Human Meibum Age, Lipid–Lipid Interactions and Lipid Saturation in Meibum from Infants
Abstract
:1. Introduction
2. Results
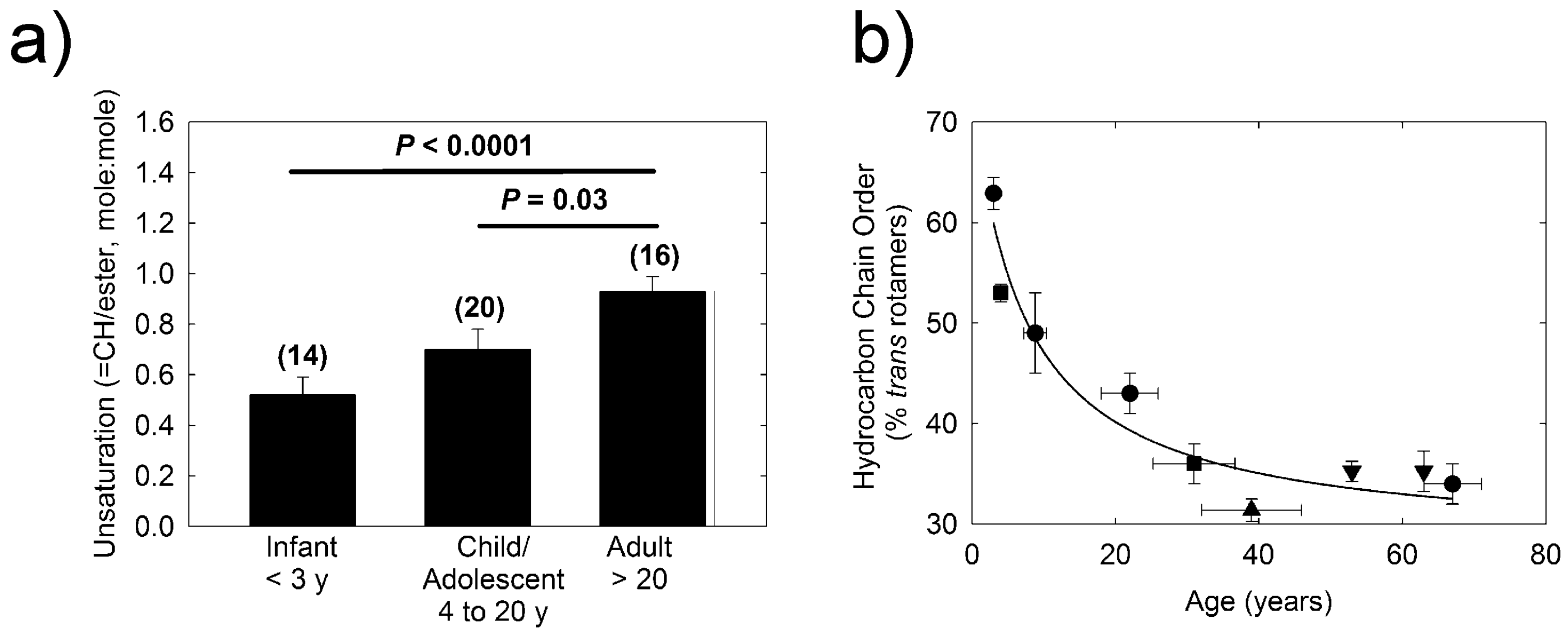
2.1. Donor Demographics
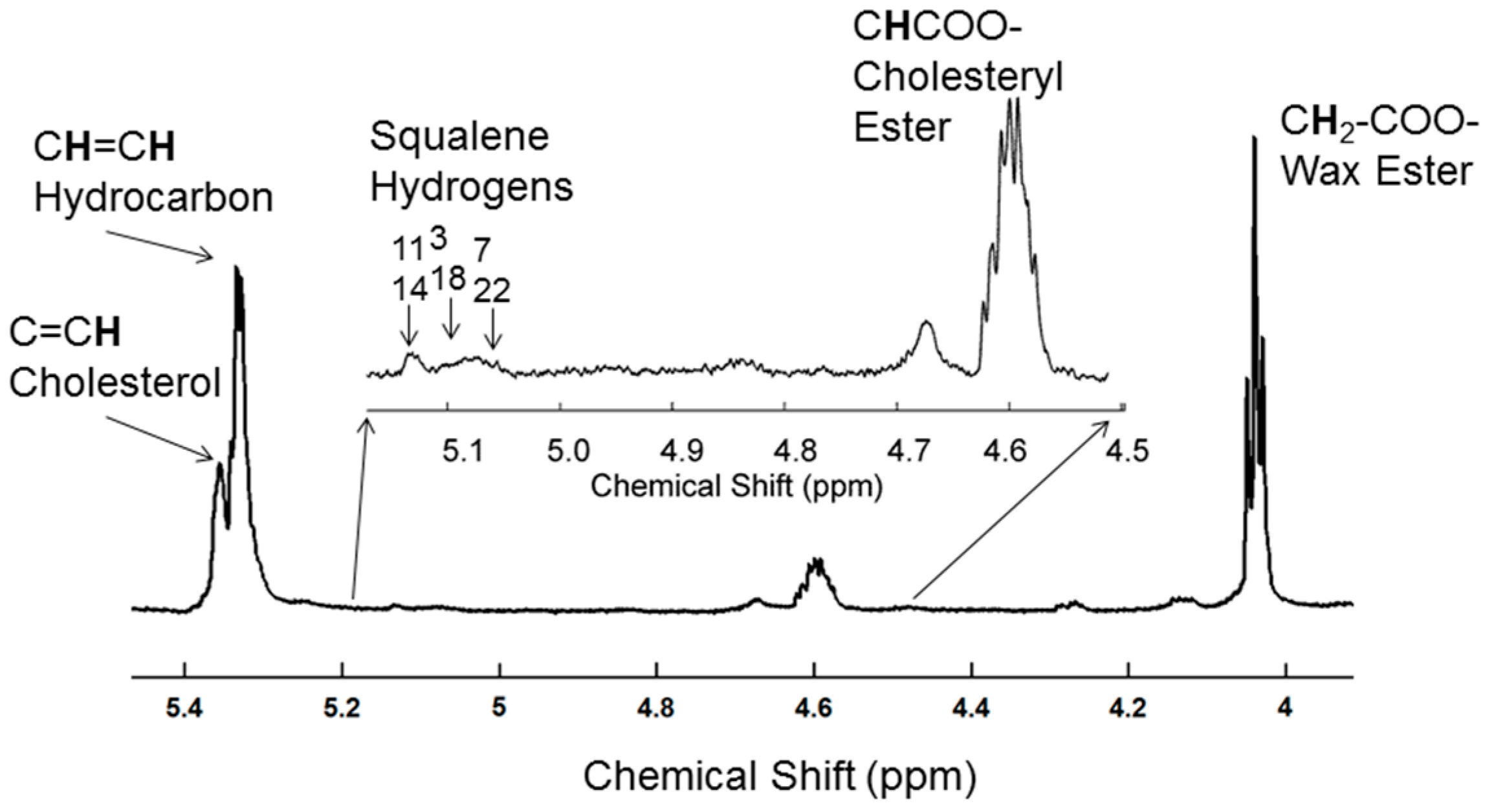
2.2. H-NMR Spectroscopy
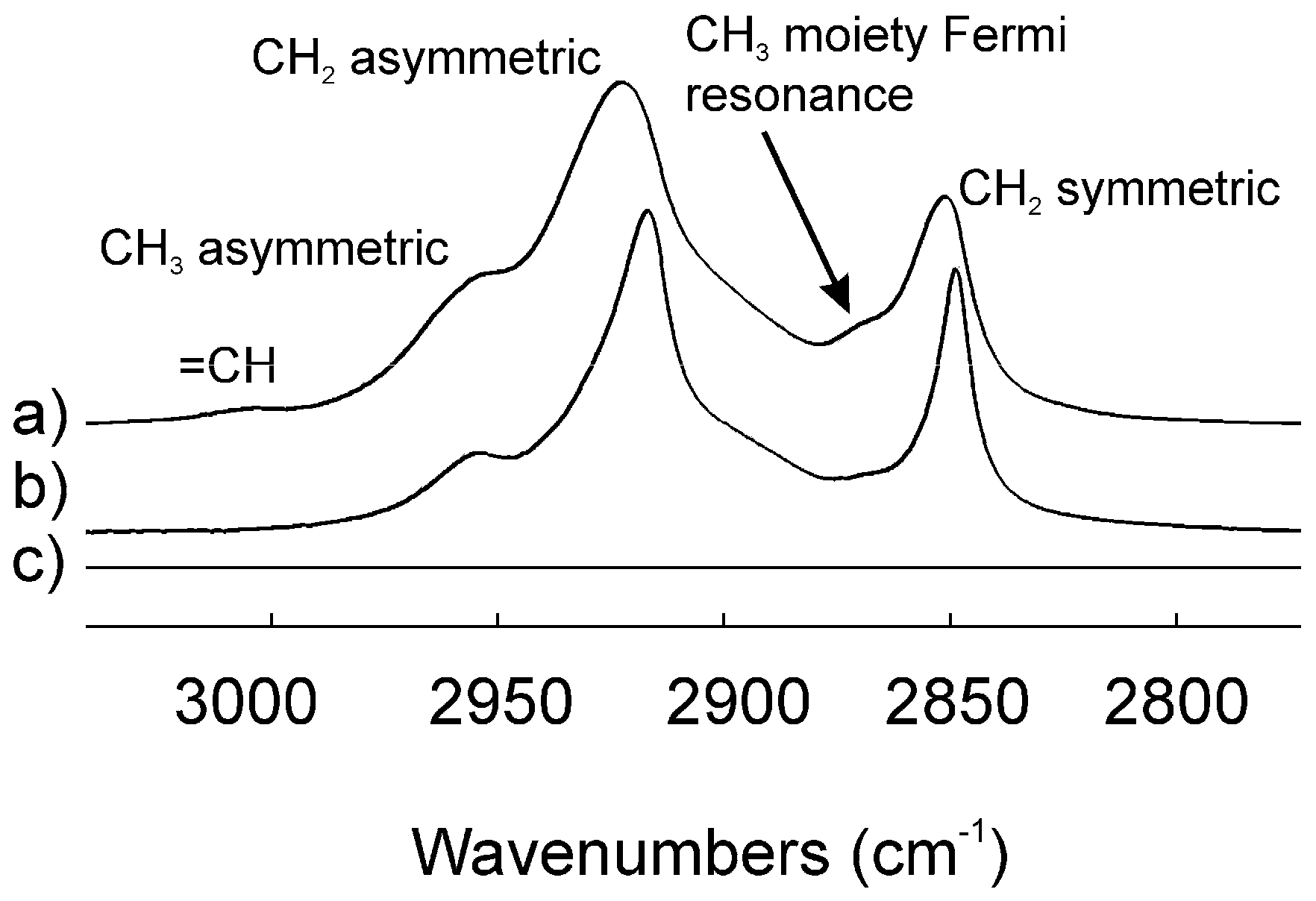
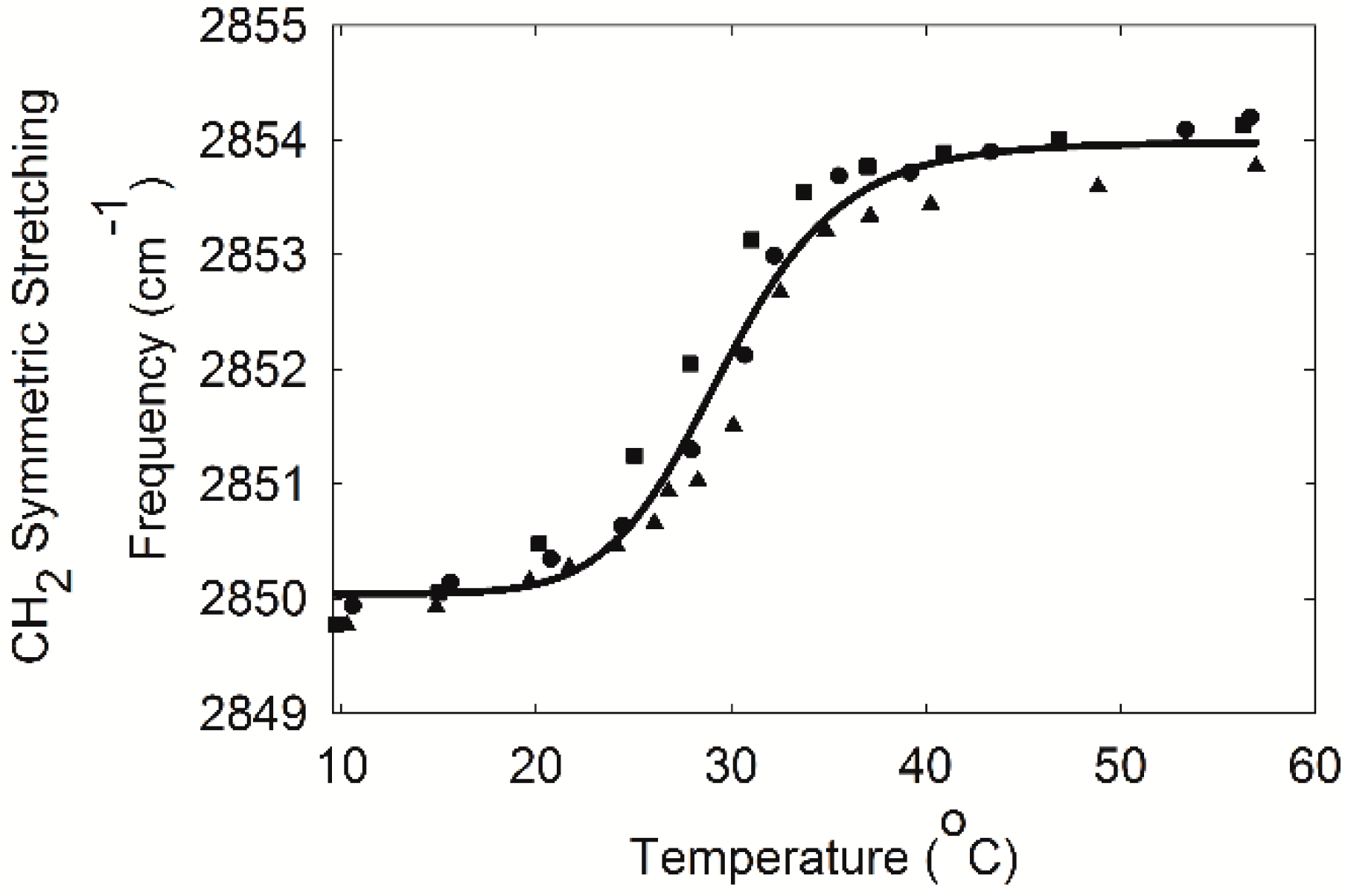
2.3. Infrared Spectroscopy
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Diagnosis of Normal Status
4.3. Collection and Extraction of Lipid from Meibum
4.4. Catalytic Hydrogenation
4.5. Saturation Analysis Using H-NMR
4.6. Measurement of Lipid Phase Transitions Using FTIR Spectroscopy
4.7. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mudgil, P.; Borchman, D.; Gerlach, D.; Yappert, M.C. Sebum/Meibum surface film interactions and phase transitional differences. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2401–2411. [Google Scholar] [CrossRef]
- Green-Church, K.B.; Butovich, I.; Willcox, M.; Borchman, D.; Paulsen, F.; Barabino, S.; Glasgow, B.J. The international workshop on meibomian gland dysfunction: Report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Foulks, G.N. The correlation between the tear film lipid layer and dry eye disease. Surv. Ophthalmol. 2007, 52, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Tiffany, J.M.; Gouveia, S.M.; Yokoi, N.; Voon, L.W. Functional aspects of the tear film lipid Layer. Exp. Eye Res. 2004, 78, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Tiberi, E.; Micera, A.; Lambiase, A.; Visintini, F.; Bonini, S. MUC5AC over expression in tear film of neonates. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, S.J.; del Signore, M.; Chen, A.; Wei, J.; Guillon, J. The lipid layer and stability of the preocular tear film in newborns and infants. Ophthalmology 2003, 110, 1408–1411. [Google Scholar] [CrossRef]
