1. Introduction
Autophagy is a lysosome-mediated degradation process in which unnecessary organelles and macromolecules are sequestered into autophagic vesicles and are delivered to lysosomes. Ultimately, various autophagic cargo molecules are degraded and recycled back to maintain cellular bioenergy homeostasis and support biosynthetic processes under metabolic stress conditions including nutrient deprivation [
1,
2]. Multiple growth-signaling pathways negatively regulate autophagy, a typical catabolic pathway. The serine/threonine protein kinase, mammalian target of rapamycin (mTOR) acts as a nutrient-sensing molecule to promote cell growth and suppress autophagy, whereas mTOR inactivation in response to nutrient deprivation induces autophagy [
3,
4]. Subsequent to autophagy activation in response to stresses, lysosomes play a pivotal role in degrading a variety of cargo molecules effectively, dependent on or independent of the autophagy pathway, using multiple trafficking routes. Moreover, lysosomes have recently received attention as a platform for regulating the mTORC1 pathway. Lysosomal function and localization can be markedly affected by mTORC1 activity. The subcellular localization of mTOR depends on intracellular nutrient status, particularly amino acid levels [
5]. In nutrient-complete conditions, mTOR with its active state is located at the lysosomal membrane. In contrast, inactive mTOR is no longer localized at the lysosomal membrane during nutrient starvation, but is more diffusely distributed throughout the cytoplasm instead [
6,
7,
8,
9,
10,
11]
Rab escort protein 1 (REP1) is essential for lipid modification of Rab proteins, directly interacting with most Rab proteins and presenting them to Rab-geranyl-geranyltransferase (GGTase2). Subsequently GGTase2 transfers the geranyl-geranyl moiety to the target proteins such as RabGTPases. This lipid modification step, called “prenylation”, dictates that Rab proteins localize at the cell membrane and further maintain their active status in a GTP-bound form at the proper locations. Therefore, since RabGTPases are involved in various steps of vesicle trafficking, it is postulated that REP1 has critical role in vesicle trafficking via regulating RabGTPase activity [
12,
13].
As an aspect of pathophysiology, mutations in
REP1 cause a disease called choroideremia (CHM), which is known as an X-linked eye disease resulting from progressive degeneration of multiple tissues including photoreceptors, retinal pigment epithelium, and choriocapillaris [
14,
15].
In addition to defective phenotypes of eye-degeneration, REP1 mutant in zebrafish have also shown excessive cell death in various organs during embryonic development [
16,
17]. Moreover, REP1 is highly expressed in various tumors and has an oncogenic effect via preventing epidermal growth factor receptor (EGFR) degradation [
18]. As another mechanism supporting for cell survival, REP1 directly interacts with fork head box O3 (Foxo3) to suppress nuclear localization of this transcription factor, which is associated with expression of the apoptotic gene set, demonstrating that REP1 prohibits cell death in response to multiple stress conditions including serum starvation or anticancer drug-5FU-treatment [
19]. Overall results from previous reports indicate that REP1 plays an important role for cell growth and survival, particularly under metabolic stress conditions including nutrient deprivation [
18,
19].
In this study, we suggested that REP1 contributes to certain types of intracellular vesicle trafficking such as autophagy, which is activated by metabolic stress conditions. Autophagy and macro pinocytosis have critical roles in supporting cell growth and survival of cancer cells. Based on increasing sensitivity of REP1 deficiency in response to nutrient deprivation, we investigated the role ofREP1 in regulation of mTORC1 activity and autophagy. Moreover, we examined the importance ofREP1 in subcellular localization of lysosome using the cells transfected with REP1 small interfering RNA (siRNA). Meanwhile, we also examined the subcellular localization of REP1, which can be affected by mTOR activity. Overall findings indicate that REP1 plays a critical role in recruiting organelles and vesicles which are involved in the degradation processes at the proper place. Since pancreatic cancer cells maintain intracellular levels of nutrients via pathways of both autophagy and macropinocytosis, REP1-mediated autophagy regulation could be a potential therapeutic target for treating pancreatic cancer.
3. Discussion
REP1 has been characterized as a crucial molecule for prenylation of the Rab protein and its mutation is tightly associated with developmental diseases as a result of a wide variety of types of tissue degeneration, including retinal and choroidal degeneration during eye development [
14,
15] and degeneration in brain development in the zebrafish model [
17]. In addition to tissue-degenerative phenotypes, knockout of the REP1 gene in zebrafish exhibited early embryonic lethality [
16]. Recent reports have demonstrated that REP1 is closely linked to cell proliferation and survival in the neoplastic stage through regulating translocation for either a growth factor receptor or a transcription factor to prevent cell death. Indeed, REP1 plays an important role in cancer progression, which is regulated by intracellular localization of EGFR or FOXO3 [
18,
19].
