Genetic Diversity and Genetic Relationships of Purple Willow (Salix purpurea L.) from Natural Locations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Efficiency of the Used Marker Systems
2.2. Genetic Diversity and Analysis of Molecular Variance (AMOVA)
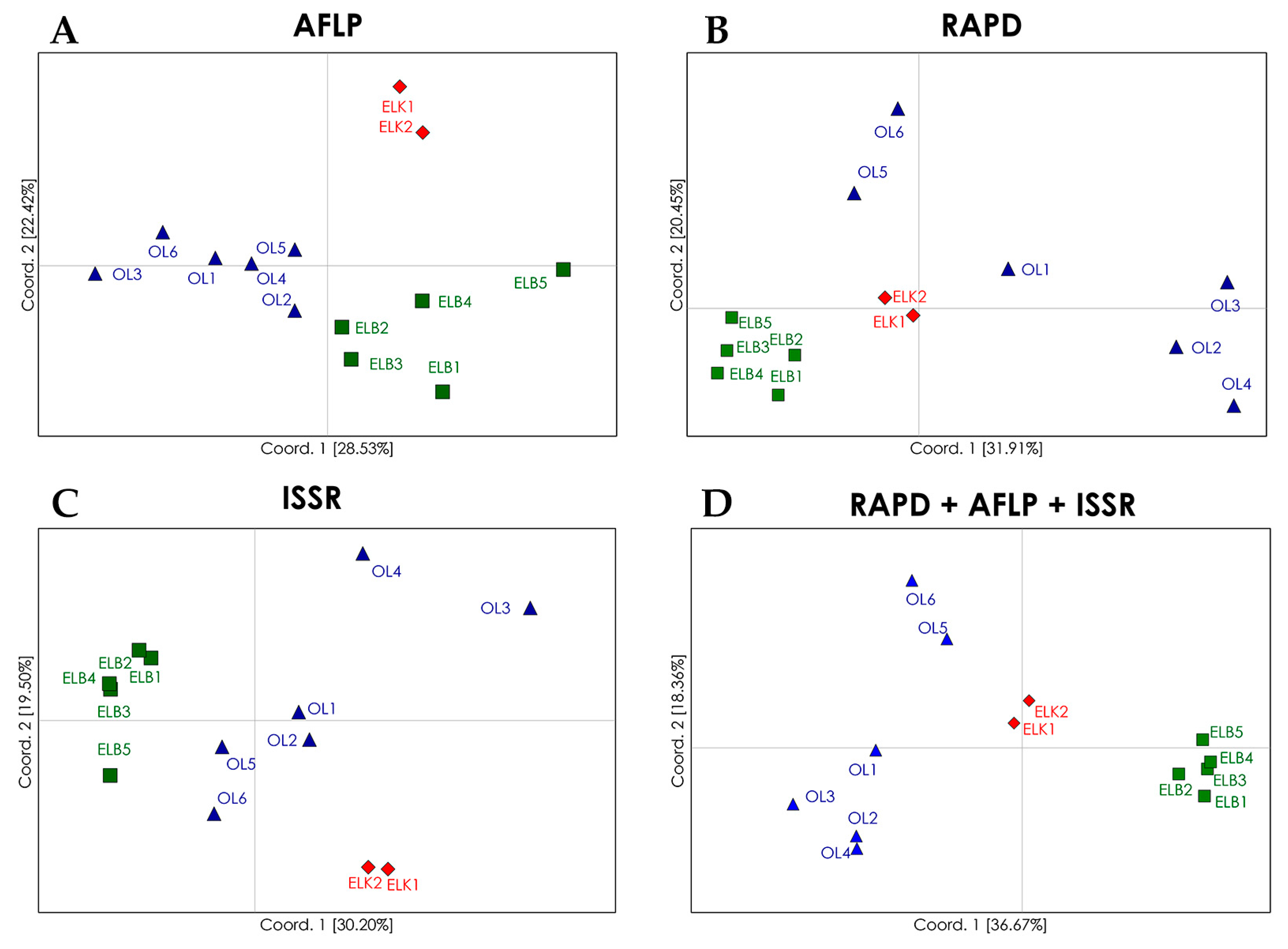
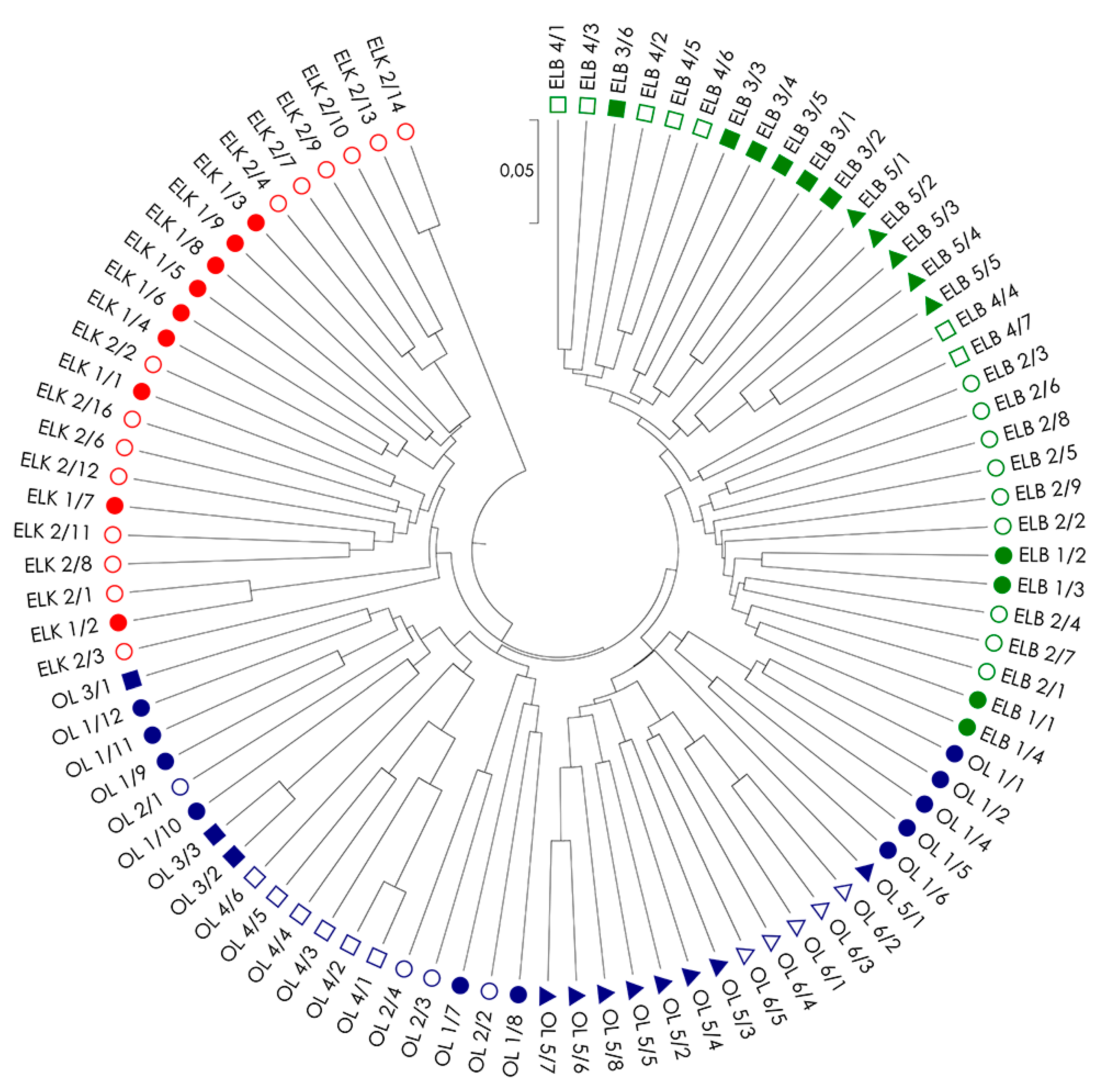
2.3. Genetic Relationships
3. Materials and Methods
3.1. Plant Material and DNA Isolation
3.2. AFLP Marker System
3.3. RAPD Marker System
3.4. ISSR Marker System
3.5. Data Collection and Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.; Cohen, M. Herbs and Natural Supplements, Volume 1: An Evidence-Based Guide, 4th ed.; Elsevier Churchill Livingstone: Sydney, Australia, 2015; pp. 1–1384. [Google Scholar]
- Chrubasik, S.; Eisenberg, E.; Balan, E.; Weinberger, T.; Luzzati, R.; Conradt, C. Treatment of low back pain exacerbations with willow bark extract: A randomized double-blind study. Am. J. Med. 2000, 109, 9–14. [Google Scholar] [CrossRef]
- Chrubasik, S.; Kunzel, O.; Model, A.; Conradt, C.; Black, A. Treatment of low back pain with a herbal or synthetic anti-rheumatic: A randomized controlled study. Willow bark extract for low back pain. Rheumatology 2001, 40, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals, 3rd ed.; CRC Press: London, UK, 2004. [Google Scholar]
- Cameron, M.; Gagnier, J.J.; Little, C.V.; Parsons, T.J.; Blümle, A.; Chrubasik, S. Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part 1: Osteoarthritis. Phytother. Res. 2009, 23, 1497–1515. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.; Gagnier, J.J.; Little, C.V.; Parsons, T.J.; Blümle, A.; Chrubasik, S. Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part 2: Rheumatoid arthritis. Phytother. Res. 2009, 23, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, J.G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J. Saudi Chem. Soc. 2010, 14, 317–322. [Google Scholar] [CrossRef]
