Event-Associated Oxygen Consumption Rate Increases ca. Five-Fold When Interictal Activity Transforms into Seizure-Like Events In Vitro
Abstract
:1. Introduction
2. Results
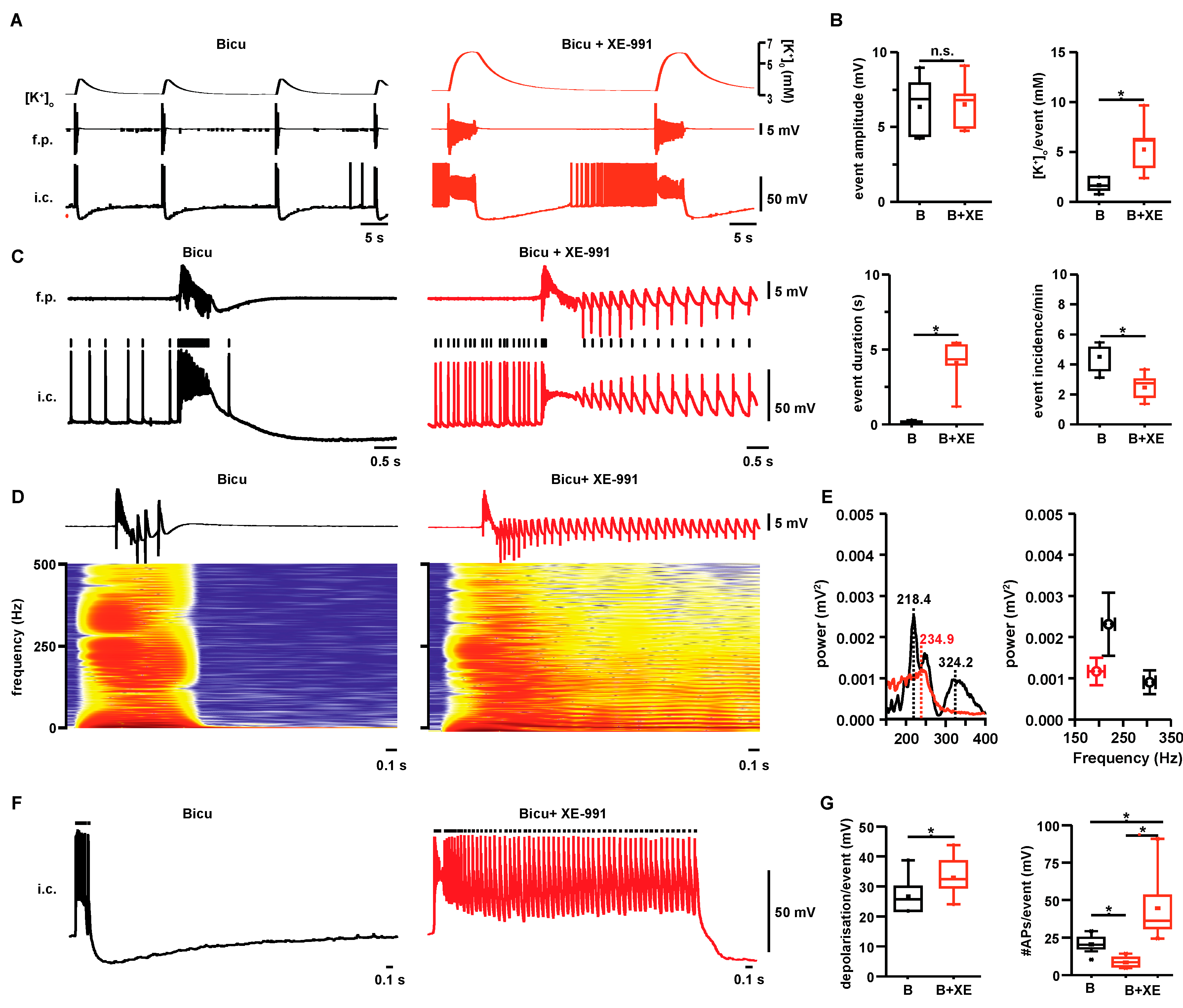
2.1. Properties of Interictal- and Seizure-Like Events in Hippocampal Area CA3
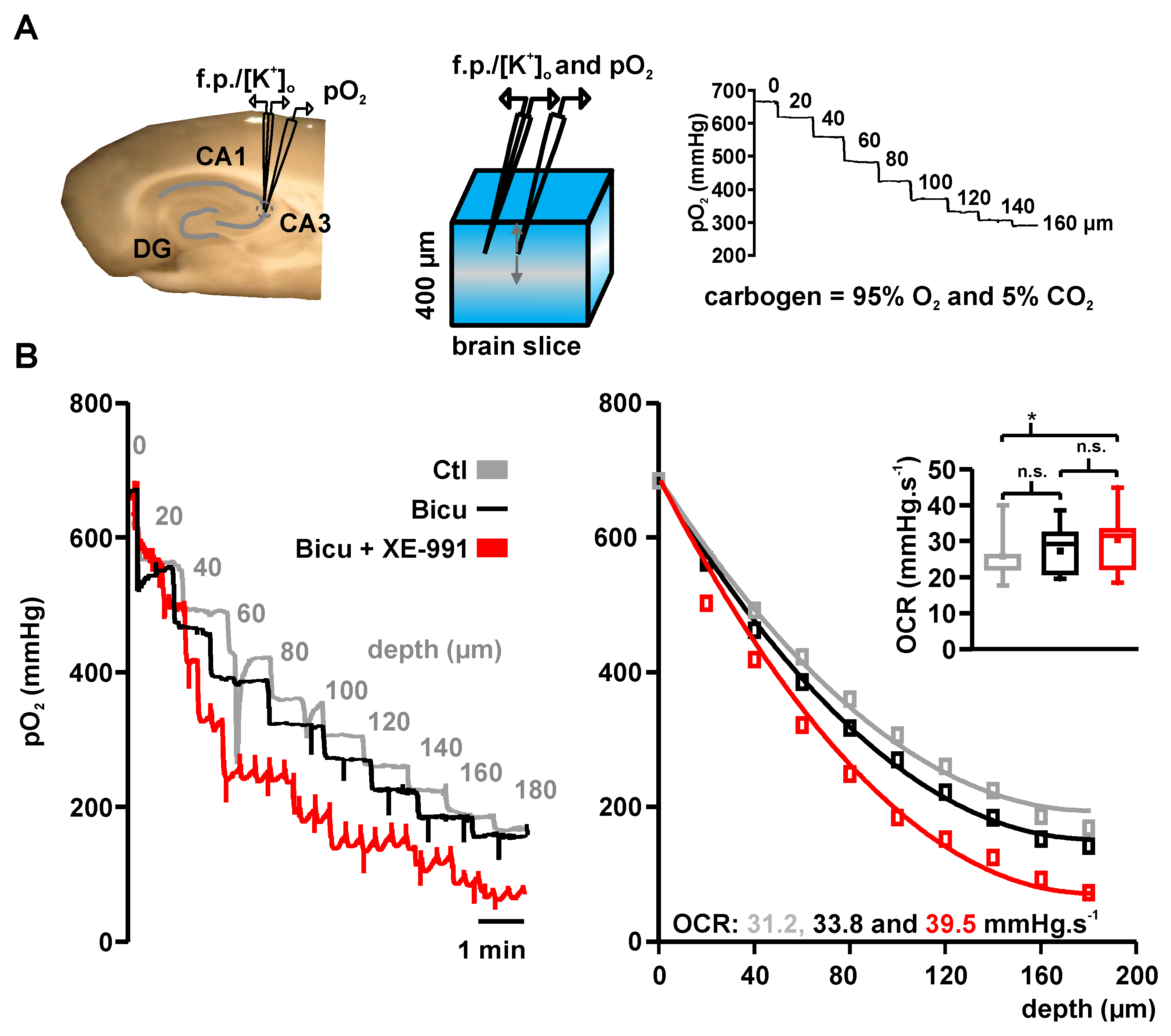
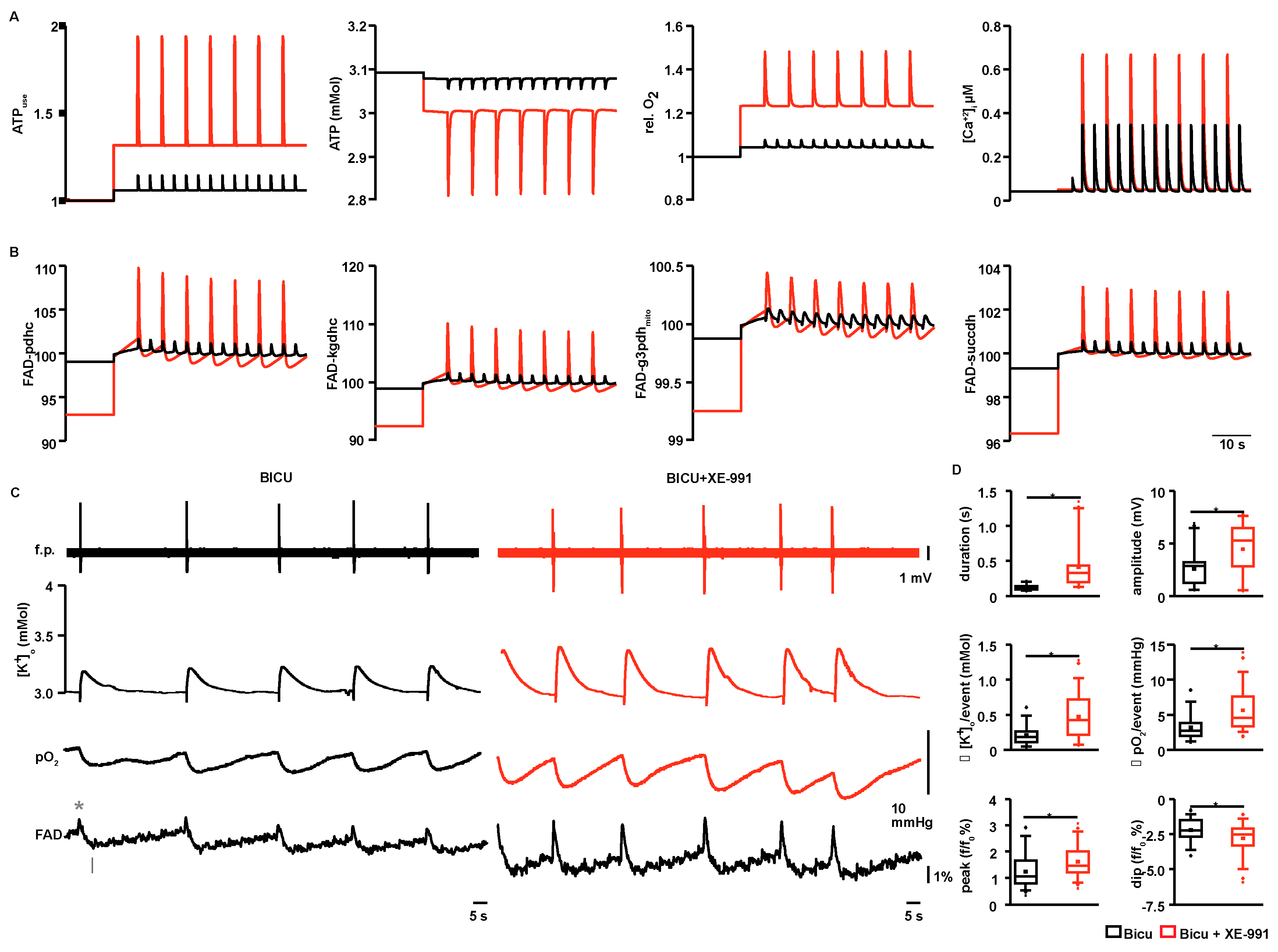
2.2. Basal Oxygen Consumption Rates under Bicuculline and Bicuculline Plus XE-991
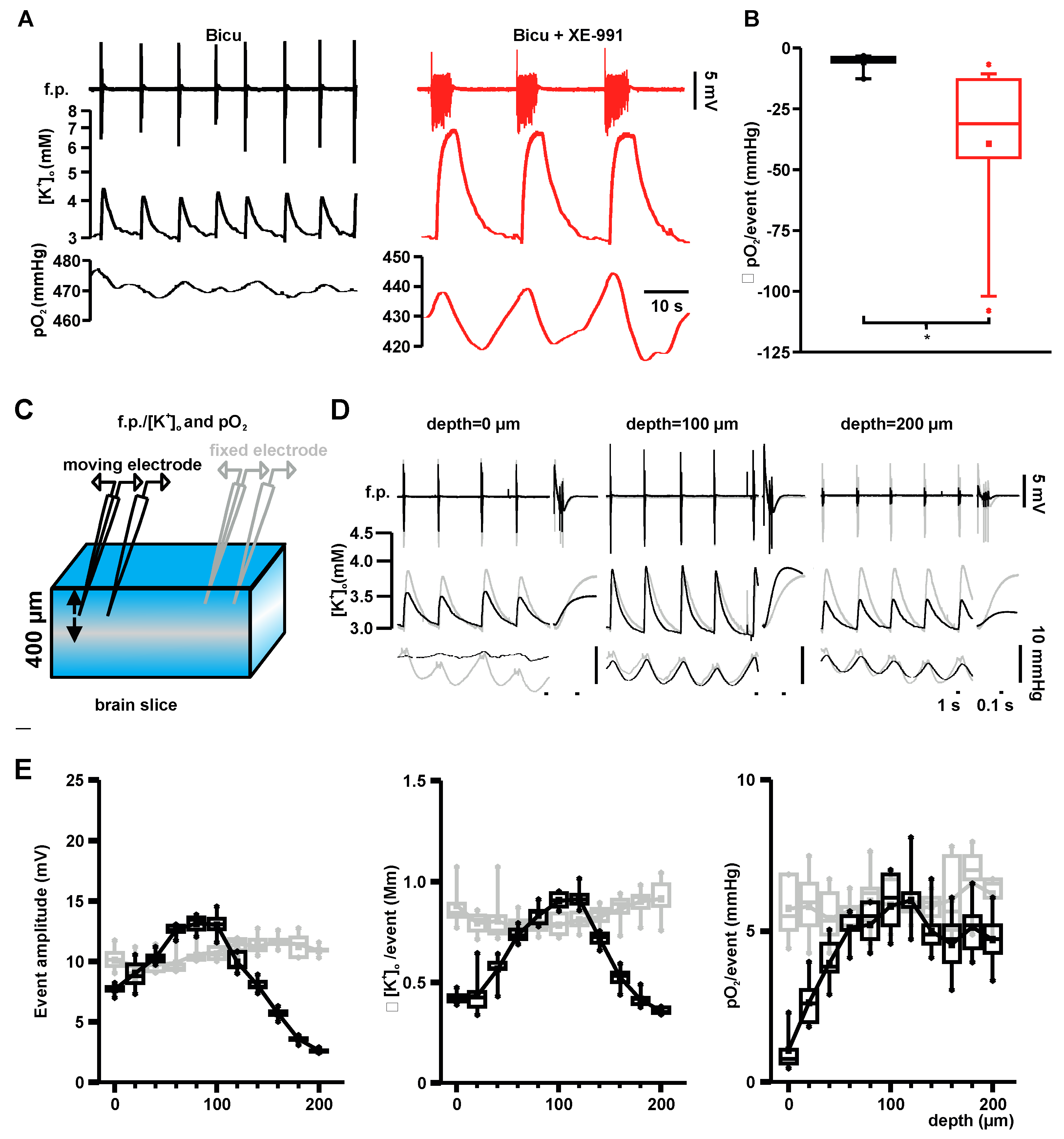
2.3. Event-Associated Oxygen Consumption Rates for ILEs and SLEs
2.4. Modeling of ATP Consumption Rates and Prediction of FAD Transients for ILEs and SLEs
3. Discussion
4. Materials and Methods
4.1. Slice Preparation and Maintenance
4.2. Electrophysiology, Oxygen Recordings and Fluorescence Recordings
4.3. Drugs
4.4. Data Acquisition and Data Analysis
4.5. Calculation of Basal Oxygen Consumption Rates of Control, Bicuculline and Bicuculline + XE-991 Condition
4.6. Calculation of Event Associated Oxygen Consumption During ILEs and SLEs
4.7. Calculating of FAD Transients and ATP Consumption Rates
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| aCSF | Artificial cerebrospinal fluid |
| ATP | Adenosine triphosphate |
| EAOCR | Event-associated oxygen consumption rate |
| FAD | Flavine adenine dinucleotide |
| f.p. | Local field potential |
| GABA | Gama-aminobutyric acid |
| HFO | High frequency oscillation |
| i.c. | Intracellular |
| ILE | Interictal-like event |
| K+ | Potassium ion |
| [K+]o | Extracellular potassium concentration |
| LED | Light emitting diode |
| Na+ | Sodium ion |
| NADH | Nicotinamide adenine dinucleotide |
| OCR | Oxygen consumption rate |
| pO2 | Partial oxygen pressure |
