Theaflavin-3,3′-Digallate Enhances the Inhibitory Effect of Cisplatin by Regulating the Copper Transporter 1 and Glutathione in Human Ovarian Cancer Cells
Abstract
:1. Introduction
2. Results
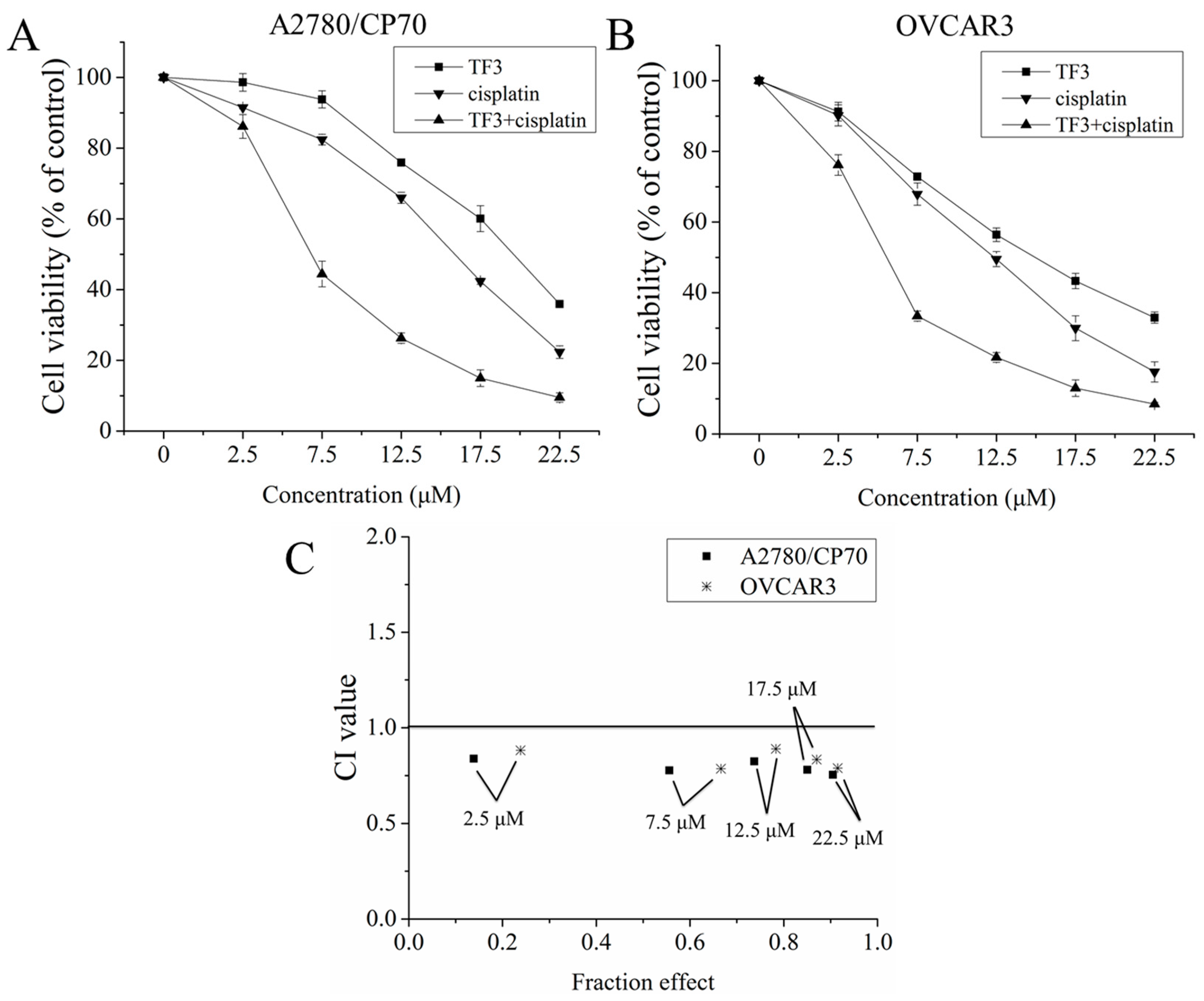
2.1. The Synergistic Inhibitory Effect of TF3 and Cisplatin Against Ovarian Cancer Cells
2.2. TF3 Increases Intracellular Accumulation of Total Pt and DNA-Pt Adducts
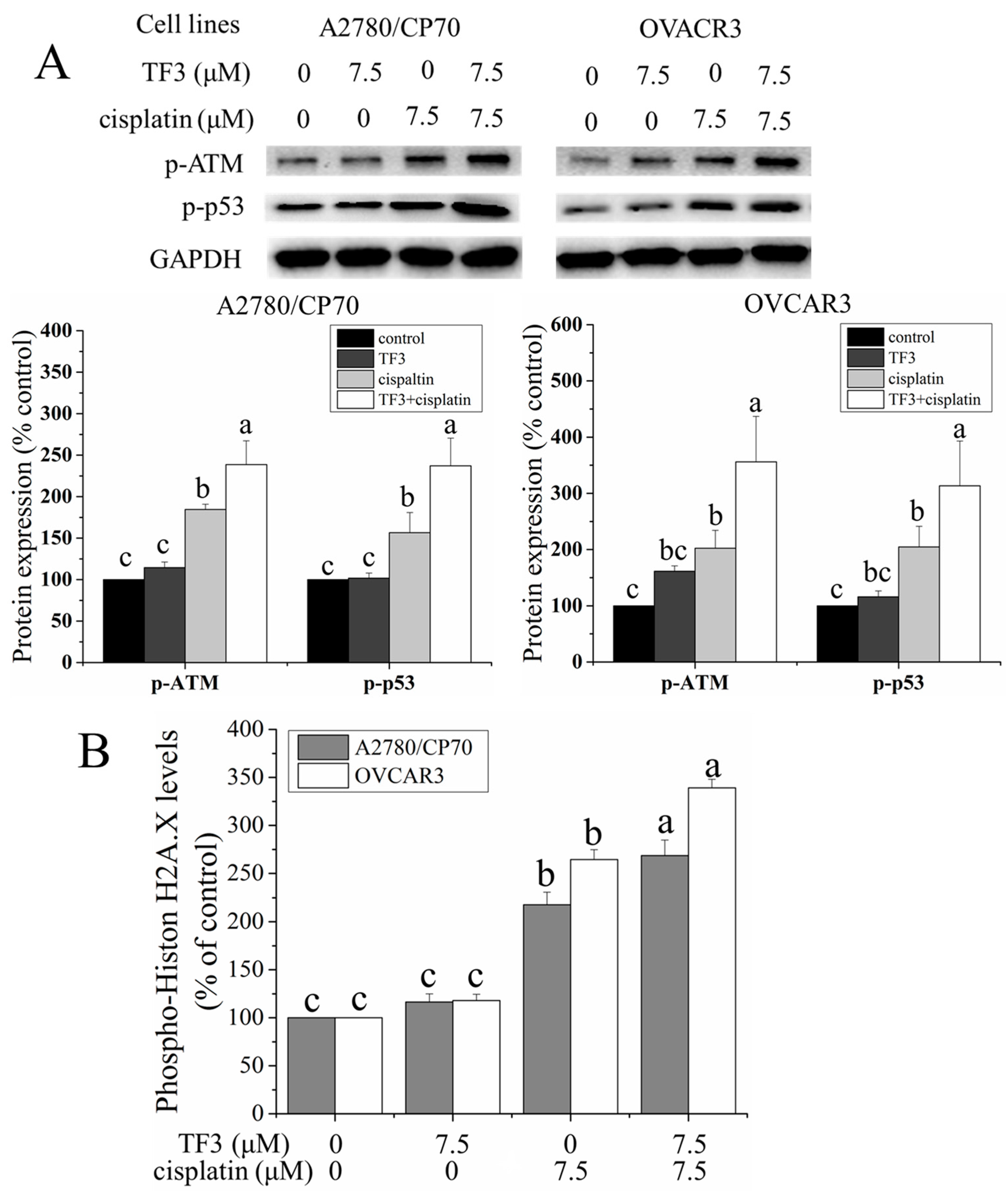
2.3. TF3 Enhanced DNA Damage Induced by Cisplatin in Ovarian Cancer Cells
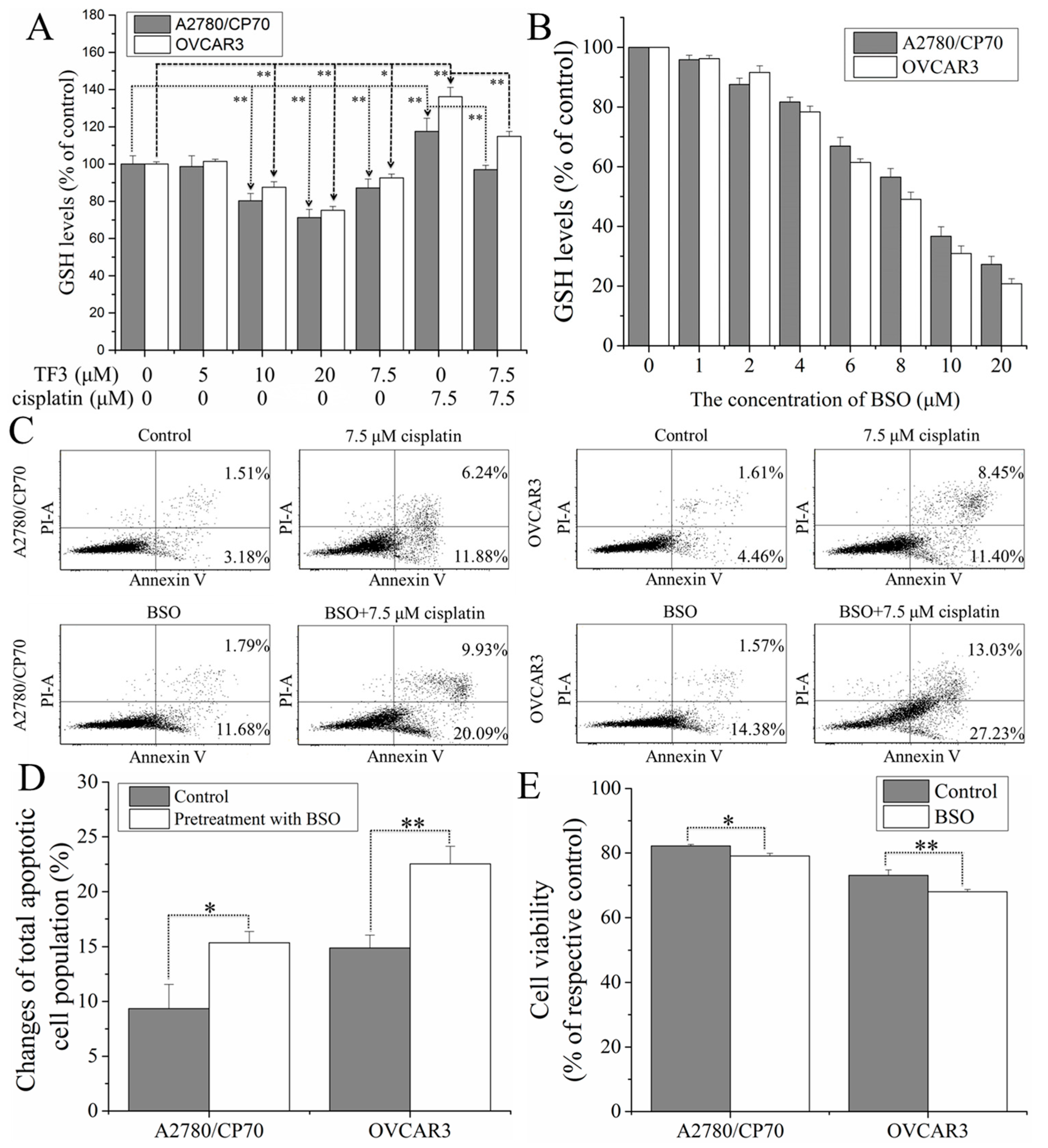
2.4. TF3 Synergistically Inhibited Ovarian Cancer Cells with Cisplatin by Reducing glutathione (GSH) Levels in the Cells
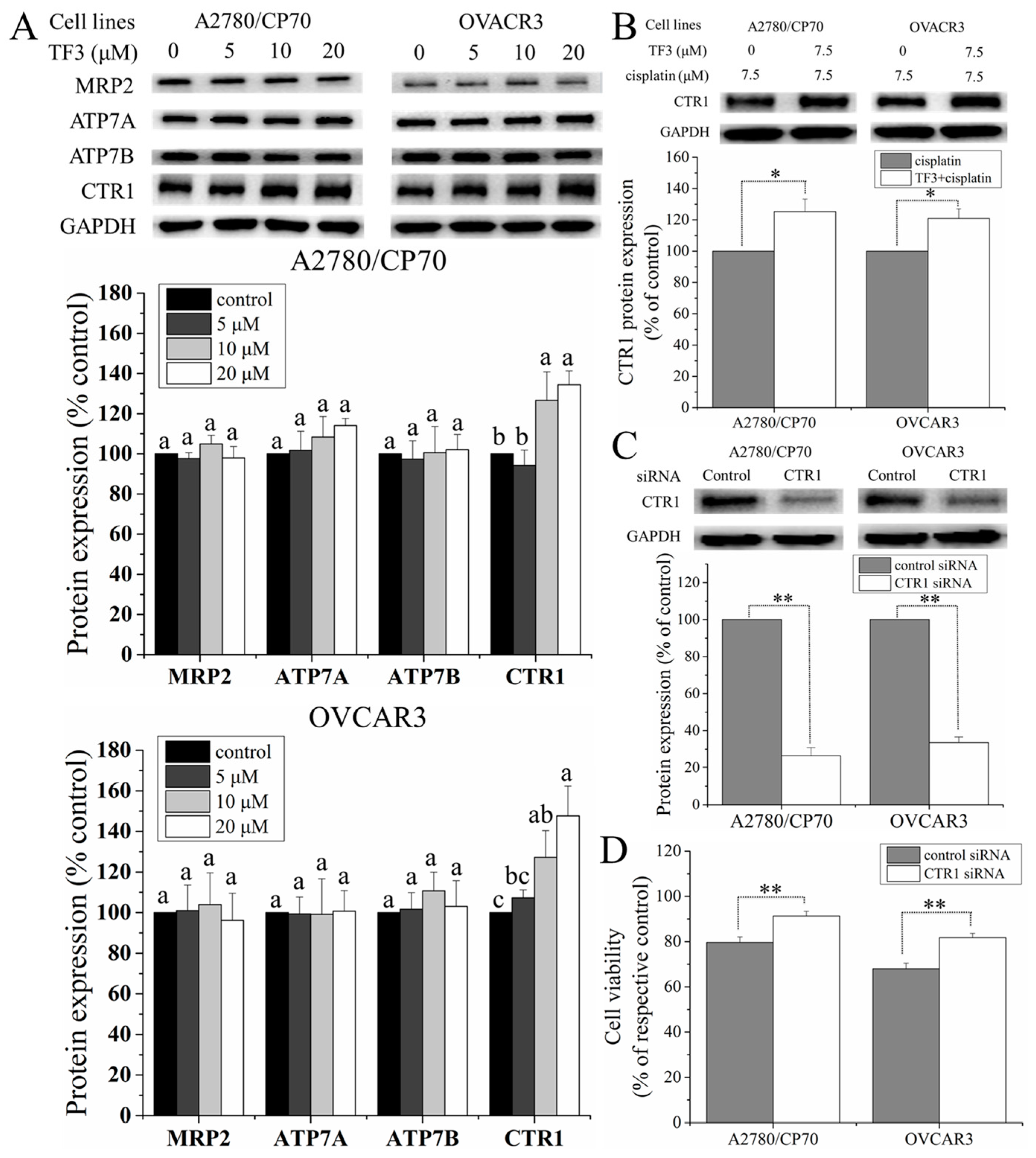
2.5. TF3 Upregulated the Protein Expression of CTR1 in Ovarian Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Viability Assay
4.3. Measurement of Intracellular Pt Accumulation
4.4. GSH Assay
4.5. Flow Cytometric Analysis of Apoptotic Cells
4.6. Cell-Based Phosphorylation ELISA Assay
4.7. Western Blotting
4.8. Transfection with Small Interfering RNA (siRNA)
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Chen, H.; Wei, D.M.; Fang, F.; Liu, G.J.; Xie, H.Y.; Wang, X.; Zou, J.; Han, X.; Feng, D. Maintenance chemotherapy for ovarian cancer. In The Cochrane Library; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Pentheroudakis, G.; Pavlidis, N. Evidence-based Oncology. Ann. Oncol. 2004, 15, 361. [Google Scholar] [CrossRef]
