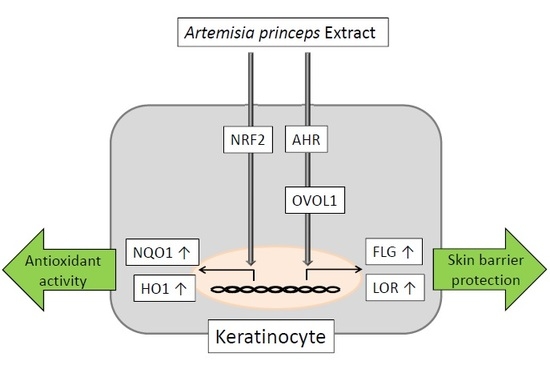
Antioxidant Artemisia princeps Extract Enhances the Expression of Filaggrin and Loricrin via the AHR/OVOL1 Pathway
Abstract
:1. Introduction
2. Results
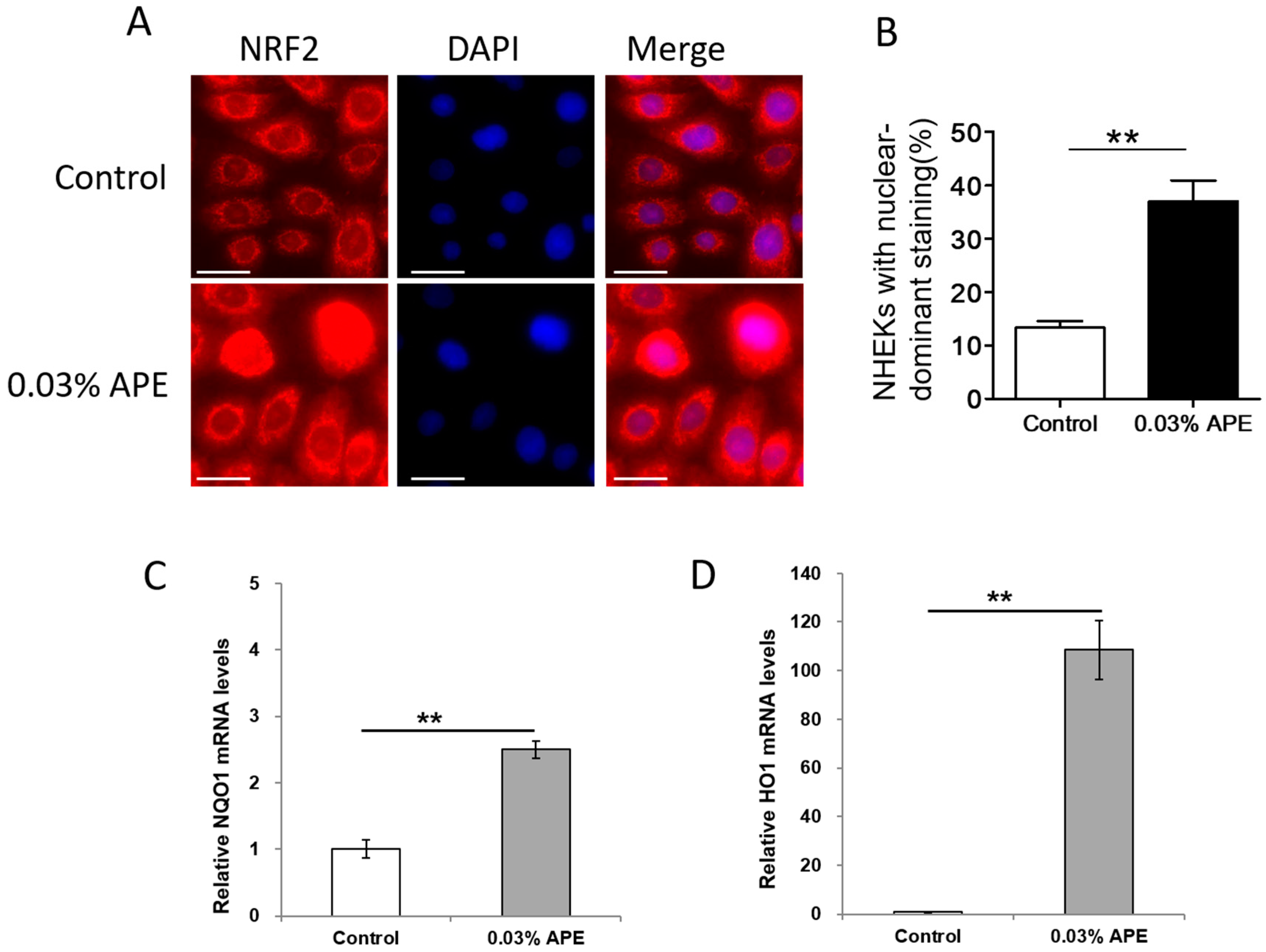
2.1. APE Upregulated Antioxidant NRF2/NQO1 Pathway in Normal Human Epidermal Keratinocytes
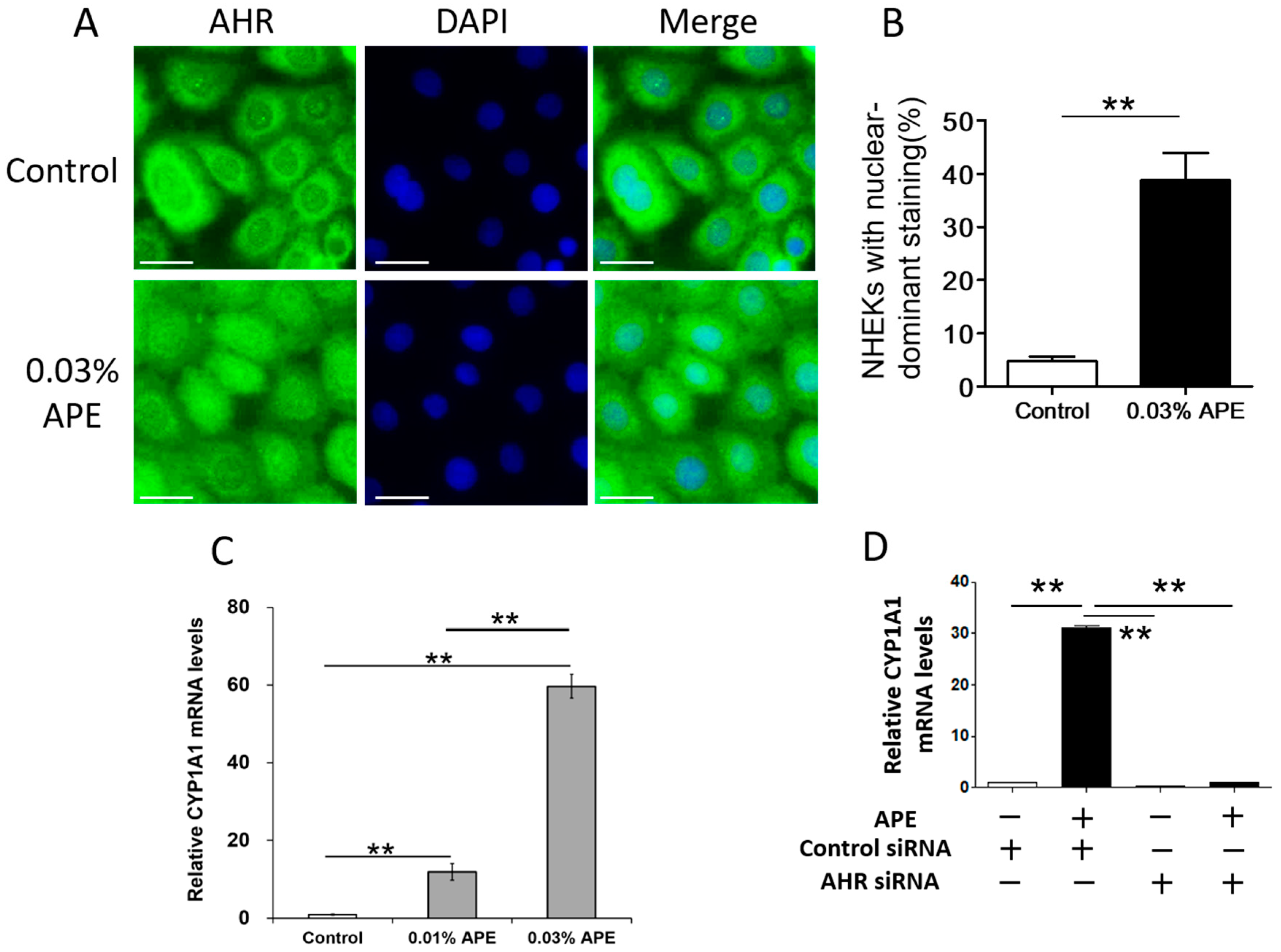
2.2. APE-Induced AHR Activation in NHEKs
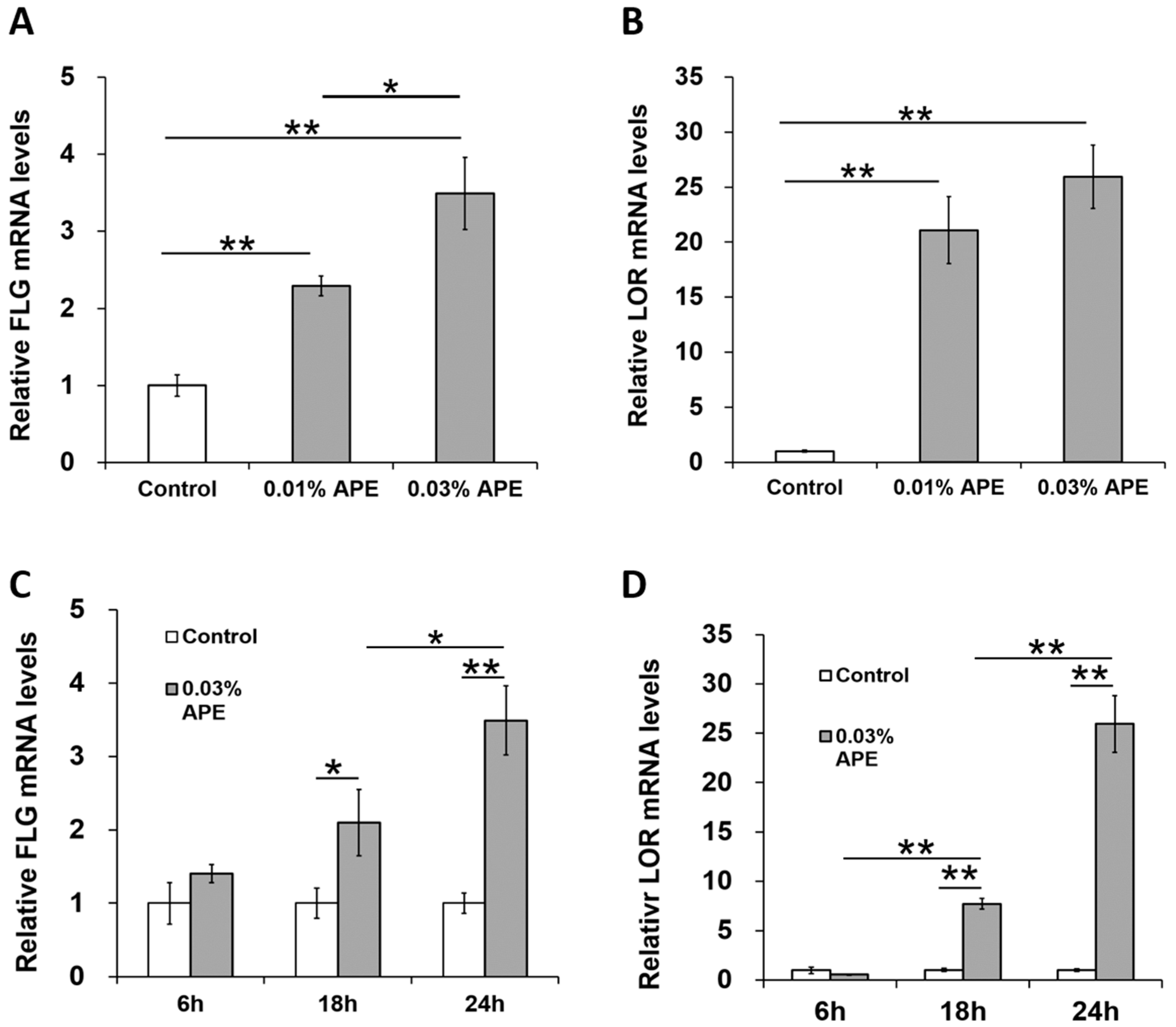
2.3. APE Induced FLG and LOR Gene Upregulation in an AHR/OVOL1-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Preparation of APE (Artemisia Princeps Extract)
4.2. Cell Culture
4.3. Cytotoxicity Evaluation
4.4. Real-Time PCR (Polymerase Chain Reaction)
4.5. Immunofluorescence Analysis
4.6. Transfection with siRNA against AHR (Aryl Hydrocarbon Receptor)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Chung, K.S.; Choi, H.E.; Shin, J.S.; Cho, E.J.; Cho, Y.W.; Choi, J.H.; Baek, N.I.; Lee, K.T. Chemopreventive effects of standardized ethanol extract from the aerial parts of Artemisia princeps Pampanini cv. Sajabal via NF-κB inactivation on colitis-associated colon tumorigenesis in mice. Food Chem. Toxicol. 2015, 275, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kanemoto, Y.; Ueda, M.; Kawasaki, K.; Fukuda, I.; Ashida, H. Anti-obesity and anti-diabetic effects of ethanol extract of Artemisia princeps in C57BL/6 mice fed a high-fat diet. Food Func. 2011, 2, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.H. Antioxidant and anticancer activity of Artemisia princeps var. orientalis extract in HepG2 and Hep3B hepatocellular carcinoma cells. Chin. J. Cancer Res. 2013, 25, 536–543. [Google Scholar] [PubMed]