- Bacher, L.F. Factors regulating eye blink rate in young infants. Optom. Vis. Sci. 2010, 87, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Lawrenson, J.G.; Birhah, R.; Murphy, P.J. Tear-film lipid layer morphology and corneal sensation in the development of blinking in neonates and infants. J. Anat. 2005, 206, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Sforza, C.; Rango, M.; Galante, D.; Bresolin, N.; Ferrario, V.F. Spontaneous blinking in healthy persons: An optoelectronic study of eyelid motion. Ophthalmic Physiol. Opt. 2008, 28, 345–353. [Google Scholar] [CrossRef]
- Cho, P.; Yap, M. Age, gender, and tear break-up time. Optom. Vis. Sci. 1993, 70, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Mohidin, N.; Bay, T.C.; Yapm, M. Non-invasive tear break-up time in normal Malays. Clin. Exp. Optom. 2002, 85, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, M.; Temizdemir, H. Age- and sex- related tear function changes in normal population. Eye 2010, 24, 79–83. [Google Scholar] [CrossRef]
- Maïssa, C.; Guillon, M. Tear film dynamics and lipid layer characteristics-effect of age and sex. Contact Lens Anterior Eye 2010, 33, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.S.; Baker, R.S.; Chuke, J.C.; Rouholiman, B.R.; Hasan, S.A.; Gaza, W.; Stava, M.W.; Porter, J.D. Age-related changes in human blinks. Passive and active changes in eyelid kinematics. Investig. Ophthalmol. Vis. Sci. 1997, 38, 92–99. [Google Scholar]
- Lavezzo, M.M.; Schellini, S.A.; Padovani, C.R. Eye blink in newborn and preschool-age children. Acta Ophthalmol. 2008, 86, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.; Tomlinson, A. Age and sex effects on the normal tear film. Adv. Exp. Med. Biol. 1998, 438, 411–415. [Google Scholar] [PubMed]
- Henderson, J.W.; Prough, W.A. Influence of age, sex on flow of tears. Arch. Ophthalmol. 1950, 43, 224–231. [Google Scholar] [CrossRef]