- Shara, M.; Stohs, S.J. Efficacy and safety of white willow bark (Salix alba) extracts. Phytother. Res. 2015, 29, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Sulima, P.; Krauze-Baranowska, M.; Przyborowski, J.A. Variations in the chemical composition and content of salicylic glycosides in the bark of Salix purpurea from natural locations and their significance for breeding. Fitoterapia 2017, 118, 118–125. [Google Scholar] [CrossRef] [PubMed]
- EMEA. Final assessment report on Salix [various species including S. purpurea L., S. daphnoides Vill., S. fragilis L.], cortex; European Medicines Agency: London, UK, 2017; pp. 4–5. [Google Scholar]
- Förster, N.; Ulrichs, C.; Zander, M.; Kätzel, R. Factors influencing the variability of antioxidative phenolic glycosides in Salix species. J. Agric. Food. Chem. 2010, 58, 8205–8210. [Google Scholar] [CrossRef] [PubMed]
- Hörandl, E.; Florineth, F.; Hadacek, F. Weiden in Österreich und Angrenzenden Gebieten; Arbeitsbereich Ingenieurbiologie u. Landschaftsbau, Univ. Bodenkultur Wien: Wien, Austria, 2002; pp. 7–150. [Google Scholar]
- Argus, G.W. Salix (Salicaceae) distribution maps and a synopsis of their classification in North America, north of Mexico. Harv. Pap. Bot. 2007, 12, 335–368. [Google Scholar] [CrossRef]
- Sulima, P.; Przyborowski, J.A.; Wiwart, M. Willow bark—Herbal raw material harvested from plants cultivated on arable lands. Herba Pol. 2006, 52, 4. [Google Scholar]
- Kenstaviciene, P.; Nenortiene, P.; Kiliuviene, G.; Zevzikovas, A.; Lukosius, A.; Kazlauskiene, D. Application of high-performance liquid chromatography for research of salicin in bark of different varieties of Salix. Medicina 2009, 45, 644–651. [Google Scholar] [PubMed]
