The Actin Cytoskeleton Is Involved in Glial Cell Line-Derived Neurotrophic Factor (GDNF)-Induced Ret Translocation into Lipid Rafts in Dopaminergic Neuronal Cells
Abstract
:1. Introduction
2. Results
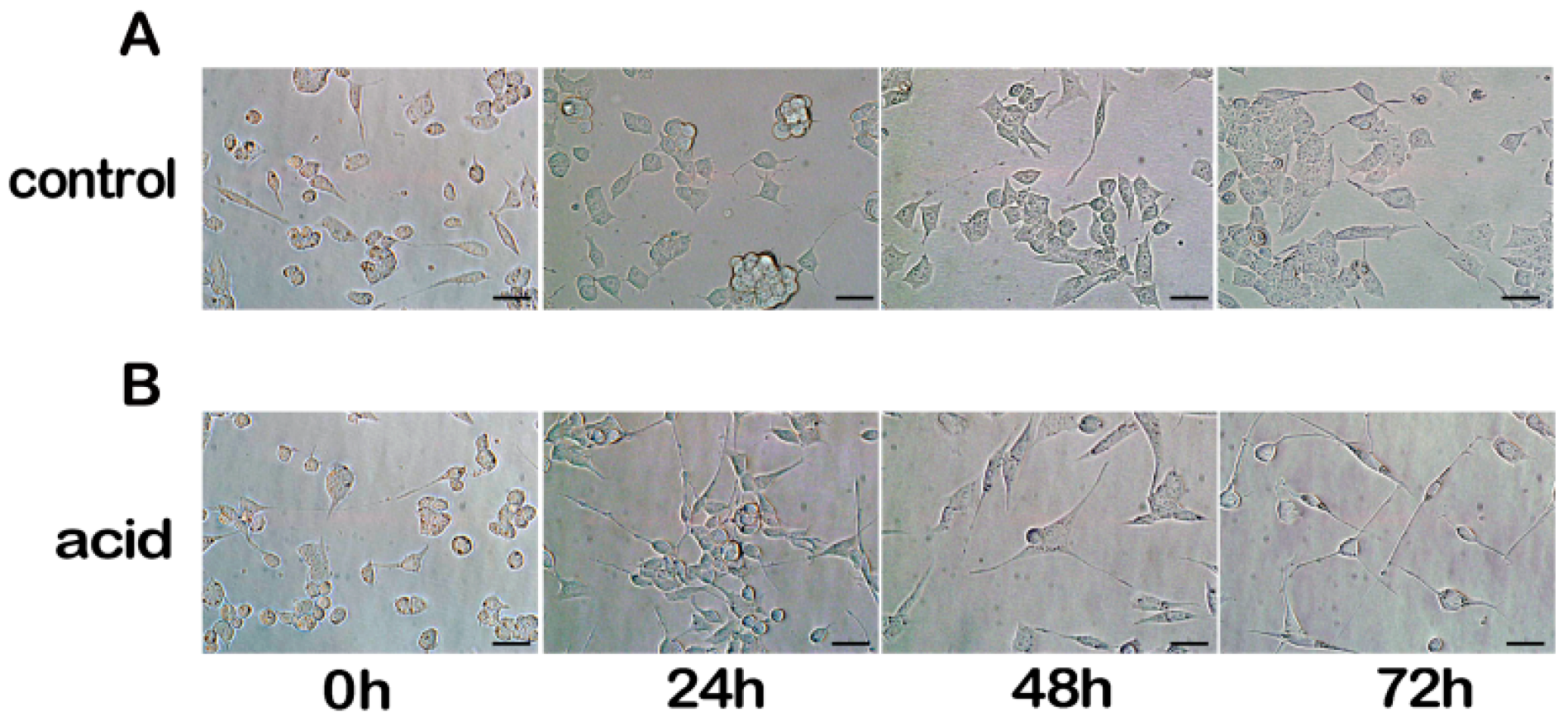
2.1. Differentiation of MN9D Neuronal Cells
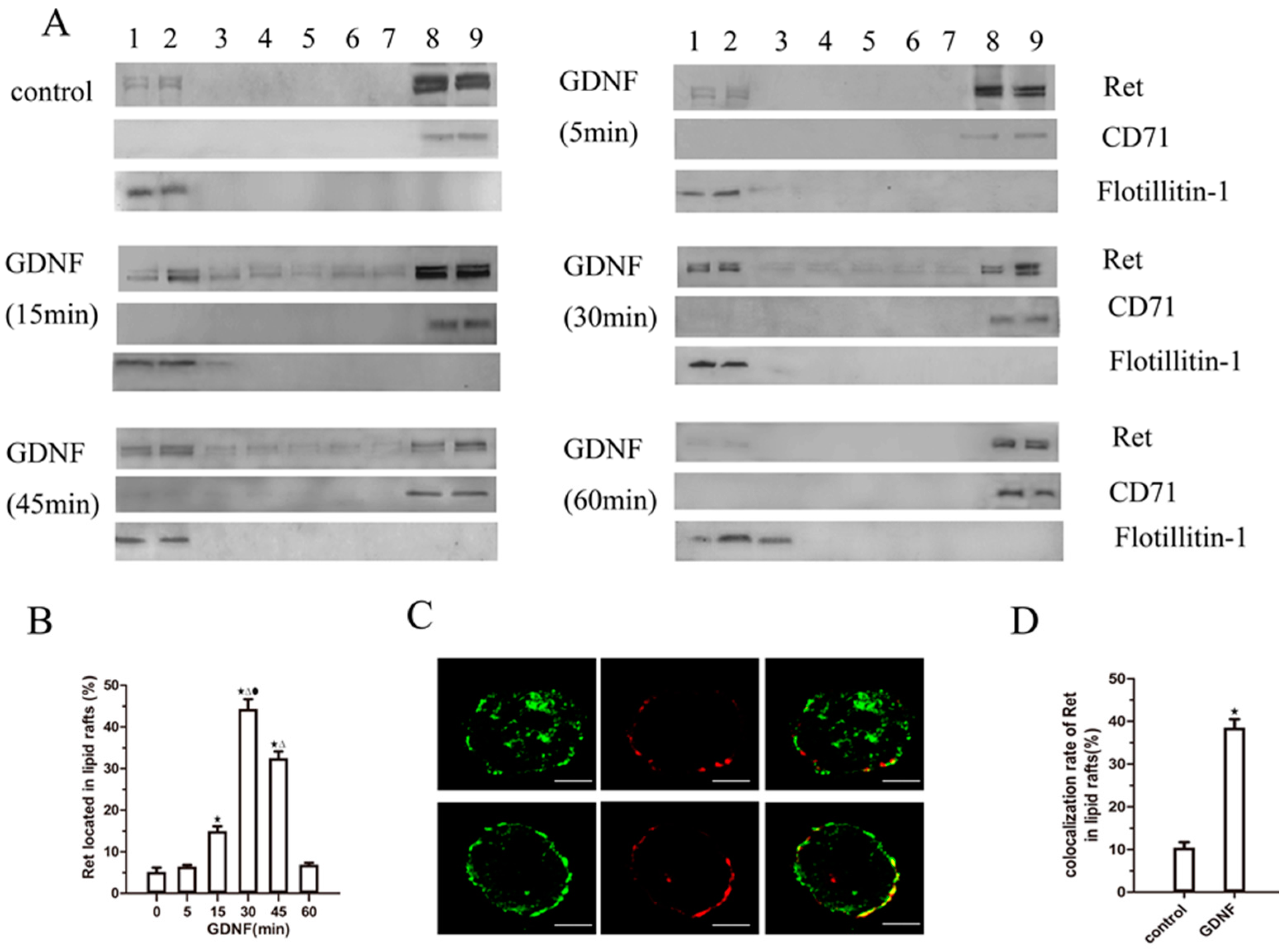
2.2. GDNF Induces Ret Translocation to Lipid Rafts
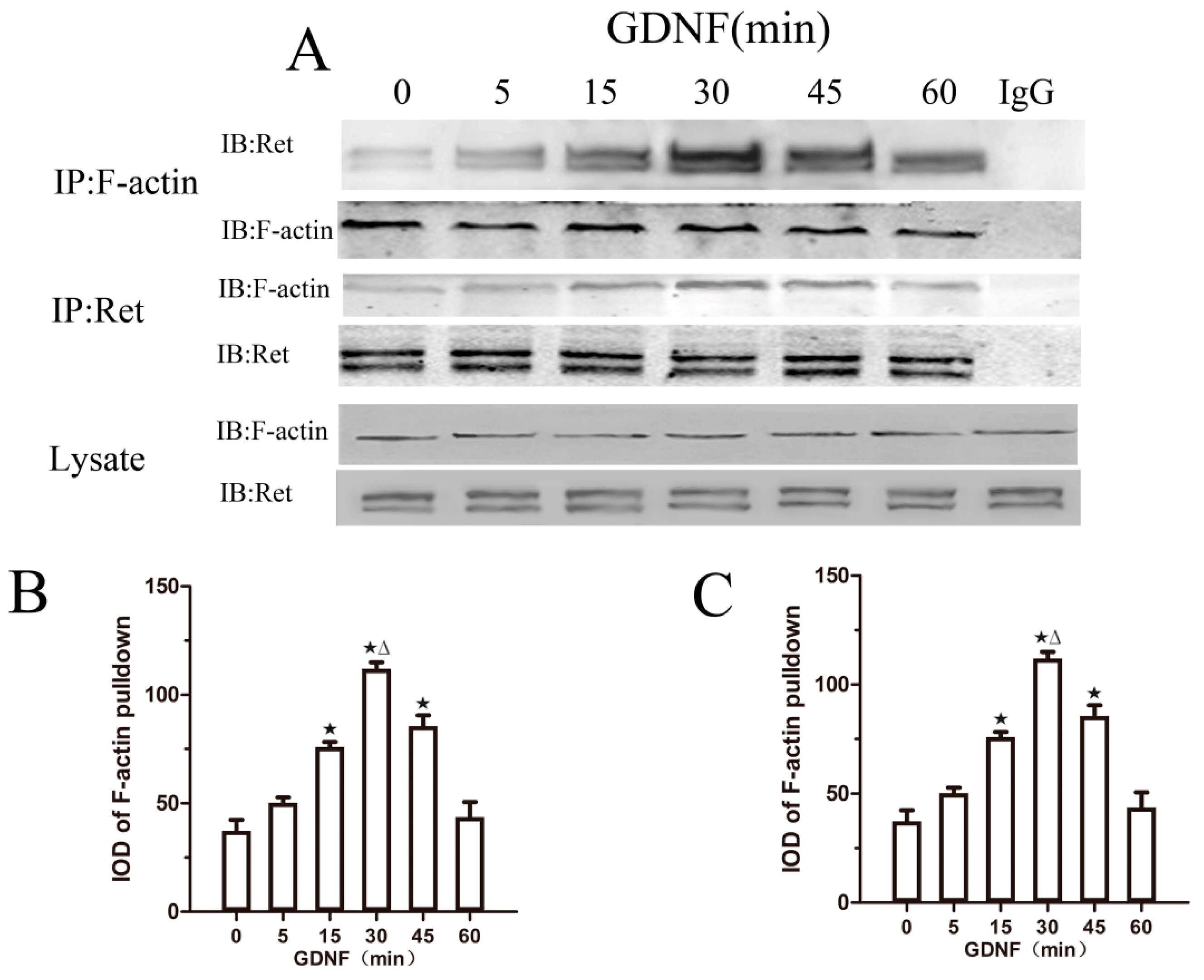
2.3. GDNF Induces the Association of Ret and F-Actin
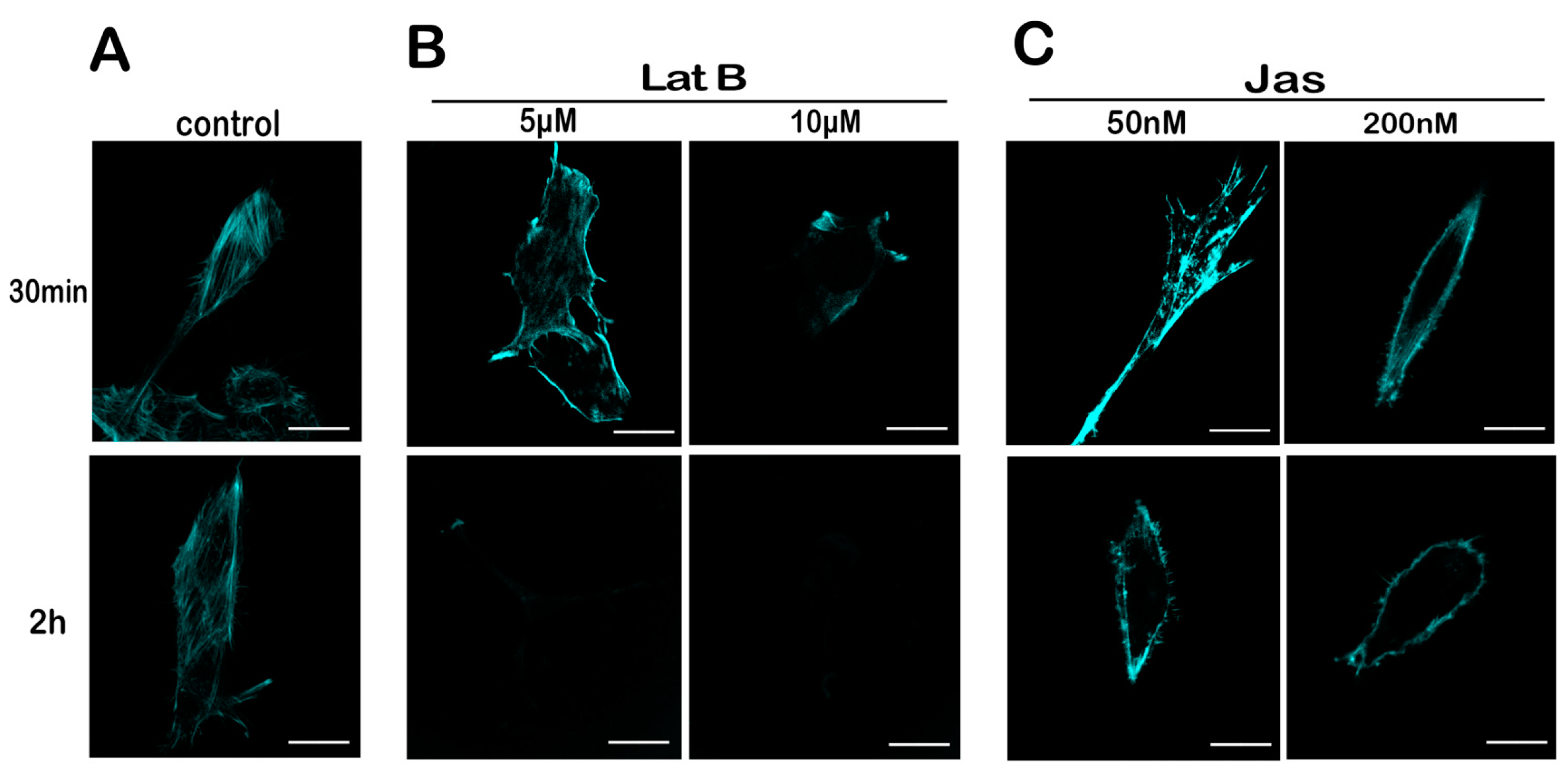
2.4. Lat B and Jas Disrupt and Enhance the Polymerization of the Actin Cytoskeleton, Respectively
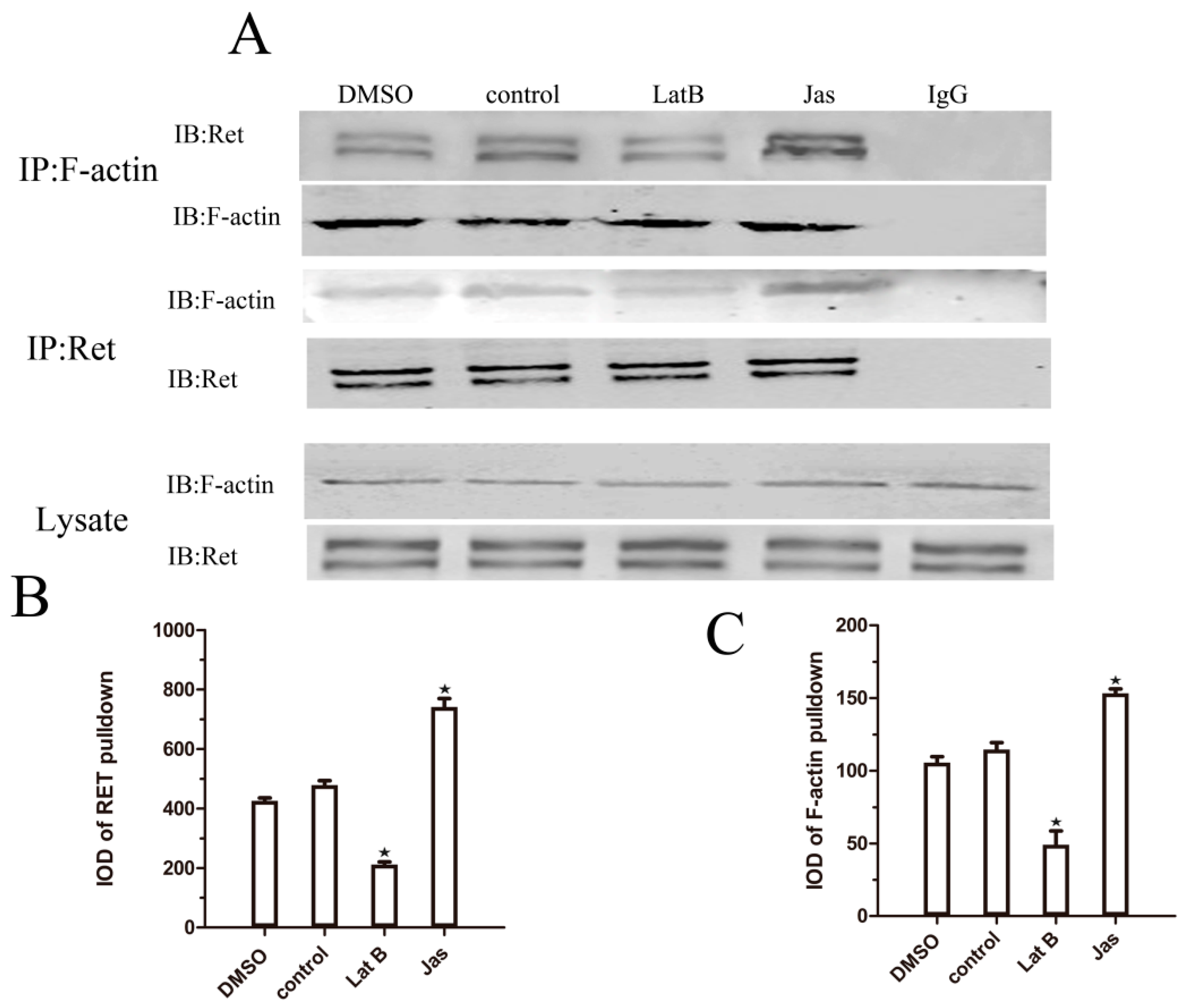
2.5. Lat B and Jas Regulate the Association between Ret and F-Actin
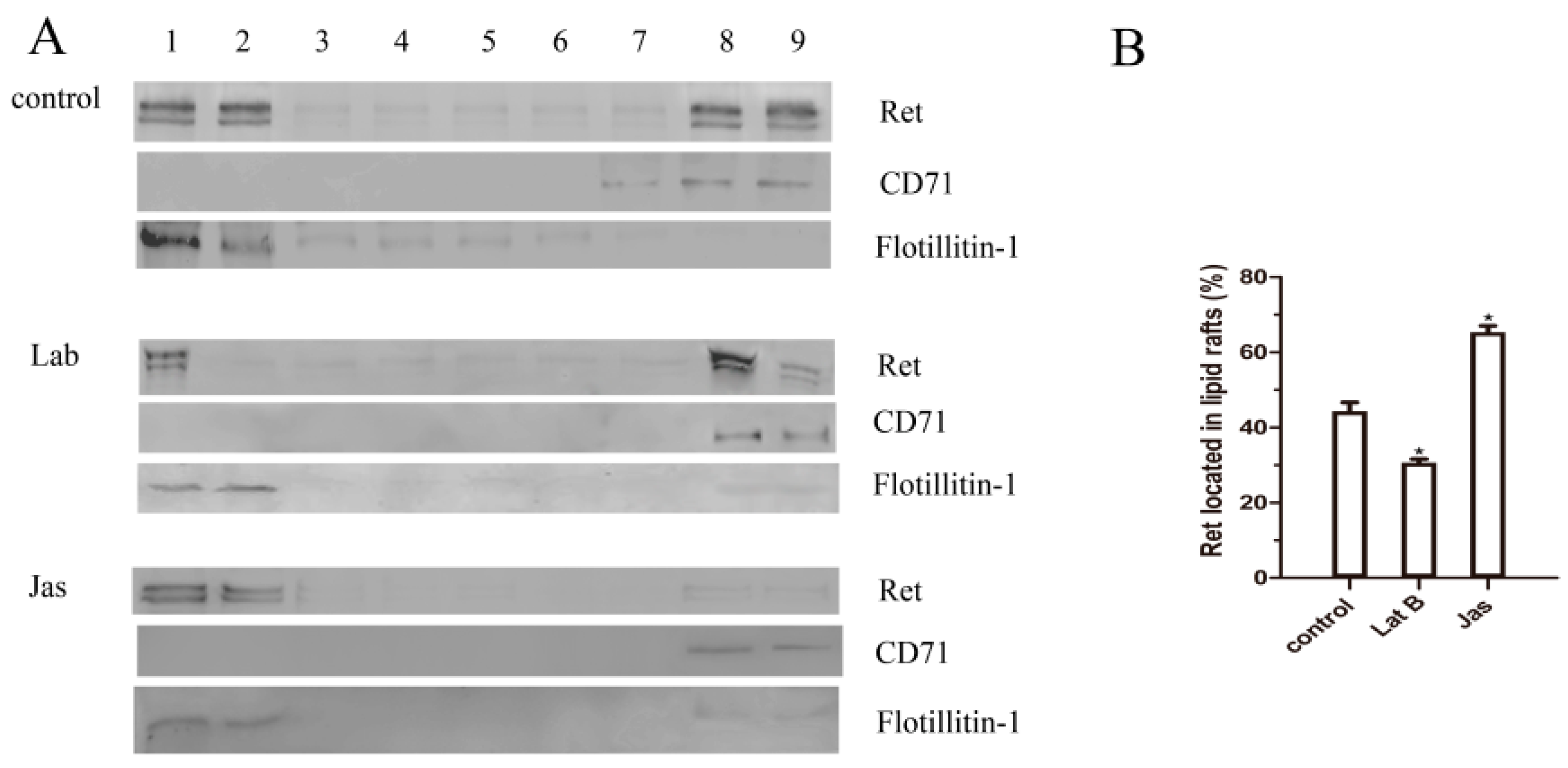
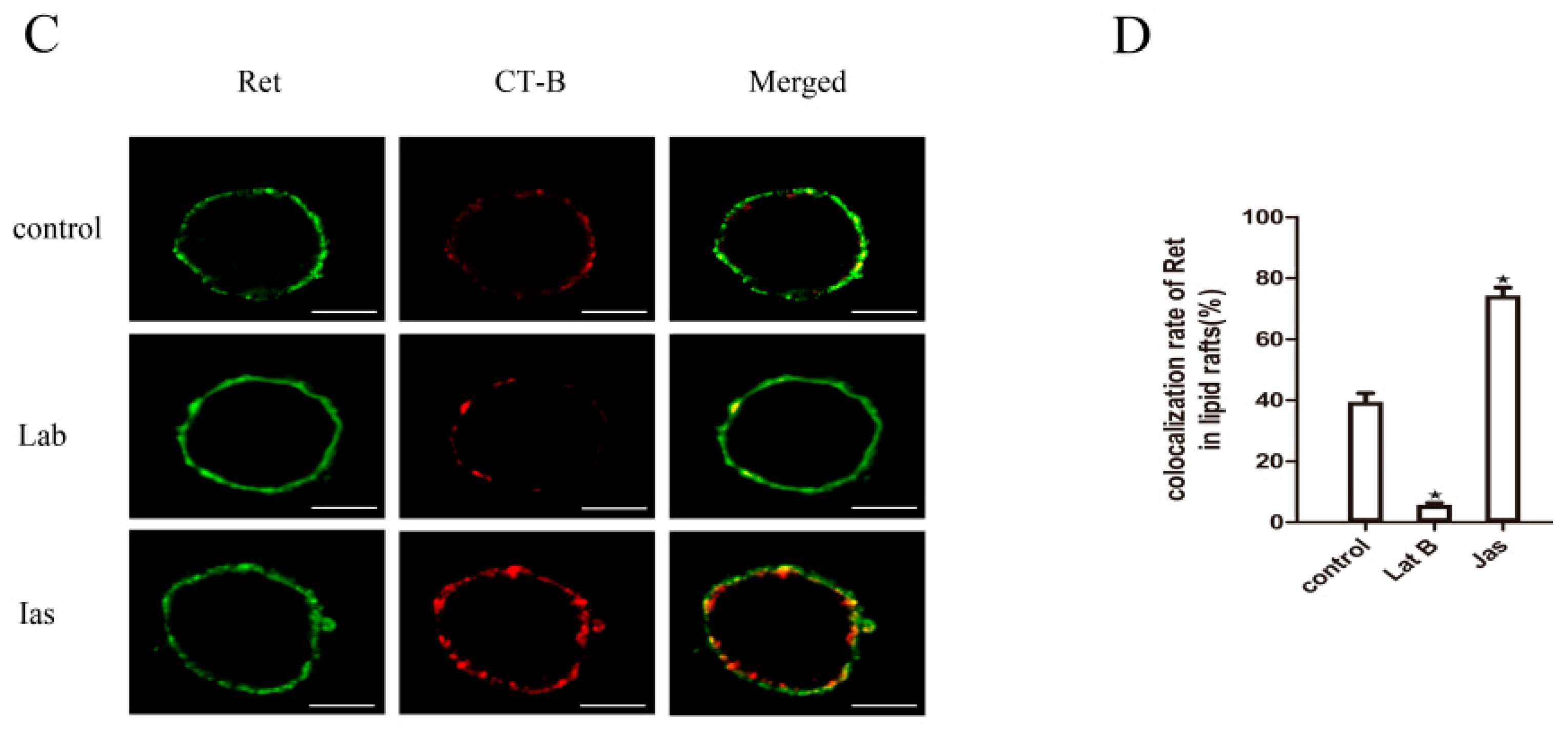
2.6. The Actin Cytoskeleton Is Involved in GDNF-Induced Ret Translocation into Lipid Rafts
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Differentiation Assay