- Bader, A.; Martini, F.; Schinella, G.R.; Rios, J.L.; Prieto, J.M. Modulation of Cox-1, 5-, 12- and 15-Lox by popular herbal remedies used in southern Italy against psoriasis and other skin diseases. Phytother. Res. 2015, 29, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nakata, K.; Monji, K.; Nayeshiro, H.; Makino, T. Studies on anti-inflammatory effects of Mugwort extracts. J. Soc. Cosmet. Chem. Jpn. 1994, 28, 178–182. [Google Scholar] [CrossRef]
- Sasaki, R.; Kishimoto, H.; Futatsuishi, Y.; Tada, M.; Yamamoto, K. Clinical evaluation of skin-care-products on atopic dermatitis. J. Pediatr. Dermatol. 1995, 14, 89–94. (In Japanese) [Google Scholar]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Wakefield, J. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Nagao, K.; Amagai, M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J. Clin. Investig. 2012, 122, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jekarl, D.W.; Kim, Y.; Kim, J.; Kim, M.; Park, Y.M. Novel FLG null mutations in Korean patients with atopic dermatitis and comparison of the mutational spectra in Asian populations. J. Dermatol. 2015, 42, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Fölster-Holst, R.; Baranowsky, A.; Schunck, M.; Winoto-Morbach, S.; Neumann, C.; Schütze, S.; Proksch, E. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J. Investig. Dermatol. 2004, 122, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogaard, E.H.; Bergboer, J.G.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.; Hato, S.V.; van der Valk, P.G.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013, 123, 917–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Uchi, H.; Takahara, H.