- Guillon, M.; Maïssa, C. Tear film evaporation: Effect of age and sex. Contact Lens Anterior Eye 2010, 33, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.A.; Garcia, D.M.; Pinto, C.T.; Cehetti, S.P. Spontaneous eyeblink activity. Ocul. Surf. 2011, 9, 29–41. [Google Scholar] [CrossRef]
- Tsubota, K.; Hata, S.; Okusawa, Y.; Egami, F.; Ohtsuki, T.; Nakamori, K. Quantitative videographic analysis of blinking in normal subjects and patients with dry eye. Arch. Ophthalmol. 1996, 114, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K. Tear dynamics and dry eye. Prog. Retin. Eye Res. 1998, 17, 565–596. [Google Scholar] [CrossRef]
- Tomlinson, A.; Khanal, S. Assessment of tear film dynamics: Quantification approach. Ocul. Surf. 2005, 3, 81–95. [Google Scholar] [CrossRef]
- Borchman, D.; Yappert, M.C.; Milliner, S.; Duran, D.; Cox, G.W.; Smith, R.J.; Bhola, R. 13C and 1H-NMR ester region resonance assignments and the composition of human infant and child meibum. Exp. Eye Res. 2013, 112, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Milliner, S.E. Changes in human meibum lipid composition with age using NMR spectroscopy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Borchman, D.; Yappert, M.C.; Duran, D.; Cox, G.W.; Smith, R.J.; Bhola, R.; Dennis, G.R.; Whitehall, J.S. Human meibum saturation and lipid order. Exp. Eye Res. 2013, 116, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Ho, D.V. Temperature-induced conformational changes in human tear lipids hydrocarbon chains. Biopolymers 2007, 87, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Kakar, S.; Podoll, N.; Rychwalski, P.; Schwietz, E. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic Res. 2010, 44, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C. Confirmation of changes in human meibum lipid infrared spectra with age using principal component analysis. Curr. Eye Res. 2010, 35, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Yappert, M.C.; Milliner, S.; Bhola, R. Confirmation of squalene in human eye lid lipid by heteronuclear single quantum correlation spectroscopy. Lipids 2013, 48, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Kóta, Z.; Debreczeny, M.; Szalontai, B. Separable contributions of ordered and disordered lipid fatty acyl chain segments to ṽCH2 bands in model and biological membranes: A fourier transform infrared spectroscopic study. Biospectroscopy 1999, 5, 169–178. [Google Scholar] [CrossRef]
- Butovich, I.A.; Arciniega, J.C.; Wojtowicz, J.C. Meibomian lipid films and the impact of temperature. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5508–5518. [Google Scholar] [CrossRef]
- Butovich, I.A.; Lu, H.; McMahon, A.; Ketelson, H.; Senchyna, M.; Meadows, D.; Campbell, E.; Molai, M.; Linsenbardt, E. Biophysical and morphological evaluation of human normal and dry eye meibum using hot stage polarized light microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wojtowicz, J.C.; Butovich, I.A. Differential scanning calorimetric evaluation of human meibomian gland secretions and model lipid mixtures: Transition temperatures and cooperativity of melting. Chem. Phys. Lipids 2013, 170–171, 55–64. [Google Scholar] [CrossRef]
- Butovich, I.A. On the lipid composition of human meibum and tears: Comparative analysis of nonpolar lipids. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3779–3789. [Google Scholar] [CrossRef] [PubMed]