| SLE | Seizure-like event |
References
- Erecińska, M.; Silver, I.A. Tissue oxygen tension and brain sensitivity to hypoxia. Respir. Physiol. 2001, 128, 263–276. [Google Scholar] [CrossRef]
- Heinemann, U.; Buchheim, K.; Gabriel, S.; Kann, O.; Kovacs, R. Coupling of electrical and metabolic activity during epileptiform discharges. Epilepsia 2002, 43 (Suppl. S5), 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ames, A. CNS energy metabolism as related to function. Brain Res. Rev. 2000, 34, 42–68. [Google Scholar] [CrossRef]
- Fisher, R.S.; Scharfman, H.E.; deCurtis, M. How Can We Identify Ictal and Interictal Abnormal Activity? Springer: Berlin, Germany, 2014; pp. 3–23. [Google Scholar]
- Fernández-Klett, F.; Offenhauser, N.; Dirnagl, U.; Priller, J. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc. Natl. Acad. Sci. USA 2010, 107, 22290–22295. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I.; Bernard, C.; Turner, D.A. Metabolic responses differentiate between interictal, ictal and persistent epileptiform activity in intact, immature hippocampus in vitro. Neurobiol. Dis. 2015, 75, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.W.; Williamson, A.; Cavus, I.; Hetherington, H.P.; Zaveri, H.; Petroff, O.A.; Spencer, D.D. Neurometabolism in human epilepsy. Epilepsia 2008, 49, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Alavi, A.; Yakir, S.; Newberg, A.B. Positron emission tomography in seizure disorders. Ann. N. Y. Acad. Sci. 2001, 1228, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, U.; Buchheim, K.; Gabriel, S.; Kann, O.; Kovacs, R.; Schuchmann, S. Cell death and metabolic activity during epileptiform discharges and status epilepticus in the hippocampus. Prog. Brain Res. 2002, 135, 197–210. [Google Scholar] [PubMed]