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Antioxidant soybean tar Glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015, 42, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Bargen, I.; Weighardt, H.; Haarmann-Stemmann, T.; Krutmann, J. Functions of the aryl hydrocarbon receptor in the skin. Semin. Immunopathol. 2013, 35, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Takahara, M.; Nakahara, T.; Uchi, H. Role of AhR/ARNT system in skin homeostasis. Arch. Dermatol. Res. 2014, 306, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Tsuji, G.; Mitoma, C.; Nakahara, T.; Chiba, T.; Morino-Koga, S.; Uchi, H. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J. Dermatol. Sci. 2015, 80, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Hashimoto-Hachiya, A.; Kiyomatsu-Oda, M.; Takemura, M.; Ohno, F.; Ito, T.; Morino-Koga, S.; Mitoma, C.; Nakahara, T.; Uchi, H.; et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 2017, 8, e2931. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Chiba, T.; Ito, T.; Nakahara, T.; Tsuji, G. Antioxidants for healthy skin: The emerging role of aryl hydrocarbon receptors and nuclear factor-erythroid 2-related factor-2. Nutrients 2017, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S.; Yasukawa, F.; Moroi, Y.; Furue, M. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: The basis of its anti-inflammatory effect. J. Investig. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Uchi, H.; Yan, X.; Hachisuka, J.; Chiba, T.; Esaki, H.; et al. Antioxidant Opuntia ficus-indica extract activates AHR-NRF2 signaling and upregulates filaggrin and loricrin expression in human keratinocytes. J. Med. Food 2015, 18, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Uchi, H.; Yasumatsu, M.; Morino-Koga, S.; Mitoma, C.; Furue, M. Inhibition of aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J. Dermatol. Sci. 2017, 85, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Mitoma, C.; Nakahara, T.; Uchi, H.