- Petrikovsky, B.M.; Kaplan, G.; Holsten, N. Eyelid movements in normal human fetuses. J. Clin. Ultrasound 2003, 31, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Bacher, L.F.; Allen, K.J. Sensitivity of the rate of spontaneous eye blinking to type of stimuli in young infants. Dev. Psychobiol. 2009, 51, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Bacher, L.F.; Smotherman, W.P. Systematic temporal variation in the rate of spontaneous eye blinking in human infants. Dev. Psychobiol. 2004, 44, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zametkin, A.J.; Stevens, J.R.; Pittman, R. Ontogeny of spontaneous blinking and of habituation of the blink reflex. Ann. Neurol. 1979, 5, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, A.R.; Bressman, S.B.; Cassetta, E.; Carretta, D.; Tonali, P.; Albanese, A. Analysis of blink rate patterns in normal subjects. Mov. Disord. 1997, 12, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, S.; Chen, E. Gravity, blink rate, and lacrimal drainage capacity. Am. J. Ophthalmol. 1997, 124, 758–764. [Google Scholar] [CrossRef]
- Zaman, M.L.; Doughty, M.J. Some methodological issues in the assessment of the spontaneous eyeblink frequency in man. Ophthalmic Physiol. Opt. 1997, 17, 421–432. [Google Scholar] [CrossRef]
- Nakamori, K.; Odawara, M.; Nakajima, T.; Mizutani, T.; Tsubota, K. Blinking is controlled primarily by ocular surface conditions. Am. J. Ophthalmol. 1997, 124, 24–30. [Google Scholar] [CrossRef]
- Biousse, V.; Skibell, B.C.; Watts, R.L.; Loupe, D.N.; Drews-Botsch, C.; Newman, N.J. Ophthalmologic features of Parkinson’s disease. Neurology 2004, 62, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Doughty, M.J. Effects of background lighting and retinal illuminance on spontaneous eyeblink activity of human subjects in primary eye gaze. Eye Contact Lens Sci. Clin. Pract. 2013, 39, 138–146. [Google Scholar] [CrossRef]
- Begley, C.G.; Port, N.; Bradley, A.; Braun, R. The effects of increasing ocular surface stimulation on blinking and tear secretion. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4211–4220. [Google Scholar]