- Al Rawahi, T.; Lopes, A.; Bristow, R.; Bryant, A.; Elattar, A.; Chattopadhyay, S.; Galaal, K. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst. Rev. 2012, 2. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, recognition, and processing of cisplatin—DNA adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Holzer, A.K.; Samimi, G.; Katano, K.; Naerdemann, W.; Lin, X.; Safaei, R.; Howell, S.B. The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol. Pharmacol. 2004, 66, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; McCormick, F.; Smith-McCune, K.; Hanahan, D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 2010, 17, 574–583. [Google Scholar] [CrossRef] [PubMed]
- More, S.S.; Akil, O.; Ianculescu, A.G.; Geier, E.G.; Lustig, L.R.; Giacomini, K.M. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. 2010, 30, 9500–9509. [Google Scholar] [CrossRef] [PubMed]
- Liedert, B.; Materna, V.; Schadendorf, D.; Thomale, J.; Lage, H. Overexpression of cMOAT (MRP2/ABCC2) is associated with decreased formation of platinum-DNA adducts and decreased G 2-arrest in melanoma cells resistant to cisplatin. J. Investig. Dermatol. 2003, 121, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Samimi, G.; Safaei, R.; Katano, K.; Holzer, A.K.; Rochdi, M.; Tomioka, M.; Goodman, M.; Howell, S.B. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin. Cancer Res. 2004, 10, 4661–4669. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Kanzaki, A.; Ogawa, K.; Miyazaki, K.; Neamati, N.; Takebayashi, Y. Copper-transporting P-type adenosine triphosphatase (ATP7B) as a cisplatin based chemoresistance marker in ovarian carcinoma: Comparative analysis with expression of MDR1, MRP1, MRP2, LRP and BCRP. Int. J. Cancer 2002, 101, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem. Sci. 1992, 17, 463–468. [Google Scholar] [CrossRef]
- Finger, A. In-vitro studies on the effect of polyphenol oxidase and peroxidase on the formation of polyphenolic black tea constituents. J. Sci. Food Agric. 1994, 66, 293–305. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, H.J.; Lee, C.Y. Antioxidant activity of black tea vs. green tea. J. Nutr. 2002, 132, 785. [Google Scholar] [PubMed]
- Gosslau, A.; Li, S.; Ho, C.T.; Chen, K.Y.; Rawson, N.E. The importance of natural product characterization in studies of their anti-inflammatory activity. Mol. Nutr. Food Res. 2011, 55, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Z.; Huang, J.; Bekhit, A.E.D.; Liu, F.; Dong, X.; Gong, Y.; Fu, D. The inhibitory effects of pure black tea theaflavins on the growth of four selected human cancer CELLS. J. Food Biochem. 2011, 35, 1561–1567. [Google Scholar] [CrossRef]