4.2. Isolation of Detergent-Resistant Membrane Fractions and Analysis of Membrane Fractions on Flotation Gradients
4.3. Patching and Immunofluorescence Confocal Microscopy
4.4. Ret/F-Actin Co-Immunoprecipitation
4.5. Western Blotting
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, H.; Wang, M.; Chen, F.; Gao, J.; Sun, S.; Li, Y.; Gao, D. NCAM-140 Translocation into lipid rafts mediates the neuroprotective effects of GDNF. Mol. Neurobiol. 2016, 54, 2739–2751. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.M.; Ortiz, R.; Dye, S.; Campbell, R.B.; Amiji, M.M.; Waszczak, B.L. Neurotrophic and neuroprotective efficacy of intranasal GDNF in a rat model of Parkinson’s disease. Neuroscience 2014, 274, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Paratcha, G.; Ledda, F.; Baars, L.; Coulpier, M.; Besset, V.; Anders, J.; Scott, R.; Ibanez, C.F. Released GFRα1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron 2001, 29, 171–184. [Google Scholar] [CrossRef]
- Tansey, M.G.; Baloh, R.H.; Milbrandt, J.; Johnson, E.M., Jr. GFRα-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron 2000, 25, 611–623. [Google Scholar] [CrossRef]
- Iwase, T.; Jung, C.G.; Bae, H.; Zhang, M.; Soliven, B. Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. J. Neurochem. 2005, 94, 1488–1499. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, T.K.; Luebke, M.; Stenqvist, A.; Ernfors, P. Differential membrane compartmentalization of Ret by PTB-adaptor engagement. FEBS J. 2008, 275, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000, 275, 17221–17224. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harbor Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef] [PubMed]
- Chichili, G.R.; Rodgers, W. Clustering of membrane raft proteins by the actin cytoskeleton. J. Biol. Chem. 2007, 282, 36682–36691. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, K.; Ghosh, S.; Saha, S.; C, R.; Mayor, S.; Rao, M. Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell 2012, 149, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.C.; Moran, M.; Chung, C.D.; Patel, V.P.; Low, T.; Zinnanti, W. Co-stimulation and counter-stimulation: Lipid raft clustering controls TCR signaling and functional outcomes. Semin. Immunol. 2001, 13, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Chichili, G.R.; Rodgers, W. Cytoskeleton-membrane interactions in membrane raft structure. Cell. Mol. Life Sci. 2009, 66, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Abid Sheikh, M.; Saeed Malik, Y.; Xing, Z.; Guo, Z.; Tian, H.; Zhu, X.; Chen, X. Polylysine-modified polyethylenimine (PEI-PLL) mediated VEGF gene delivery protects dopaminergic neurons in cell culture and in rat models of Parkinson’s Disease (PD). Acta Biomater. 2016, 54, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.S.; Hermanson, E.; Joseph, B.; Wallén, A.; Aarnisalo, P.; Heller, A.; Perlmann, T. Induction of cell cycle arrest and morphological differentiation by Nurr1 and retinoids in dopamine MN9D cells. J. Biol. Chem. 2001, 276, 43277–43284. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.M.; Brugge, J.S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997, 13, 513–609. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.J.; Goodman, L.; de Sauvage, F.; Stone, D.M.; Poulsen, K.T.; Beck, C.D.; Gray, C.; Armanini, M.P.; Pollock, R.A.; Hefti, F. Characterization of a multicomponent receptor for GDNF. Nature 1996, 382, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; London, E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998, 14, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Brdicka, T.; Pavlistova, D.; Leo, A.; Bruyns, E.; Korinek, V.; Angelisova, P.; Scherer, J.; Shevchenko, A.; Hilgert, I.; Cerny, J.; et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 2000, 191, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Lenne, P.F.; Wawrezinieck, L.; Conchonaud, F.; Wurtz, O.; Boned, A.; Guo, X.J.; Rigneault, H.; He, H.T.; Marguet, D. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J. 2006, 25, 3245–3256. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, W.; Glaser, M. Distributions of proteins and lipids in the erythrocyte membrane. Biochemistry 1993, 32, 12591–12598. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, W.; Zavzavadjian, J. Glycolipid-enriched membrane domains are assembled into membrane patches by associating with the actin cytoskeleton. Exp. Cell Res. 2001, 267, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Rodgers, W. T cell glycolipid-enriched membrane domains are constitutively assembled as membrane patches that translocate to immune synapses. J. Immunol. 2003, 171, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.N.; Gangaraju, S.; Aylsworth, A.; Hou, S.T. Membrane raft disruption results in neuritic retraction prior to neuronal death in cortical neurons. Biosci. Trends 2012, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Goudenege, S.; Dargelos, E.; Claverol, S.; Bonneu, M.; Cottin, P.; Poussard, S. Comparative proteomic analysis of myotube caveolae after milli-calpain deregulation. Proteomics 2007, 7, 3289–3298. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Fu, Y.; Gu, M.; Zhang, L.; Li, D.; Li, H.; Chien, S.; Shyy, J.Y.; Zhu, Y. Activation of integrin α5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 2016, 113, 201524523. [Google Scholar] [CrossRef] [PubMed]
- Fra, A.M.; Williamson, E.; Simons, K.; Parton, R.G. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 1994, 269, 30745. [Google Scholar] [PubMed]
- Janes, P.W.; Ley, S.C.; Magee, A.I. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 1999, 147, 447–461. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Song, H.; Mu, P.; Xu, M.; Liu, C.; Wang, Y.; Qin, Y.; Sun, S.; Gao, J.; Wang, T.; et al. The Actin Cytoskeleton