- Liotta, A.; Caliskan, G.; ul Haq, R.; Hollnagel, J.O.; Rösler, A.; Heinemann, U.; Behrens, C.J. Partial disinhibition is required for transition of stimulus-induced sharp wave-ripple complexes into recurrent epileptiform discharges in rat hippocampal slices. J. Neurophysiol. 2001, 105, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, J.J.; Heinemann, U. Extracellular K+ and Ca2+ changes during epileptiform discharges in the immature rat neocortex. Dev. Brain Res. 1987, 36, 299–303. [Google Scholar] [CrossRef]
- Dreier, J.P.; Heinemann, U. Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp. Brain Res. 1991, 87, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Passmore, G.M. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 2009, 156, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Miceli, F.; Soldovieri, M.V.; Ambrosino, P.; Barrese, V.; Migliore, M.; Cilio, M.R.; Taglialatela, M. Genotype-phenotype correlations in neonatal epilepsies caused by mutations in the voltage sensor of K(v)7.2 potassium channel subunits. Proc. Natl. Acad. Sci. USA 2013, 110, 4386–4391. [Google Scholar] [CrossRef] [PubMed]
- Soldovieri, M.V.; Boutry-Kryza, N.; Milh, M.; Doummar, D.; Heron, B.; Bourel, E.; Auvin, S. Novel KCNQ2 and KCNQ3 mutations in a large, cohort of families with benign, neonatal epilepsy: First evidence for an altered channel regulation by syntaxin-1A. Hum. Mutat. 2014, 35, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Angamo, E.A.; Rösner, J.; Liotta, A.; Kovács, R.; Heinemann, U. A neuronal lactate uptake inhibitor slows recovery of extracellular ion concentration changes in the hippocampal CA3 region by affecting energy metabolism. J. Neurophysiol. 