Since REP1 is involved in Rab protein recruitment in proper vesicles during vesicle trafficking, and the REP1mutation induces cell death which might cause tissue degeneration, we hypothesized that REP1 plays a critical role in the regulation of autophagy, which requires a variety of RabGTPases at distinct steps of vesicle trafficking processes [
12]. In addition, overall cellular phenotypes of Rep1 depletion or mutation are similar to that of those seen in autophagy defects. Moreover, a number of Rab proteins are known to be involved in distinct steps of the autophagy pathway, which is a lysosome-mediated degradation process and is induced by typical metabolic stresses including growth factor- or nutrient-deprivation and accumulation of reactive oxygen species (ROS) [
24]. Rab proteins that influence autophagy—such as Rab1, Rab5, Rab7, and Rab11—can be target proteins for lipid modification regulated by REP1 [
25]. Since nutrient deprivation eliminates mTOR activity completely, we hypothesized that REP1 work sin a distinct mechanism to maintain cell growth and survival in response to nutrient deprivation.
According to the data that REP1knockdown sensitizes cancer cells, especially under nutrient-deprivation conditions, we hypothesized that target of rapamycin (TOR) signaling can be closely related to REP1-mediated cell growth and survival to sustain metabolic stress conditions. TOR signaling is conserved as a nutrient-sensing protein from yeast to humans which controls cell growth and proliferation via regulating ribosome biogenesis, protein translation, and even autophagy. Furthermore, a yeast homolog of the vertebrate
choroideremia (
chm,
REP1) gene, named
MRS6, has a role in regulating TOR signaling and ribosome biogenesis, which is dependent on extracellular nutrient levels in the yeast model system [
26,
27], implying that REP1 has a conserved role in association with the nutrient signaling pathway, TOR.
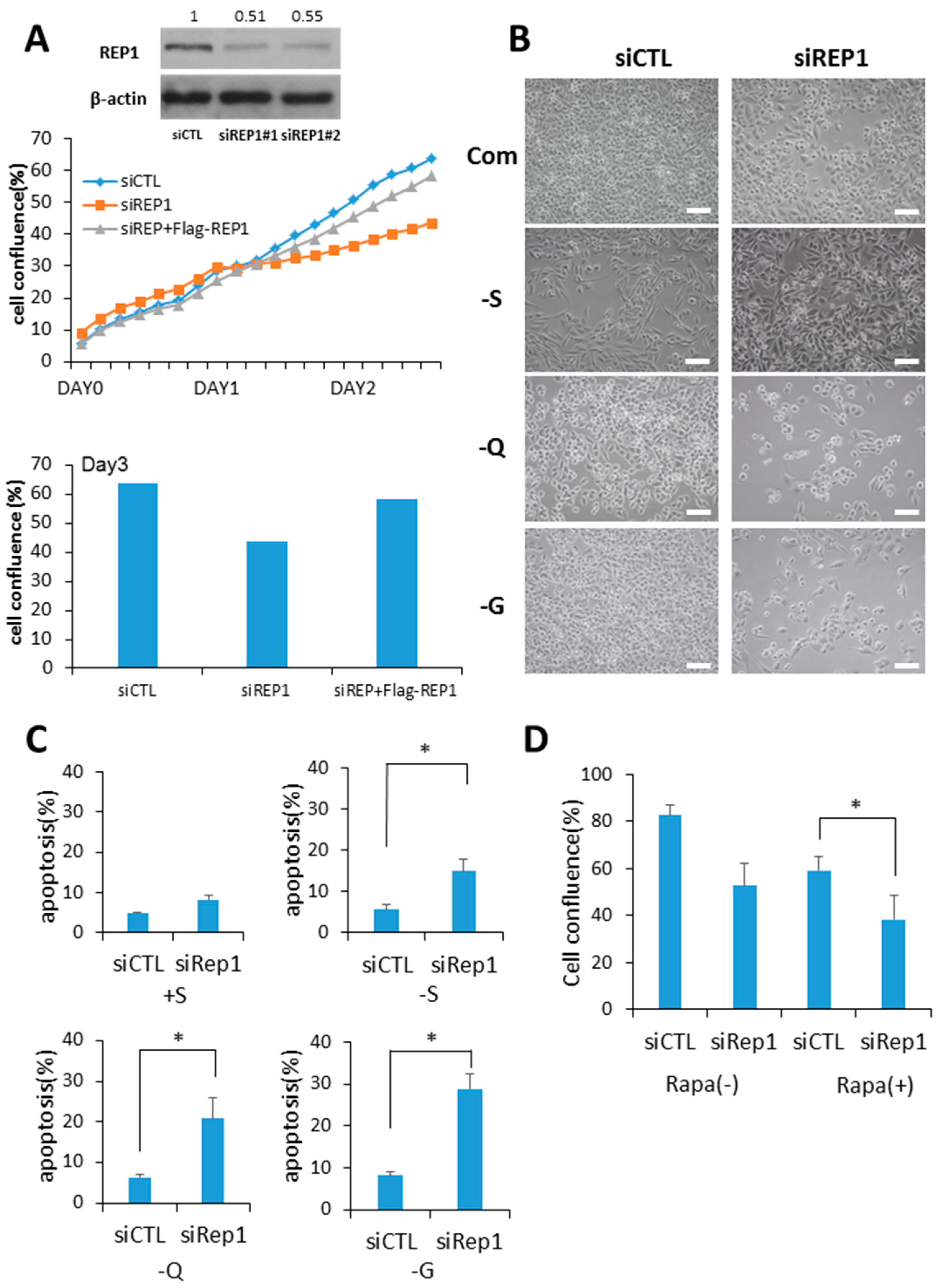
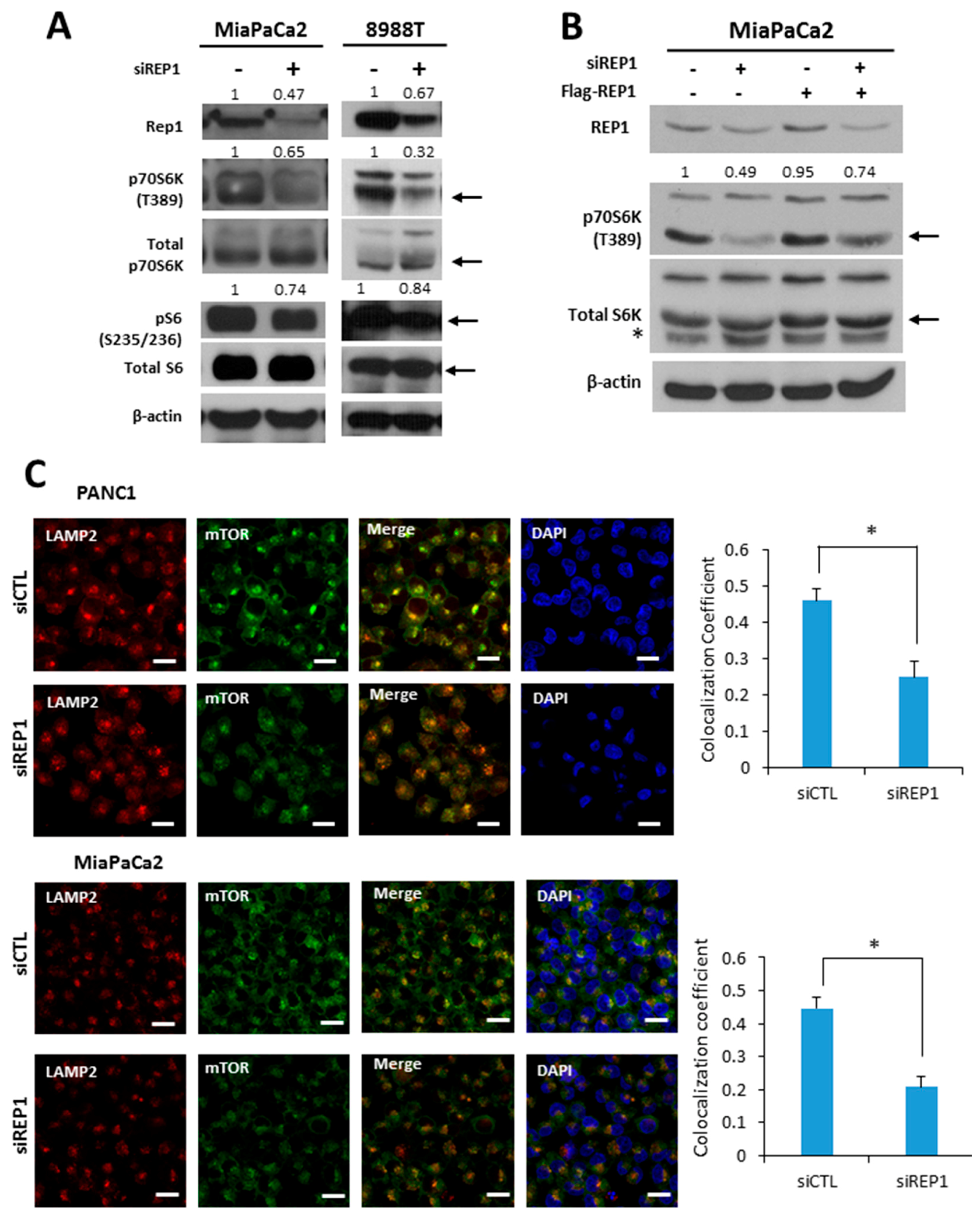