- Meneghetti, S.; Barcaccia, G.; Paiero, P.; Lucchin, M. Genetic characterization of Salix alba L. and Salix fragilis L. by means of different PCR-derived marker systems. Plant Biosyst. 2007, 141, 283–291. [Google Scholar] [CrossRef]
- Chen, J.H.; Sun, H.; Wen, J.; Yang, Y.P. Molecular phylogeny of Salix L. (Salicaceae) inferred from three chloroplast datasets and its systematic implications. Taxon 2010, 59, 29–37. [Google Scholar]
- Przyborowski, J.A.; Sulima, P.; Kuszewska, A.; Załuski, D.; Kilian, A. Phylogenetic relationships between four Salix L. species based on DArT markers. Int. J. Mol. Sci. 2013, 14, 24113–24125. [Google Scholar] [CrossRef] [PubMed]
- Sulima, P.; Przyborowski, J.A.; Załuski, D. RAPD markers reveal genetic diversity in Salix purpurea L. Crop. Sci. 2009, 49, 857–863. [Google Scholar] [CrossRef]
- Aravanopoulos, F. Clonal identification based on quantitative, co-dominant, and dominant marker data: A comparative analysis of selected willow (Salix L.) clones. Int. J. For. Res. 2010. [Google Scholar] [CrossRef]
- Ngantcha, A.C. DNA Fingerprinting and Genetic Relationships among Willow (Salix spp.). Master’s Thesis, University of Saskatchewan, Saskatoon, Canada, 2010. [Google Scholar]
- Sulima, P.; Przyborowski, J.A. Genetic diversity of Salix purpurea L. genotypes and interspecific hybrids. Acta Biol. Crac. Ser. Bot. 2013, 55, 29–36. [Google Scholar] [CrossRef]
- The Genome Sequence of Salix purpurea Female Clone 94006. Salix purpurea v1.0, DOE-JGI. Available online: http://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Spurpurea (accessed on 9 December 2017).