2016, 116, 2420–2430. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, C.W. Use of NAD(P)H and flavoprotein autofluorescence transients to probe neuron and astrocyte responses to synaptic activation. Neurochem. Int. 2010, 56, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Huchzermeyer, C.; Berndt, N.; Holzhütter, H.-G.; Kann, O. Oxygen consumption rates during three different neuronal activity states in the hippocampal CA3 network. J. Cereb. Blood Flow Metab. 2013, 33, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Berndt, N.; Kann, O.; Holzhütter, H.-G. Physiology-based kinetic modeling of neuronal energy metabolism unravels the molecular basis of NAD(P)H fluorescence transients. J. Cereb. Blood Flow Metab. 2015, 35, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E. Synchronization of area CA3 hippocampal pyramidal cells and non-granule cells of the dentate gyrus in bicuculline-treated rat hippocampal slices. Neuroscience 1994, 59, 245–257. [Google Scholar] [CrossRef]
- Wong, R.K.; Traub, R.D. Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2–CA3 region. J. Neurophysiol. 1983, 49, 442–458. [Google Scholar] [PubMed]
- Treven, M.; Koenig, X.; Assadpour, E.; Gantumur, E.; Meyer, C.; Hilber, K.; Kubista, H. The anticonvulsant retigabine is a subtype selective modulator of GABA A receptors. Epilepsia 2015, 56, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Johnson, B.N.; Tallent, M.K. K+ M-Current regulates the transition to seizures in immature and adult hippocampus. Epilepsia 2007, 48, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Masamoto, K.; Tanishita, K. Oxygen transport in brain tissue. J. Biomech. Eng. 2009, 131, 74002. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 2001, 17, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.A.; Beaver, C.J.; Turner, D.A. Interaction between tissue oxygen tension and NADH imaging during synaptic stimulation and hypoxia in rat hippocampal slices. Neuroscience 2005, 132, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Hájos, N.; Ellender, T.J.; Zemankovics, R.; Mann, E.O.; Exley, R.; Cragg, S.J.; Paulsen, O. Maintaining network activity in submerged hippocampal slices: Importance of oxygen supply. Eur. J. Neurosci. 2009, 29, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Galeffi, F.; Somjen, G.G.; Foster, K.A.; Turner, D.A. Simultaneous monitoring of tissue PO2 and NADH fluorescence during synaptic stimulation and spreading depression reveals a transient dissociation between oxygen utilization and mitochondrial redox state in rat hippocampal slices. J. Cereb. Blood Flow Metab. 2011, 31, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Rösner, J.; Liotta, A.; Angamo, E.A.; Spies, C.; Heinemann, U.; Kovács, R. Minimizing photodecomposition of flavin adenine dinucleotide fluorescence by the use of pulsed LEDs. J. Microsc. 2016, 264, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kann, O.; Huchzermeyer, C.; Kovács, R.; Wirtz, S.; Schuelke, M. Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria. Brain 2001, 134, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Liotta, A.; Rösner, J.; Huchzermeyer, C.; Wojtowicz, A.; Kann, O.; Schmitz, D.; Kovács, R. Energy demand of synaptic transmission at the hippocampal Schaffer-collateral synapse. J. Cereb. Blood Flow Metab. 2012, 32, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Berndt, N.; Bulik, S.; Holzhütter, H.