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, M.; Furue, M. Antioxidant Houttuynia cordata extract upregulates filaggrin expression in an aryl hydrocarbon-dependent manner. Fukuoka Igaku Zasshi 2014, 105, 205–213. [Google Scholar] [PubMed]
- Ikuta, T.; Namiki, T.; Fujii-Kuriyama, Y.; Kawajiri, K. AhR protein trafficking and function in the skin. Biochem. Pharmacol. 2009, 77, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Zaher, H.; Yang, T.J.; Gelboin, H.V.; Fernandez-Salguero, P.; Gonzalez, F.J. Effect of phenobarbital on hepatic CYP1A1 and CYP1A2 in the Ahr-null mouse. Biochem. Pharmacol. 1998, 55, 235–238. [Google Scholar] [CrossRef]
- Egawa, G.; Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 2016, 138, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Galactomyces fermentation filtrate prevents T helper 2-mediated reduction of filaggrin in an aryl hydrocarbon receptor-dependent manner. Clin. Exp. Dermatol. 2015, 40, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Sutter, C.H.; Bodreddigari, S.; Campion, C.; Wible, R.S.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol. Sci. 2011, 124, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.H.; Sutter, C.H.; Leon Carrion, S.; Tran, Q.T.; Bodreddigari, S.; Kensicki, E.; Mohney, R.P.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol. Sci. 2013, 132, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.; Weighardt, H.; Deenen, R.; Köhrer, K.; Clausen, B.; Zahner, S.; Boukamp, P.; Bloch, W.; Krutmann, J.; Esser, C. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J. Investig. Dermatol. 2016, 136, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Nagao, K.; Kubo, A.; Hata, T.; Shimizu, A.; Mizuno, H.; Yamada, T.; Amagai, M. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J. Allergy Clin. Immunol. 2012, 129, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Man, M.; Man, W.; Zhu, W.; Hupe, M.; Park, K.; Crumrine, D.; Elias, P.M.; Man, M.Q. Topical hesperidin improves epidermal permeability barrier function and epidermal differentiation in normal murine skin. Exp. Dermatol. 2012, 21, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Sun, R.; Hupe, M.; Kim, P.L.; Park, K.; Crumrine, D.; Lin, T.K.; Santiago, J.L.; Mauro, T.M.; Elias, P.M.; et al. Topical apigenin improves epidermal permeability barrier homoeostasis in normal murine skin by divergent mechanisms. Exp. Dermatol. 2013, 22, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Suárez-Fariñas, M.; Chiricozzi, A.; Nograles, K.E.; Shemer, A.; Fuentes-Duculan, J.; Cardinale, I.; Lin, P.; Bergman, R.; Bowcock, A.M.; et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J. Allergy Clin. Immunol. 2009, 124, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Frauenstein, K.; Sydlik, U.; Tigges, J.; Majora, M.; Wiek, C.; Hanenberg, H.; Abel, J.; Esser, C.; Fritsche, E.; Krutmann, J.; et al. Evidence for a novel anti-apoptotic pathway in human keratinocytes involving the aryl hydrocarbon receptor, E2F1, and checkpoint kinase 1. Cell Death Differ. 2013, 20, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Poulin, Y.; Zhou, Y.; Tan, J.; Hong, H.C.; Webster, J.; Ip, W.; Tang, L.; Lyle, M. Efficacy and safety of topical WBI-1001 in patients with mild to severe atopic dermatitis: Results from a 12-week, multicentre, randomized, placebo-controlled double-blind trial. Br. J. Dermatol. 