- Martin, T.; Rönnberg-Wästljung, A.C.; Stenlid, J.; Samils, B. Identification of a differentially expressed TIR-NBS-LRR gene in a major QTL associated to leaf rust resistance in Salix. PLoS ONE 2016, 11, e0168776. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, X.; Bi, C.; Xu, Y.; Wu, D.; Ye, N. Assembly and analysis of the complete Salix purpurea L. (Salicaceae) mitochondrial genome sequence. SpringerPlus 2016, 5, 1894. [Google Scholar] [CrossRef] [PubMed]
- Bracci, T.; Busconi, M.; Fogher, C.; Sebastiani, L. Molecular studies in olive (Olea europaea L.): Overview on DNA markers applications and recent advances in genome analysis. Plant Cell Rep. 2011, 30, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.B.; Cetin, O.; Kaya, H.; Sahin, M.; Sefer, F.; Kahraman, A.; Tanyolac, B. SNP discovery by Illumina-based transcriptome sequencing of the olive and the genetic characterization of Turkish olive genotypes revealed by AFLP, SSR and SNP markers. PLoS ONE 2013, 8, e73674. [Google Scholar] [CrossRef] [PubMed]
- Frascaroli, E.; Schrag, T.A.; Melchinger, A.E. Genetic diversity analysis of elite European maize (Zea mays L.) inbred lines using AFLP, SSR, and SNP markers reveals ascertainment bias for a subset of SNPs. Theor. Appl. Genet. 2013, 126, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Skvortsov, A.K. Willows of Russia and Adjacent Countries. Taxonomical and Geographical Revision; University of Joensuu: Joensuu, Finland, 1999; pp. 10–254. [Google Scholar]
- Dolatowski, J.; Seneta, W. Dendrologia, 3rd ed.; PWN: Warszawa, Poland, 2017; ISBN 978-8-30-115369-4. [Google Scholar]
- USDA, NRCS. The PLANTS Database. National Plant Data Team, Greensboro, NC, USA. Available online: https://plants.usda.gov/core/profile?symbol=SAPU2 (accessed on 9 December 2017).