- Cardona, G.; Perez-Cabre, E.; Rodriguez, M. Blink rate and incomplete blinks in six different controlled hard-copy and electronic reading conditions. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6679–6685. [Google Scholar]
- Michael, J.; Doughty, M.J. Assessment of short-term variability in human spontaneous blink rate during video observation with or without head/chin support. Clin. Exp. Optom. 2016, 99, 135–141. [Google Scholar]
- Kruis, A.; Slagter, H.A.; Bachhuber, D.R.W.; Davidson, R.J.; Lutz, A. Effects of meditation practice on spontaneous eyeblink rate. Psychophysiology 2016, 53, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Nosch, D.S.; Pult, H.; Albon, J.; Purslow, C.; Murphy, P.J. Relationship between corneal sensation, blinking, and tear film quality. Optom. Vis. Sci. 2016, 93, 471–481. [Google Scholar] [CrossRef]
- Lane, K.J.; Ousler, G.W., III; Angjeli, E.; Smith, L.M.; Rodriguez, J.D.; Bateman, K.M.; Abelson, M.B. Diurnal tracking of blink and relationship to signs and symptoms of dry eye. Cornea 2016, 35, 1104–1111. [Google Scholar]
- Nakano, T. The right angular gyrus controls spontaneous eyeblink rate: A combined structural MRI and TMS study. Cortex 2017, 88, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Frohnert, B.; Jacobs, D.R., Jr.; Steinberger, J.; Moran, A.; Steffen, L.M.; Sinaiko, A.R. Relation between serum free fatty acids and adiposity, insulin resistance, and cardiovascular risk factors from adolescence to adulthood. Diabetes 2013, 62, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Felix, P.; Heald, G.A.; Seakold, W.; Morrison, D. Plasma levels of free fatty acids in adolescents. Am. J. Clin. Nutr. 1967, 20, 1010–1014. [Google Scholar]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Bell, J.; Wells, E.; Neravetla, S.; Greenstone, V. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3805–3817. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970, 45, 3–23. [Google Scholar] [CrossRef]