-G. Kinetic modeling of the mitochondrial energy metabolism of neuronal cells: The impact of reduced α-ketoglutarate dehydrogenase activities on ATP production and generation of reactive oxygen species. Int. J. Cell Biol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.G.; Meldrum, B.S.; Siesiö, B.K. Cerebral metabolic changes during prolonged epileptic seizures in rats. J. Neurochem. 1977, 28, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Hinzman, J.M.; Andaluz, N.; Shutter, L.A.; Okonkwo, D.O.; Pahl, C.; Strong, A.J.; Hartings, J.A. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain 2014, 137, 2960–2972. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.K.L.; Chassidim, Y.; Lublinsky, S.; Revankar, G.S.; Major, S.; Kang, E.J.; Friedman, A. Impaired neurovascular coupling to ictal epileptic activity and spreading depolarization in a patient with subarachnoid hemorrhage: Possible link to blood-brain barrier dysfunction. Epilepsia 2012, 53, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, D.H. Epilepsy after head injury: An overview. Epilepsia 2009, 50, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.T. Early poststroke seizures: Is it time for prospective treatment trials? Neurology 2011, 77, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.W.; Kuzniecky, R.I. Utility of magnetic resonance spectroscopic imaging for human epilepsy. Quant. Imaging Med. Surg. 2015, 5, 313–322. [Google Scholar] [PubMed]
- Xu, M.Y.; Ergene, E.; Zagardo, M.; Tracy, P.T.; Wang, H.; Liu, W.; Machens, N.A. Proton MR spectroscopy in patients with structural MRI-negative temporal lobe epilepsy. J. Neuroimaging 2015, 25, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, U.; Arens, J. Production and Calibration of Ion-Sensitive Microelectrodes. In Practical Electrophysiological Methods; Kettenmann, H., Grantyn, R., Eds.; Wiley: New York, NY, USA, 1992; pp. 206–212. [Google Scholar]