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Friters, A.; Pot, J.; Paleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.G.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990, 18, 6531–6535. [Google Scholar] [CrossRef] [PubMed]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Penner, G.A.; Bush, A.; Wise, R.; Kim, W.; Domier, L.; Kasha, K.; Laroche, A.; Scoles, G.; Molnar, S.J.; Fedak, G. Reproducibility of random amplified polymorphic DNA (RAPD) analysis among laboratories. Methods Appl. 1993, 2, 341–345. [Google Scholar] [CrossRef]
- Powell, W.; Morgante, M.; Andre, C.M.; Hanafey, M.; Vogel, J.; Tingey, S.; Rafalski, A. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol. Breed. 1996, 2, 225–238. [Google Scholar] [CrossRef]
- Saliba-Colombani, V.; Causse, M.; Gervais, L.; Philouze, J. Efficiency of RFLP, RAPD, and AFLP markers for the construction of an intraspecific map of the tomato genome. Genome 2000, 43, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Pereira, G.; Garrido, I.; Tavares-de-Sousa, M.M.; Espinosa, F. Comparison of RAPD, ISSR, and AFLP molecular markers to reveal and classify orchardgrass (Dactylis glomerata L.) germplasm variations. PLoS ONE 2016, 11, e0152972. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.H.A.; Matthes, M.; Arnold, G.M.; Edwards, K.J.; Ahman, I.; Larsson, S.; Karp, A. Characterization of genetic diversity in potential biomass willows (Salix spp.) by RAPD and AFLP analyses. Genome 1999, 42, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Van Puyvelde, K.; Triest, L. ISSRs indicate isolation by distance and spatial structuring in Salix alba populations along Alpine upstream rivers (Alto Adige and Upper Rhine). Belg. J. Bot. 2007, 140, 100–108. [Google Scholar]
- Trybush, S.O.; Jahodová, Š.; Macalpine, W.; Karp, A. A genetic study of a Salix germplasm resource reveals new insights into relationships among sub-genera, sections and species. Bioenergy Res. 2008, 1, 67–79. [Google Scholar] [CrossRef]
- Lin, J.; Gibbs, J.P.; Smart, L.B. Population genetic structure of native versus naturalized sympatric shrub willows (Salix; Salicaceae). Am. J. Bot. 2009, 96, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Przyborowski, J.A.; Sulima, P. The analysis of genetic diversity of Salix viminalis genotypes as a potential source of biomass by RAPD markers. Ind. Crop. Prod. 2010, 31, 395–400. [Google Scholar] [CrossRef]
- Trybush, S.O.; Jahodová, Š.; Čížková, L.; Karp, A.; Hanley, S.J. High levels of genetic diversity in Salix viminalis of the Czech Republic as revealed by microsatellite markers. Bioenergy Res. 2012, 5, 969–977. [Google Scholar] [CrossRef]
- Berlin, S.; Ghelardini, L.; Bonosi, L.; Weih, M.; Rönnberg-Wästljung, A.C. QTL mapping of biomass and nitrogen economy traits in willows (Salix spp.) grown under contrasting water and nutrient conditions. Mol. Breed. 2014, 34, 1987–2003. [Google Scholar] [CrossRef]
- Lauron-Moreau, A.; Pitre, F.E.; Argus, G.W.; Labrecque, M.; Brouillet, L. Phylogenetic relationships of american willows (Salix L., Salicaceae). PLoS ONE 2015, 10, e0121965. [Google Scholar]
- Zhai, F.; Mao, J.; Liu, J.; Peng, X.; Han, L.; Sun, Z. Male and female subpopulations of Salix viminalis present high genetic diversity and high long-term migration rates between them. Front. Plant Sci. 2016, 7, 330. [Google Scholar] [CrossRef] [PubMed]
- Alsos, I.G.; Alm, T.; Normand, S.; Brochmann, C. Past and future range shifts and loss of diversity in dwarf willow (Salix herbacea L.) inferred from genetics, fossils and modelling. Glob. Ecol. Biogeogr. 2009, 18, 223–239. [Google Scholar] [CrossRef]
- Berlin, S.; Trybush, S.O.; Fogelqvist, J.; Gyllenstrand, N.; Hallingbäck, H.R.; Åhman, I.; Nordh, N.E.; Shield, I.; Powers, S.J.; Weih, M.; et al. Genetic diversity, population structure and phenotypic variation in European Salix viminalis L. (Salicaceae). Tree Genet. Genomes 2014, 10, 1595–1610. [Google Scholar] [CrossRef]
- Nybom, H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol. Ecol. 2004, 13, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, J.L.; Godt, M.J.W.; Sherman-Broyles, S.L. Factors influencing levels of genetic diversity in woody plant species. New For. 1992, 6, 95–124. [Google Scholar] [CrossRef]
- Sulima, P.; Przyborowski, J.A. Natural Occurrence of Purple Willow (Salix purpurea). Available online: https://www.google.com/maps/d/u/0/edit?mid=1lFNsWPj63seNFPMTADXIsZP41Yw (accessed on 9 December 2017).