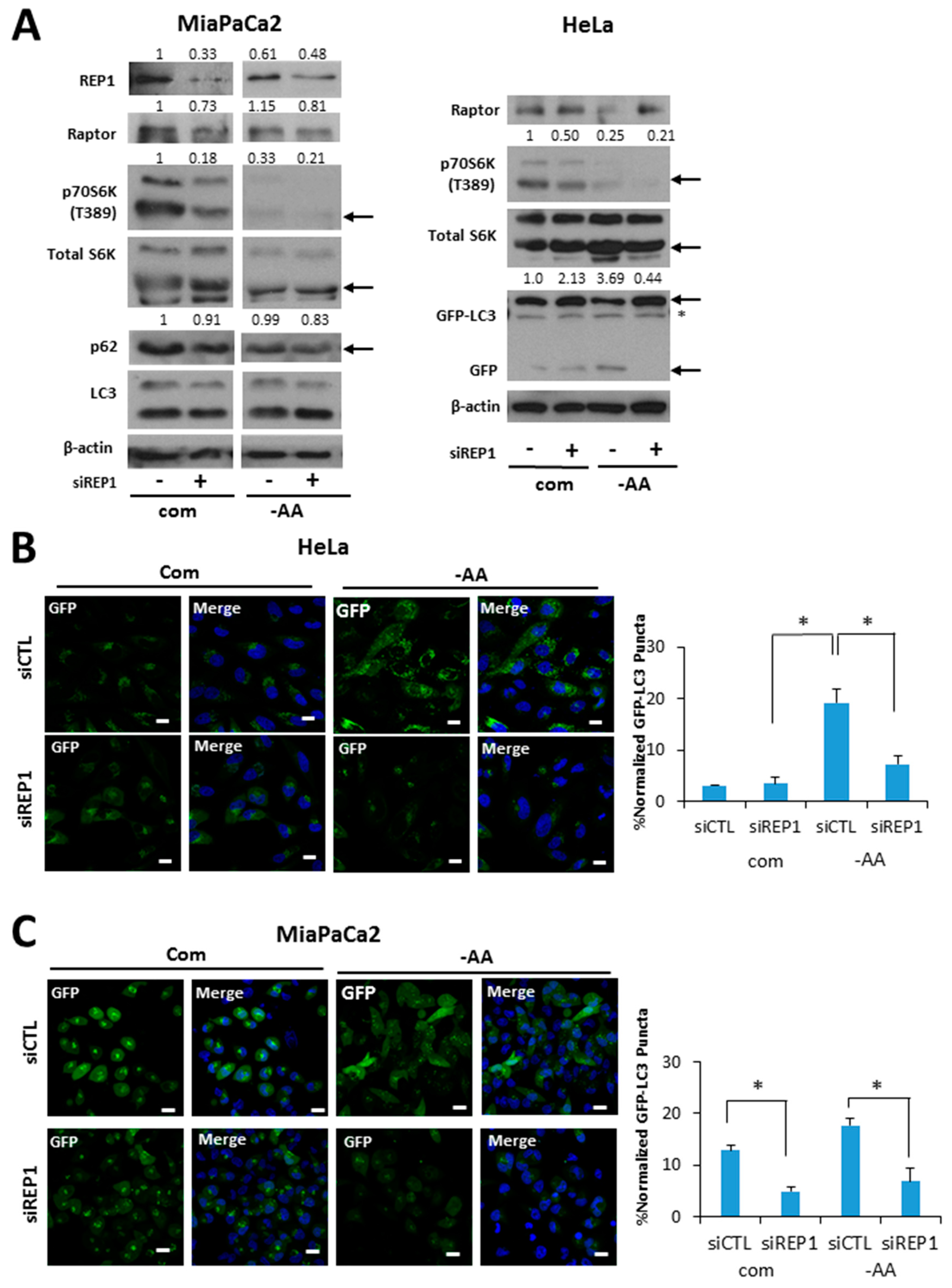
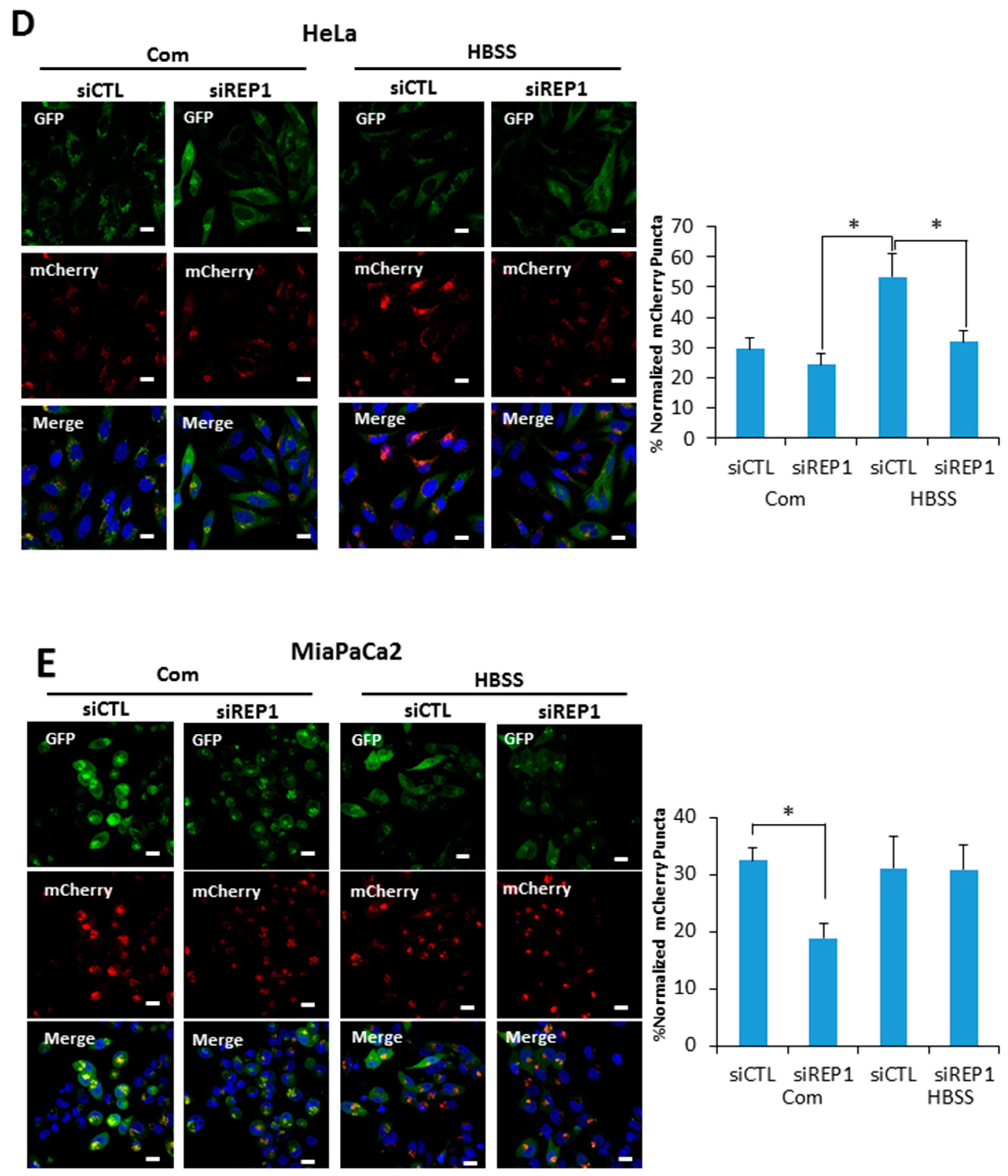
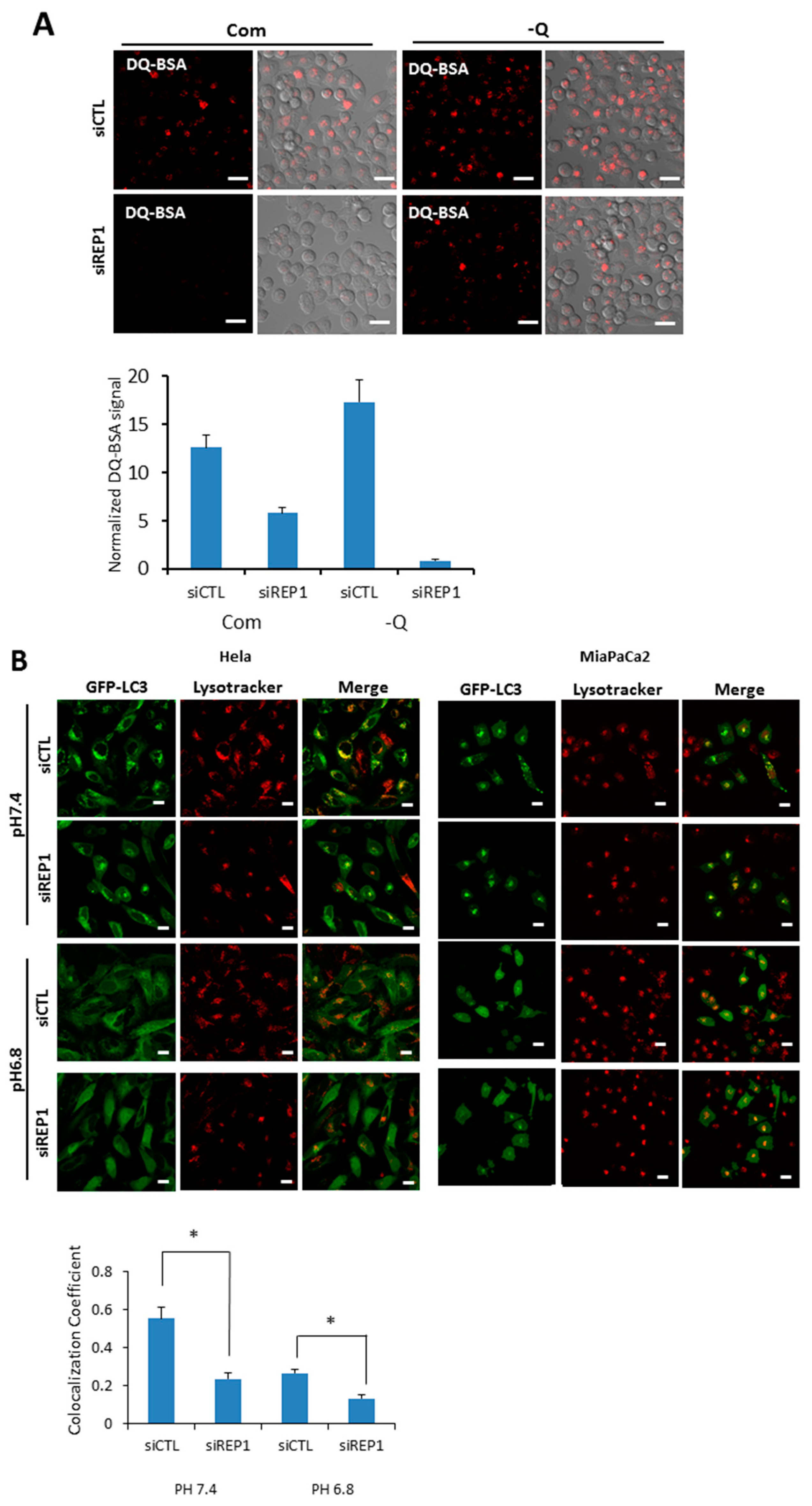
Based on our data, there is a relationship between REP1 and autophagy which is mediated by the regulation of mTORC1 signaling and lysosomes. REP1 knockdown suppressed mTORC1 activity, a negative modulator of autophagy in normal growing conditions. During nutrient starvation, REP1 depletion clearly blocked autophagy stimulated by various metabolic stresses, thereby exhibiting severe suppression of cell proliferation and survival in REP1 knockdown cells in response to metabolic stress, commonly shown in cancer development.