- Rösner, J.; Liotta, A.; Schmitz, D.; Heinemann, U.; Kovács, R. A LED-based method for monitoring NAD(P)H and FAD fluorescence in cell cultures and brain slices. J. Neurosci. Methods 2013, 212, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Kasischke, K.A.; Lambert, E.M.; Panepento, B.; Sun, A.; Gelbard, H.A.; Burgess, R.W. Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions. J. Cereb. Blood Flow Metab. 2011, 31, 68–81. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoknecht, K.; Berndt, N.; Rösner, J.; Heinemann, U.; Dreier, J.P.; Kovács, R.; Friedman, A.; Liotta, A. Event-Associated Oxygen Consumption Rate Increases ca. Five-Fold When Interictal Activity Transforms into Seizure-Like Events In Vitro. Int. J. Mol. Sci. 2017, 18, 1925. https://doi.org/10.3390/ijms18091925
Schoknecht K, Berndt N, Rösner J, Heinemann U, Dreier JP, Kovács R, Friedman A, Liotta A. Event-Associated Oxygen Consumption Rate Increases ca. Five-Fold When Interictal Activity Transforms into Seizure-Like Events In Vitro. International Journal of Molecular Sciences. 2017; 18(9):1925. https://doi.org/10.3390/ijms18091925
Chicago/Turabian StyleSchoknecht, Karl, Nikolaus Berndt, Jörg Rösner, Uwe Heinemann, Jens P. Dreier, Richard Kovács, Alon Friedman, and Agustin Liotta. 2017. "Event-Associated Oxygen Consumption Rate Increases ca. Five-Fold When Interictal Activity Transforms into Seizure-Like Events In Vitro" International Journal of Molecular Sciences 18, no. 9: 1925. https://doi.org/10.3390/ijms18091925