- Neumann, A. Die Mitteleuropäischen Salix-Arten; Mitt Forstlichen Bundes-Versuchsanstalt: Wien, Austria, 1981; pp. 9–144. [Google Scholar]
- Milligan, B.G. Total DNA isolation. In Molecular Genetic Analysis of Populations: A Practical Approach; Hoelzel, A.R., Ed.; Oxford University Press: Oxford, UK, 1998; pp. 29–64. [Google Scholar]
- Hantula, J.; Dusabenygasani, M.; Hamelin, R.C. Random Amplified Microsatellites (RAMS)—A novel method for characterizing genetic variation within fungi. For. Pathol. 1996, 26, 159–166. [Google Scholar] [CrossRef]
- McGregor, C.E.; Lambert, C.A.; Greyling, M.N.; Louw, J.H.; Warnich, L. A comparative assessment of DNA fingerprinting techniques (RAPD, ISSR, AFLP and SSR) in tetraploid potato (Solanum tuberosum L.) germplasm. Euphytica 2000, 113, 135–144. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Vekemans, X. AFLPsurv V.1.0. A Software for Genetic Diversity Analysis with AFLP Population Data; Université Libre de Bruxelles: Brussel, Belgium, 2001; pp. 1–15. [Google Scholar]
- Yeh, F.C.; Yang, R.C.; Boyle, T.B.J.; Ye, Z.H.; Mao, J.X. POPGENE, the User-Friendly Shareware for Population Genetic Analysis; University of Alberta: Edmonton, Canada. Available online: https://sites.ualberta.ca/~fyeh/popgene_download.html (accessed on 9 December 2017).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.L.; Clark, A.G. Principles of Population Genetics, 4th ed.; Sinauer: Sunderland, MA, USA, 2007. [Google Scholar]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; pp. 3–125. [Google Scholar]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [PubMed]
- Gower, J.C. Principal Coordinates Analysis. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Chichester, UK, 2005. [Google Scholar]
- Nei, M. Genetic distance between populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification; W.H. Freeman: San Francisco, CA, USA, 1973. [Google Scholar]
| Parameter | AFLP | RAPD | ISSR | All Products |
|---|---|---|---|---|
| Total number of products (L) | 159 | 574 | 221 | 954 |
| Percent of polymorphic loci (%p) | 74.8 | 89.9 | 87.3 | 86.8 |
| Scored products ratio (SPR) | 79.5 | 9.3 | 11.1 | 11.4 |
| Shannon diversity index (I) | 0.187 | 0.264 | 0.258 | 0.247 |
| Unbiased expected heterozygosity (uHe) | 0.134 | 0.192 | 0.187 | 0.179 |
| Minimum uHe within a location (MinuHe) | 0.096 | 0.114 | 0.091 | 0.110 |
| Maximum uHe within a location (MaxuHe) | 0.170 | 0.232 | 0.220 | 0.218 |
| Genetic differentiation between locations (ΦST) | 0.150 | 0.159 | 0.225 | 0.212 |