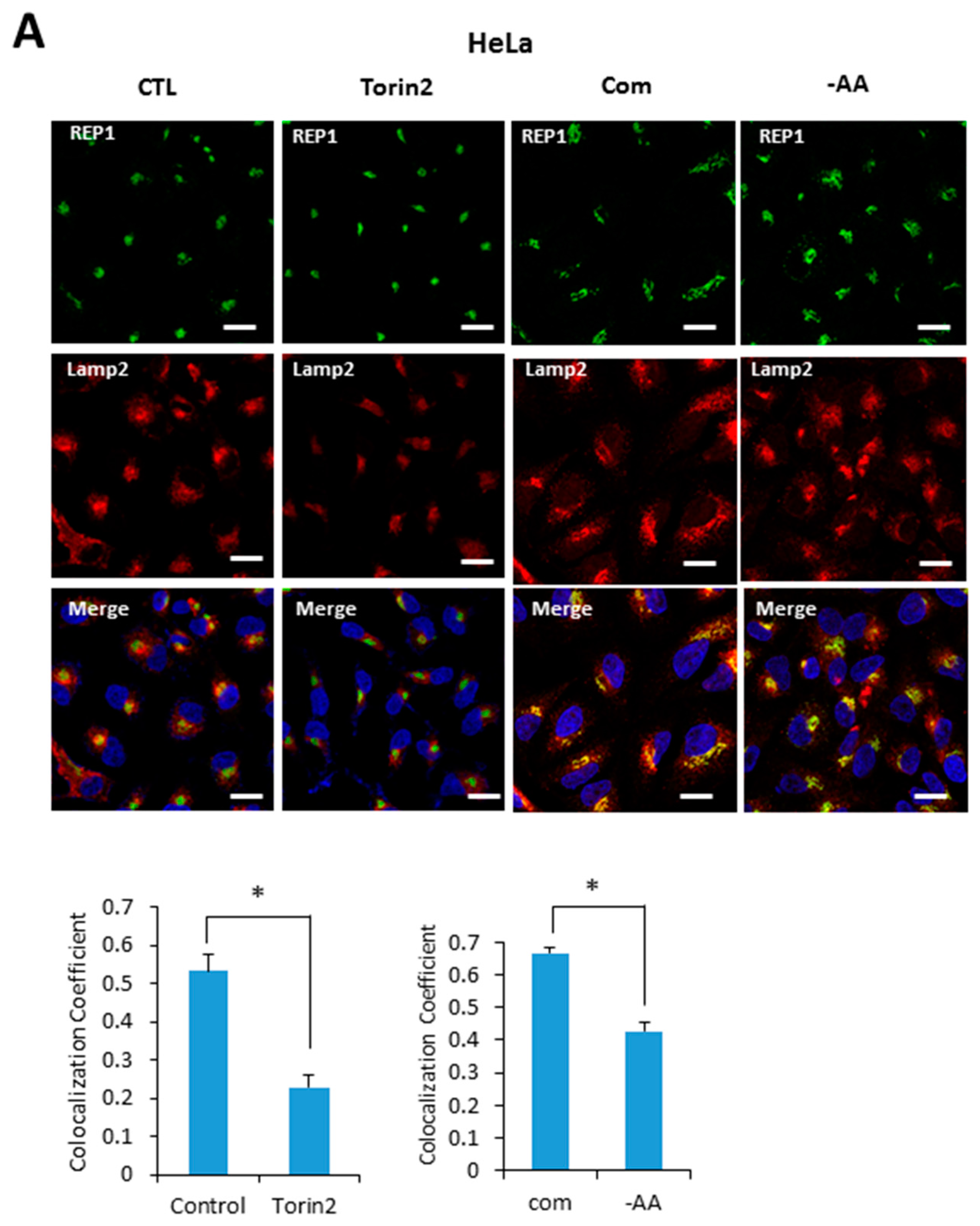
Although this detailed mechanism needs to be defined further, our results suggested that REP1 might modulate intracellular localization of the lysosome as well as autophagy pathway during starvation, which is affected by mTOR activity. Moreover, normal recruitment of REP1 itself to the lysosomes is significantly impaired in mTOR inactivation or nutrient deprivation, including deprivation of amino acids and growth factors. Unexpectedly, REP1 knockdown increases macropinocytosis, a nonselective endocytosis process for utilization of extracellular macromolecules. However this phenotype—increased level of uptake of macropinocytic marker—might be a feedback phenotype to compensate autophagy suppression shown in REP1-depleted cells.
Autophagy is generally necessary for modulating metabolic disturbances caused by a variety of stresses, including lack of nutrient or oxygen levels and oxidative stress. Dysregulation of autophagy can be closely linked to the progression of human diseases, including cancer, neurodegenerative disorders, and metabolic diseases. Therefore, understanding the regulatory mechanisms of macromolecule degradation pathways including autophagy can assist us in exerting therapeutic effects for several disorders. Particularly, autophagy plays context-dependent roles in tumor progression, relying on developmental stages or the microenvironment of tumor [
4].