- Borchman, D.; Foulks, G.N.; Yappert, M.C. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp. Eye Res. 2010, 91, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Wenk, M.R.; Tong, L. Lipid-based therapy for ocular surface inflammation and disease. Trends Mol. Med. 2015, 21, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Tang, D.; Ho, D.V. Spectroscopic evaluation of human tear lipids. Chem. Phys. Lipids 2007, 147, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Svitova, T.F.; Lin, M.C. Dynamic interfacial properties of human tear-lipid films and their interactions with model-tear proteins in vitro. Adv. Colloid Interface Sci. 2016, 233, 4–24. [Google Scholar] [CrossRef]
- Pucker, A.D.; Haworth, K.M. The presence and significance of polar meibum and tear lipids. Ocul. Surf. 2015, 13, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Kilp, H.; Schmid, E.; Kirchner, L.; Pohl, A. Tear Film Observation by Reflecting Microscopy and Differential Interference Contrast Microscopy. In The Preocular Tear Film in Health, Disease and Contact Lens Wear; Holly, F.J., Ed.; The Dry Eye Institute: Lubbock, TX, USA, 1986; pp. 564–569. [Google Scholar]
- Ferguson, S.R.; Borchman, D.; Yappert, M.C. Confirmation of the identity of the major phospholipid in human lens membranes. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1703–1706. [Google Scholar]
- Ferguson-Yankey, S.; Borchman, D.; Taylor, K.G.; DuPre, D.B.; Yappert, M.C. Conformational studies of sphingolipids by NMR spectroscopy. I. dihydrosphingomyelin. Biochim. Biophys. Acta 2000, 1467, 307–325. [Google Scholar] [CrossRef]
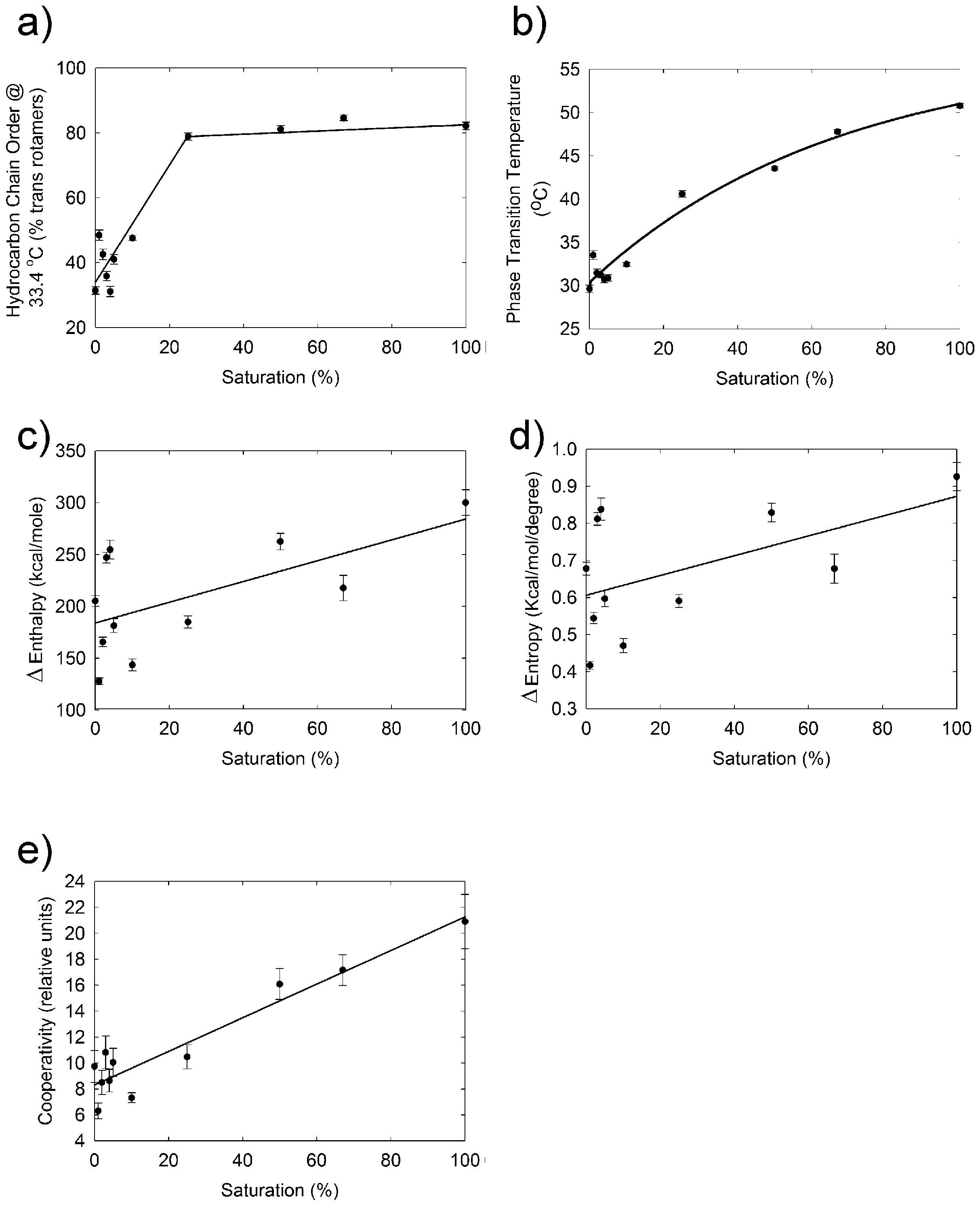
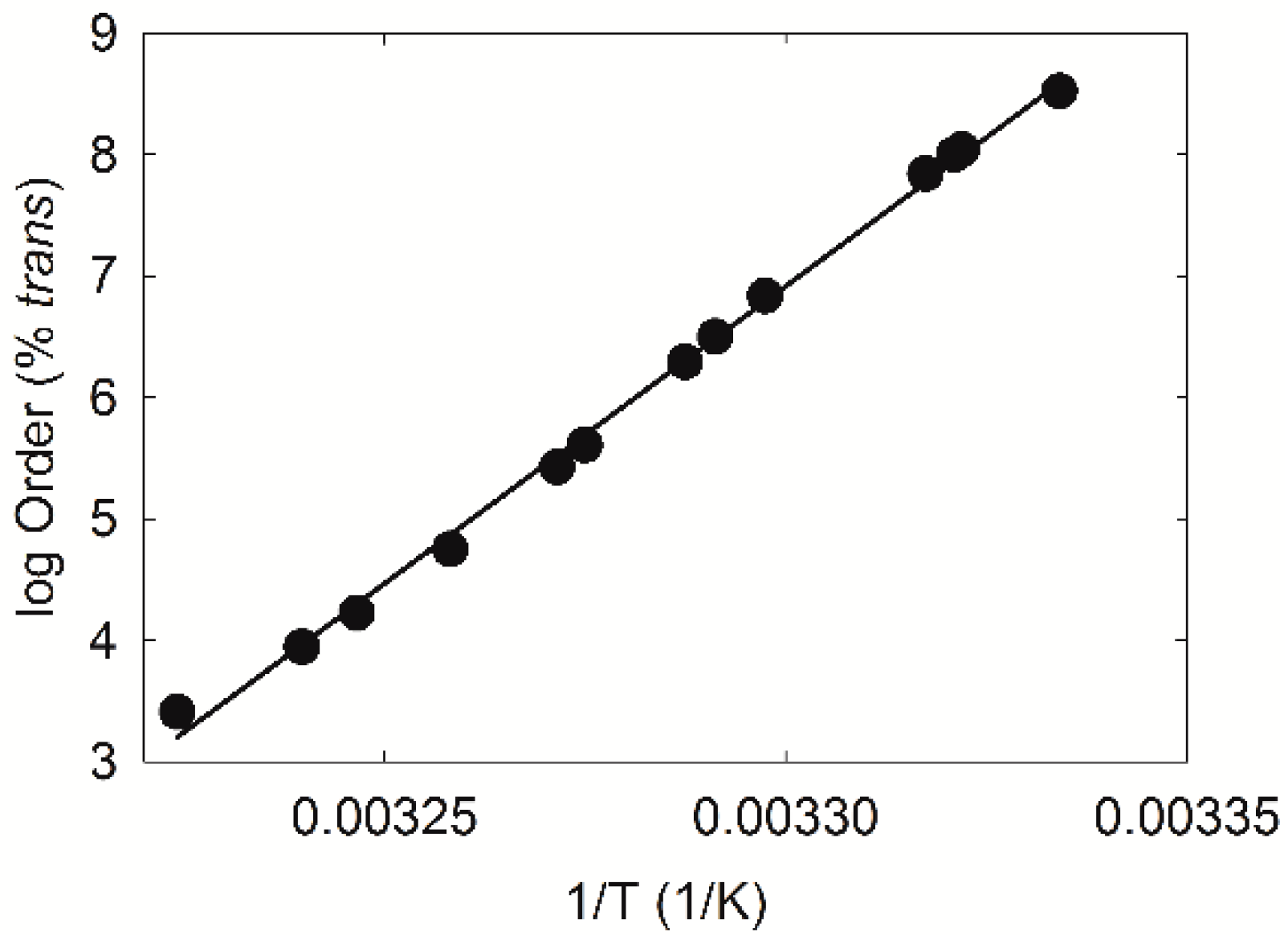