| Location | Parameter | AFLP | RAPD | ISSR | All Marker Systems |
|---|---|---|---|---|---|
| ELK1 | pP | 125 | 481 | 186 | 792 |
| pM | 0 | 2 | 1 | 3 | |
| ELK2 | pP | 138 | 517 | 199 | 854 |
| pM | 2 | 7 | 0 | 9 | |
| ELB1 | pP | 108 | 390 | 154 | 652 |
| pM | 1 | 0 | 2 | 3 | |
| ELB2 | pP | 124 | 469 | 186 | 779 |
| pM | 0 | 2 | 1 | 3 | |
| ELB3 | pP | 109 | 444 | 171 | 724 |
| pM | 1 | 0 | 1 | 2 | |
| ELB4 | pP | 116 | 449 | 171 | 736 |
| pM | 0 | 3 | 0 | 3 | |
| ELB5 | pP | 102 | 404 | 158 | 664 |
| pM | 0 | 1 | 1 | 2 | |
| OL1 | pP | 119 | 492 | 185 | 796 |
| pM | 4 | 1 | 1 | 6 | |
| OL2 | pP | 98 | 404 | 158 | 660 |
| pM | 0 | 1 | 2 | 3 | |
| OL3 | pP | 95 | 342 | 125 | 562 |
| pM | 1 | 1 | 1 | 3 | |
| OL4 | pP | 108 | 382 | 142 | 632 |
| pM | 0 | 1 | 1 | 2 | |
| OL5 | pP | 116 | 456 | 168 | 740 |
| pM | 2 | 2 | 0 | 4 | |
| OL6 | pP | 107 | 395 | 159 | 661 |
| pM | 1 | 1 | 2 | 4 | |
| Average | pP | 112.7 | 432.7 | 166.3 | 711.7 |
| pM | 0.9 | 1.7 | 1.0 | 3.6 | |
| %p | 70.9 | 75.4 | 75.3 | 74.6 | |
| %pM | 7.5 | 3.8 | 5.9 | 4.9 |
| Location | N | Na | Ne | I | uHe | %p |
|---|---|---|---|---|---|---|
| ELK1 | 9 | 1.421 | 1.325 | 0.295 | 0.205 | 59.78 |
| ELK2 | 14 | 1.582 | 1.348 | 0.322 | 0.218 | 69.16 |
| ELB1 | 4 | 1.057 | 1.250 | 0.212 | 0.164 | 38.04 |
| ELB2 | 9 | 1.382 | 1.323 | 0.288 | 0.202 | 57.61 |
| ELB3 | 6 | 1.220 | 1.286 | 0.248 | 0.181 | 46.88 |
| ELB4 | 7 | 1.285 | 1.300 | 0.266 | 0.190 | 51.77 |
| ELB5 | 5 | 1.082 | 1.261 | 0.220 | 0.165 | 39.40 |
| OL1 | 11 | 1.454 | 1.339 | 0.306 | 0.211 | 62.36 |
| OL2 | 4 | 1.090 | 1.270 | 0.228 | 0.178 | 40.76 |
| OL3 | 3 | 0.829 | 1.157 | 0.134 | 0.110 | 23.51 |
| OL4 | 6 | 1.056 | 1.249 | 0.212 | 0.157 | 38.99 |
| OL5 | 8 | 1.317 | 1.306 | 0.273 | 0.193 | 53.94 |
| OL6 | 5 | 1.067 | 1.245 | 0.209 | 0.156 | 38.45 |
| Average | 7.0 | 1.218 | 1.282 | 0.247 | 0.179 | 47.74 |
| Location | ELK1 | ELK2 | ELB1 | ELB2 | ELB3 | ELB4 | ELB5 | OL1 | OL2 | OL3 | OL4 | OL5 | OL6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELK1 | 0.000 | ||||||||||||
| ELK2 | 0.028 | 0.000 | |||||||||||
| ELB1 | 0.194 | 0.173 | 0.000 | ||||||||||
| ELB2 | 0.166 | 0.146 | 0.004 | 0.000 | |||||||||
| ELB3 | 0.188 | 0.167 | 0.081 | 0.068 | 0.000 | ||||||||
| ELB4 | 0.187 | 0.154 | 0.106 | 0.082 | 0.045 | 0.000 | |||||||
| ELB5 | 0.222 | 0.188 | 0.166 | 0.131 | 0.132 | 0.131 | 0.000 | ||||||
| OL1 | 0.160 | 0.149 | 0.190 | 0.172 | 0.184 | 0.191 | 0.219 | 0.000 | |||||
| OL2 | 0.221 | 0.233 | 0.244 | 0.235 | 0.265 | 0.291 | 0.312 | 0.098 | 0.000 | ||||