In summary, we proposed a novel mechanism in which REP1 plays a critical role in cancer cell growth and survival mediated through regulating mTORC1 activity. As a downstream effector of mTORC1, lysosomal degradation pathways including autophagy and macropinocytosis can be modulated by REP1, which directly influences intracellular localization of mTOR and lysosomes to determine their activities. Therefore, understanding the molecular mechanism for inter-regulation among REP1, mTORC1, and lysosomal degradation pathways would provide an intriguing rationale for targeting for pancreatic cancers with high capacities for lysosomal degradation. Taken together, our study suggested that the molecular mechanisms of REP1 in regulating mTOR and subsequent lysosomal degradation pathways, as well as targeting autophagy, might be possible therapeutic options for cancer patients with high expression of REP1 levels.
4. Materials and Methods
4.1. Chemicals and Reagents
Primary antibodies against phospho-S6 (S235/236), S6 ribosomal protein, phospho-p70S6K (T389), p70S6K, and LC3B, respectively were purchased from Cell Signaling Technology (Beverly, MA, USA). Primary antibodies were purchased against LAMP2 from Abcam (Cambridge, UK), P62 from Progen (Heidelberg, Germany), β-actin from Sigma-Aldrich (St. Louis, MO, USA) and tubulin, cathepsin B, and cathepsin D from Santa Cruz (Dallas, TX, USA). The secondary antibodies, Alexa Fluor-488 conjugated anti-rabbit (A21206) and Alexa Fluor-592 anti-mouse (A11005), were from Life Technologies (Carlsbad, CA, USA). Horseradish peroxidase (HRP)-linked anti-rabbit and anti-mouse antibodies were from Bethyl Laboratories (Montgomery, TX, USA). DAPI, dextran, fluorescein (70 KDa; D1820), and dextran, tetramethylrhodamine (70 K Da; D1817) were from Life Technologies (Carlsbad, CA, USA). Mounting media (S3023) was from DAKO (Carpinteria, CA, USA). Bovine serum albumin (BSA; A1470), chloroquine (CQ; C6628), 5-(N-Ethyl-N-isopropyl), amiloride (EIPA; A3085), and the phosphatase inhibitor cocktail were from Sigma-Aldrich (St. Louis, MO, USA). The protease inhibitor cocktail tablet was from Roche Applied Bioscience, Penzberg, Upper Bavaria, Germany Rapamycin (S1039) and Torin2 were purchased from Selleck Chemicals (Houston, TX, USA).
Non-essential amino acids (1567906), essential amino acids (1542383), glutamine (25030081), HEPES (1627660), vitamin solution (1567870), and sodium bicarbonate (1546264) were from Life Technologies (Carlsbad, CA, USA). Mounting media (S3023) was from DAKO (Carpinteria, CA, USA).
4.2. Cell Lines and Culture Conditions
The Hela cells, and Panc1, 8988T, and MiaPaCa2 human pancreatic cancer cell lines from the American Type Culture Collection (ATCC; Manassas, VA, USA) were kindly provided by Kyung-Tae Kim and Yun-Hee Kim (National Cancer Center, Goyang-si, Korea). All cells were maintained under 5% CO2 at 37 °C in medium supplemented with 10% fetal bovine serum (FBS) (Hyclone), 100 U/mL penicillin, and 100 μg/mL streptomycin (Life Technologies). Mouse embryonic fibroblast (MEFs) and MiaPaCa2 were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Life technologies). For glutamine starvation, DMEM without glutamine (Life Technologies) was supplemented with 10% dialyzed FBS (Life Technologies). For the amino acid-starvation medium, Hank’s balanced saline solution (HBSS) was supplemented with 10% dialyzed FBS, glucose, vitamins, HEPES and minerals at the same concentrations as in DMEM.
GFP-LC3 in the MigRI-based retroviral vector was generously provided by Craig Thompson andpBabemCherry-GFP-LC3 (#22418) was a gift from Jayanta Debnarth through Addgene [
28]. Hela and MiaPaCa2 stably expressing GFP-LC3or mCherry-GFP-LC3 were generated following standard protocols for retrovirus transduction. The sequences of siRNA for REP1 are 5′-CCGGAGAGUUCUGCAUGUU-3′ (#1) and 5′GCAUGAAAGGCACCUAUUU-3′ (#2).
4.3. Western Blotting
For Western blotting, cells were rinsed with ice-cold phosphate buffered saline (PBS) and harvested using ice-cold lysis buffer (RIPA buffer). Samples were then incubated in RIPA buffer (50 mM TRIS-Cl pH 7.4, 150 mMNaCl, 1% NP-40, 0.5% Na-deoxycholate, 0.1% Sodium dodecyl sulfate, (SDS), and 1 mM ethylenediaminetetraacetic acid (EDTA) with protease inhibitor cocktail (Roche Applied Bioscience, Penzberg, Upper Bavaria, Germany) and phosphatase inhibitor (Sigma-Aldrich, St. Louis, MI, USA) for 15 min, and soluble lysate fractions were isolated by centrifugation at 16,000× g for 15 min. Protein concentrations were determined with the Pierce BCA Protein Assay (Thermo Scientific, Waltham, MA, USA), and equal amounts of protein were analyzed by SDS gel electrophoresis and Western blotting following standard protocols.