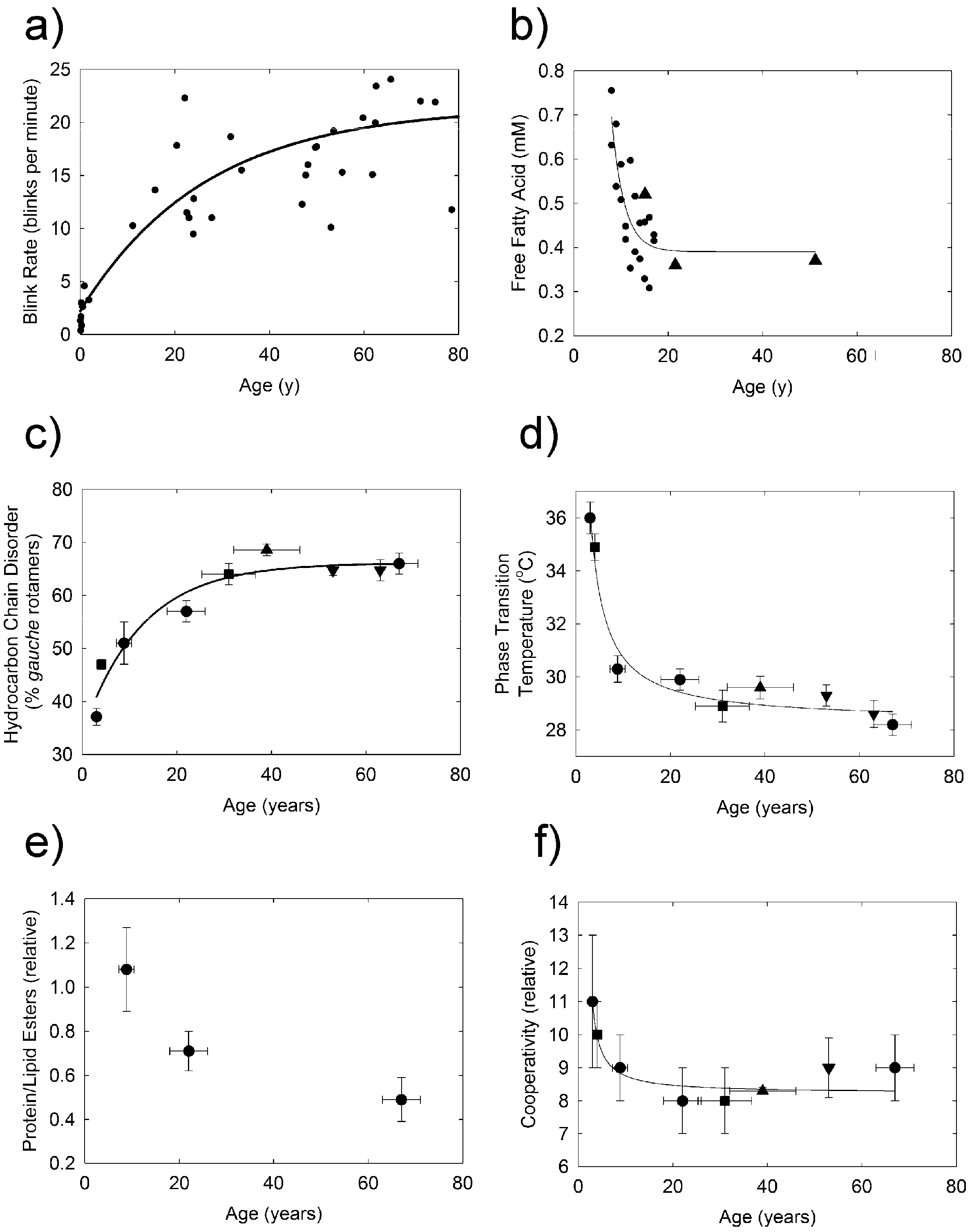
| Parameter | This Study FTIR | FTIR [1] | Microscopy [31] | Birefringence [32] | DSC [33] | Light Reflectance [34] |
|---|---|---|---|---|---|---|
| Hydrocarbon Chain Order (% trans) | 31 ± 2 | 36 ± 2 | ||||
| Phase Transition Temperature (°C) | 29.6 ± 0.4 | 28.9 ± 0.6 | 32.1 ± 0.1 | 32 ± 2 | 30.2 ± 0.1 | 32 ± 1 |
| Cooperativity | 10 ± 1 | 8 ± 1 | 19.3 ± 0.1 | 7 ± 1, 34 ± 10 | 16, 40, 100 | |
| Change in enthalpy (Kcal/mol) | 205 ± 5 | 196 ± 2 | 6 ± 1 | |||
| Change in entropy (Kcal/mol/deg) | 0.68 ± 0.02 | 0.650 ± 0.008 | ||||
| Age (years) | 39 ± 7 | 31 ± 6 | 29, 50 | 53, 38 ± 4 | 50 ± 2 | 47 |
| Sample demographics | 1 pool of 8 | 1 pool of 7 | 1 pool of 2 | 1 donor and 1 pool of 4 | 1 pool of 2 | 1 pool of 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sledge, S.; Henry, C.; Borchman, D.; Yappert, M.C.; Bhola, R.; Ramasubramanian, A.; Blackburn, R.; Austin, J.; Massey, K.; Sayied, S.; et al. Human Meibum Age, Lipid–Lipid Interactions and Lipid Saturation in Meibum from Infants. Int. J. Mol. Sci. 2017, 18, 1862. https://doi.org/10.3390/ijms18091862
Sledge S, Henry C, Borchman D, Yappert MC, Bhola R, Ramasubramanian A, Blackburn R, Austin J, Massey K, Sayied S, et al. Human Meibum Age, Lipid–Lipid Interactions and Lipid Saturation in Meibum from Infants. International Journal of Molecular Sciences. 2017; 18(9):1862. https://doi.org/10.3390/ijms18091862
Chicago/Turabian StyleSledge, Samiyyah, Collin Henry, Douglas Borchman, Marta C. Yappert, Rahul Bhola, Aparna Ramasubramanian, Ryan Blackburn, Jonathan Austin, Kayla Massey, Shanzeh Sayied, and et al. 2017. "Human Meibum Age, Lipid–Lipid Interactions and Lipid Saturation in Meibum from Infants" International Journal of Molecular Sciences 18, no. 9: 1862. https://doi.org/10.3390/ijms18091862