| OL3 | 0.244 | 0.224 | 0.322 | 0.257 | 0.319 | 0.318 | 0.369 | 0.101 | 0.222 | 0.000 | |||
| OL4 | 0.241 | 0.238 | 0.287 | 0.260 | 0.269 | 0.280 | 0.317 | 0.158 | 0.190 | 0.222 | 0.000 | ||
| OL5 | 0.181 | 0.157 | 0.216 | 0.172 | 0.209 | 0.202 | 0.229 | 0.098 | 0.234 | 0.223 | 0.242 | 0.000 | |
| OL6 | 0.249 | 0.201 | 0.294 | 0.239 | 0.257 | 0.280 | 0.300 | 0.143 | 0.271 | 0.296 | 0.292 | 0.119 | 0.000 |
| Source of Variation | Df | Sum of Squares | Mean Square | Estimated Variation | Total Variance |
|---|---|---|---|---|---|
| Between regions | 2 | 1314.12 | 657.06 | 14.17 | 10% |
| Between locations | 10 | 2005.46 | 200.55 | 14.46 | 11% |
| Within locations | 78 | 8319.15 | 106.66 | 106.66 | 79% |
| Total | 90 | 11,638.74 | – | 135.28 | 100% |
| Location | Latitude/Longitude | Geographic Region | Number of Genotypes |
|---|---|---|---|
| ELK1 | 53°48′59.94″/22°23′12.06″ | Ełk Lakeland—ELK | 9 |
| ELK2 | 53°50′23.52″/22°22′41.62″ | Ełk Lakeland—ELK | 14 |
| ELB1 | 54°8′37.35″/19°23′4.98″ | Delta of the Vistula River—ELB | 4 |
| ELB2 | 54°7′34.18″/19°18′28.95″ | Delta of the Vistula River—ELB | 9 |
| ELB3 | 54°11′17.02″/19°12′29.94″ | Delta of the Vistula River—ELB | 6 |
| ELB4 | 54°15′30.93″/19°14′18.96″ | Delta of the Vistula River—ELB | 7 |
| ELB5 | 54°9′14.92″/19°1′10.77″ | Delta of the Vistula River—ELB | 5 |
| OL1 | 53°45′30.02″/20°29′12.62″ | Olsztyn Lakeland—OL | 11 |
| OL2 | 53°46′18.72″/20°26′36.12″ | Olsztyn Lakeland—OL | 4 |
| OL3 | 53°46′43.26″/20°30′42.54″ | Olsztyn Lakeland—OL | 3 |
| OL4 | 53°43′3.06″/20°28′18.72″ | Olsztyn Lakeland—OL | 6 |
| OL5 | 53°52′31.56″/20°21′25.14″ | Olsztyn Lakeland—OL | 8 |
| OL6 | 53°51′54.90″/20°22′45.30″ | Olsztyn Lakeland—OL | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulima, P.; Prinz, K.; Przyborowski, J.A. Genetic Diversity and Genetic Relationships of Purple Willow (Salix purpurea L.) from Natural Locations. Int. J. Mol. Sci. 2018, 19, 105. https://doi.org/10.3390/ijms19010105
Sulima P, Prinz K, Przyborowski JA. Genetic Diversity and Genetic Relationships of Purple Willow (Salix purpurea L.) from Natural Locations. International Journal of Molecular Sciences. 2018; 19(1):105. https://doi.org/10.3390/ijms19010105
Chicago/Turabian StyleSulima, Paweł, Kathleen Prinz, and Jerzy A. Przyborowski. 2018. "Genetic Diversity and Genetic Relationships of Purple Willow (Salix purpurea L.) from Natural Locations" International Journal of Molecular Sciences 19, no. 1: 105. https://doi.org/10.3390/ijms19010105