2012, 166, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof Is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans. J. Investig. Dermatol. 2017. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ACTB | 5′-TTGTTACAGGAAGTCCCTTGCC-3′ | 5′-ATGCTATCACCTCCCCTGTGTG-3′ |
| CYP1A1 | 5′-TAGACACTGATCTGGCTGCAG-3′ | 5′-GGGAAGGCTCCATCAGCATC-3′ |
| NQO1 | 5′-GGATTGGACCGAGCTGGAA-3′ | 5′-AATTGCAGTGAAGATGAAGGCAAC-3′ |
| HO1 | 5′-AAGACTGCGTTCCTGCTCAAC-3′ | 5′-AAAGCCCTACAGCAACTGTCG-3′ |
| FLG | 5′-CATGGCAGCTATGGTAGTGCAGA-3′ | 5′-ACCAAACGCACTTGCTTTACAGA-3′ |
| LOR | 5′-GGCTGCATCTAGTTCTGCTGTTTA-3′ | 5′-CAAATTTATTGACTGAGGCACTGG-3′ |
| OVOL1 | 5′-ACGATGCCCATCCACTACCTG-3′ | 5′-TTTCTGAGGTGCTGGTCATCATTC-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, A.; Goto, M.; Mitsui, T.; Hashimoto-Hachiya, A.; Tsuji, G.; Furue, M. Antioxidant Artemisia princeps Extract Enhances the Expression of Filaggrin and Loricrin via the AHR/OVOL1 Pathway. Int. J. Mol. Sci. 2017, 18, 1948. https://doi.org/10.3390/ijms18091948
Hirano A, Goto M, Mitsui T, Hashimoto-Hachiya A, Tsuji G, Furue M. Antioxidant Artemisia princeps Extract Enhances the Expression of Filaggrin and Loricrin via the AHR/OVOL1 Pathway. International Journal of Molecular Sciences. 2017; 18(9):1948. https://doi.org/10.3390/ijms18091948
Chicago/Turabian StyleHirano, Akiko, Masashi Goto, Tsukasa Mitsui, Akiko Hashimoto-Hachiya, Gaku Tsuji, and Masutaka Furue. 2017. "Antioxidant Artemisia princeps Extract Enhances the Expression of Filaggrin and Loricrin via the AHR/OVOL1 Pathway" International Journal of Molecular Sciences 18, no. 9: 1948. https://doi.org/10.3390/ijms18091948