Is Involved in Glial Cell Line-Derived Neurotrophic Factor (GDNF)-Induced Ret Translocation into Lipid Rafts in Dopaminergic Neuronal Cells. Int. J. Mol. Sci. 2017, 18, 1922. https://doi.org/10.3390/ijms18091922
Li L, Song H, Mu P, Xu M, Liu C, Wang Y, Qin Y, Sun S, Gao J, Wang T, et al. The Actin Cytoskeleton Is Involved in Glial Cell Line-Derived Neurotrophic Factor (GDNF)-Induced Ret Translocation into Lipid Rafts in Dopaminergic Neuronal Cells. International Journal of Molecular Sciences. 2017; 18(9):1922. https://doi.org/10.3390/ijms18091922
Chicago/Turabian StyleLi, Li, Haijing Song, Peipei Mu, Ming Xu, Chaoxia Liu, Ying Wang, Yingsong Qin, Shen Sun, Jin Gao, Ting Wang, and et al. 2017. "The Actin Cytoskeleton Is Involved in Glial Cell Line-Derived Neurotrophic Factor (GDNF)-Induced Ret Translocation into Lipid Rafts in Dopaminergic Neuronal Cells" International Journal of Molecular Sciences 18, no. 9: 1922. https://doi.org/10.3390/ijms18091922