4.4. siRNA and Transfection
REP1 siRNA and negative control siRNA (non-targeting pool) were purchased from Genolution Inc. (Seoul, Korea); cells were transfected with lipofectamine 2000 for siRNA in Opti-MEM media. Cells were then resuspended in complete DMEM medium, incubated for 24–48 h, and used for further experiments.
4.5. Immunofluorescence Analysis
To determine the localization of mTOR, immunostaining against mTOR and LAMP2 was performed using MiaPaCa2 and Hela cells. After they were plated onto glass coverslips in complete medium for 18 h, cells were incubated in control (CTL) and REP1 siRNA treatment for 24 h. For immunostaining, cells were rinsed with ice-cold PBS, fixed with 3.7% formaldehyde for 30 min, and permeabilized with 0.05% Triton X-100 in PBS (PBS-T) for 5 min. After rinsing with PBS-T, cells were blocked with blocking buffer (0.5% BSA in PBS-T) for 1 h, incubated with primary antibodies in blocking buffer for 16 h in 4 °C, washed three times with PBS-T and then incubated with secondary antibodies conjugated with fluorescent probe in PBS-T for 1 h at room temperature. After three PBS-T washes, cells were stained with DAPI for 10 min and mounted on microscope slides. A confocal fluorescence microscope (Zeiss LSM 780, Oberkochen, Germany) was used for imaging and analysis. Microscopic images were generated through capturing at least five different areas per culture condition from the cells with either 400× or 630× magnification. The experiments were repeated at three independent days. Images were quantified by either counting function of pixel area or co-localization function ofimage-based software (ZEN black edition), which is provided by the confocal microscope system (LSM780).
Pearson’s correlation coefficient was used for calculating co-localization.
Pearson’s correlation coefficient provides information on the intensity distribution within the co-localizing region. Actual value ranges from−1 to +1. 0 mean pixels in the scattergram distribute in a cloud with no preferential direction. −1 and +1 mean all pixels are found on straight line in the scattergram.
4.6. Lysosome Trafficking Assay
For imaging of lysosome trafficking mediated by extracellular pH, Hela, and MiaPaCacells stably expressing mCherry-GFP-LC3were transfected with scrambled siRNA(CTL) or REP1 siRNA following the reverse transfection protocol, and incubated at 37 °C for 24 h in an 8-well Lab-Tek chamber. Subsequently, cells were changed with pH7.4 and pH6.8 DMEM media for 16 h. Then, nuclei were stained using DAPI. For lysotracker staining, LysoTracker Red was treated on the cells 30 min prior to imaging. Images were captured using an LSM780 fluorescent microscope (Zeiss, Oberkochen, Germany) and quantified using the software ZEN (Zeiss, Oberkochen, Germany). Auto lysosome signals (Red) from mCherry-GFP-LC3 were captured in at least five distinct fields from different regions for an individual experimental set. The total puncta area per cell was normalized by the area of DAPI-stained nucleus of that cell.
4.7. Macropinocytosis Analysis
For imaging of macropinocytosis, cells were incubated in the media without FBS for 4 h to increase macro pinocytosis. For starvation, the medium was replaced with either serum- or glutamine-complete or serum- or glutamine-deplete medium. These cells were incubated with 0.5 mg/mL dextran conjugated with FITC for the indicated periods at 37 °C. Subsequently, cells were washed three times with ice-cold PBS and fixed with 3.7% formaldehyde in PBS for 15 min. Nuclei were then stained using DAPI and mounted using mounting media (DAKO). Images were captured using an LSM780 confocal fluorescent microscope (Zeiss, Oberkochen, Germany) and quantified using software ZEN (Zeiss, Oberkochen, Germany). The total macropinosome area per cell was normalized by the area of DAPI-stained nucleus of that cell. Macropinosome areas were quantified in at least five distinct fields captured from different regions for an individual experimental set. In addition, subcellular localization of GFP-LC3 or mCherry-GFP-LC3 for measuring autophagy activity was monitored by LSM780 confocal fluorescent microscope (Zeiss, Oberkochen, Germany).
4.8. Cell Growth and Viability
Cells were plated in complete media in 24-well plates prior to starvation. Twenty-four hours after seeding, cells were washed with PBS and incubated in the indicated glutamine- or serum-starvation media with 10% dialyzed FBS. For rescue experiments, the cells were incubated in the media supplemented with either 0% BSA or 4% BSA. Negative control cells for macropinocytosiswere treated with EIPA. The number of viable cells was assessed using automated cell proliferation detector using IncuCyteTM (Essen Instruments, Ann Arbor, MI, USA). Proliferation was measured through quantitative kinetic processing metrics derived from time-lapse image acquisition and presented as percentage of culture confluence over time. The number of cells wasalso counted either using a Coulter Counter (Beckman Coulter, Brea, CA, USA) and/or using trypan blue exclusion and an automated cell counter (Nano-Entek, Seoul, Korea). Cell viability was determined by Annexin V and propidium iodide (PI) staining following standard protocols at indicated periods of time (BD Biosciences). Cells negative for both Annexin V and PI staining were considered live cells.