Scholar] [CrossRef]
- Clynes, M. Basic Cell Culture—A Practical Approach; Davis, J.M., Ed.; IRL Press at Oxford University Press: Oxford, UK, 1996; p. 301. ISBN 0-199-63433-5. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakic, R.; Bourdon, B.; Hervieu, M.; Branly, T.; Legendre, F.; Saulnier, N.; Audigié, F.; Maddens, S.; Demoor, M.; Galera, P. RNA Interference and BMP-2 Stimulation Allows Equine Chondrocytes Redifferentiation in 3D-Hypoxia Cell Culture Model: Application for Matrix-Induced Autologous Chondrocyte Implantation. Int. J. Mol. Sci. 2017, 18, 1842. https://doi.org/10.3390/ijms18091842
Rakic R, Bourdon B, Hervieu M, Branly T, Legendre F, Saulnier N, Audigié F, Maddens S, Demoor M, Galera P. RNA Interference and BMP-2 Stimulation Allows Equine Chondrocytes Redifferentiation in 3D-Hypoxia Cell Culture Model: Application for Matrix-Induced Autologous Chondrocyte Implantation. International Journal of Molecular Sciences. 2017; 18(9):1842. https://doi.org/10.3390/ijms18091842
Chicago/Turabian StyleRakic, Rodolphe, Bastien Bourdon, Magalie Hervieu, Thomas Branly, Florence Legendre, Nathalie Saulnier, Fabrice Audigié, Stéphane Maddens, Magali Demoor, and Philippe Galera. 2017. "RNA Interference and BMP-2 Stimulation Allows Equine Chondrocytes Redifferentiation in 3D-Hypoxia Cell Culture Model: Application for Matrix-Induced Autologous Chondrocyte Implantation" International Journal of Molecular Sciences 18, no. 9: 1842. https://doi.org/10.3390/ijms18091842