- Lee, H.-H.; Ho, C.-T.; Lin, J.-K. Theaflavin-3, 3′-digallate and penta-O-galloyl-β-d-glucose inhibit rat liver microsomal 5α-reductase activity and the expression of androgen receptor in LNCaP prostate cancer cells. Carcinogenesis 2004, 25, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Pan, S.; Miao, A.; Ling, C.; Pang, S.; Tang, J.; Chen, D.; Zhao, C. Active extracts of black tea (Camellia Sinensis) induce apoptosis of PC-3 prostate cancer cells via mitochondrial dysfunction. Oncol. Rep. 2013, 30, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Kim, E.; Gao, Y.; Rankin, G.O.; Li, B.; Chen, Y.C. Theaflavin-3, 3′-digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin-resistant ovarian cancer A2780/CP70 cells. Int. J. Oncol. 2016, 48, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Rankin, G.O.; Tu, Y.; Chen, Y.C. Theaflavin-3, 3′-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways. Int. J. Oncol. 2016, 48, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, P.; Wang, P.; Yang, C.S.; Wang, X.; Feng, Q. EGCG enhances cisplatin sensitivity by regulating expression of the copper and cisplatin influx transporter CTR1 in ovary cancer. PLoS ONE 2015, 10, e0125402. [Google Scholar]
- Jiang, P.; Wu, X.; Wang, X.; Huang, W.; Feng, Q. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget 2016, 7, 43337. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, T.; Nakahashi, Y.; Hachimine, D.; Seki, T.; Okazaki, K. The combination of glycyrrhizin and lamivudine can reverse the cisplatin resistance in hepatocellular carcinoma cells through inhibition of multidrug resistance-associated proteins. Int. J. Oncol. 2007, 31, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Jamali, B.; Nakhjavani, M.; Hosseinzadeh, L.; Amidi, S.; Nikounezhad, N.; Shirazi, F.H. Intracellular GSH alterations and its relationship to level of resistance following exposure to cisplatin in cancer cells. Iran. J. Pharm. Res. 2015, 14, 513. [Google Scholar] [PubMed]
- Mistry, P.; Kelland, L.; Abel, G.; Sidhar, S.; Harrap, K. The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight human ovarian carcinoma cell lines. Br. J. Cancer 1991, 64, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Godwin, A.K.; Meister, A.; O’Dwyer, P.J.; Huang, C.S.; Hamilton, T.C.; Anderson, M.E. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc. Natl. Acad. Sci. USA 1992, 89, 3070–3074. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hutter, K.-J.; Zeller, W. Positive correlation between cellular glutathione and acquired cisplatin resistance in human ovarian cancer cells. Cell Biol. Toxicol. 1995, 11, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Schuck, A.G.; Ausubel, M.B.; Zuckerbraun, H.L.; Babich, H. Theaflavin-3, 3′-digallate, a component of black tea: An inducer of oxidative stress and apoptosis. Toxicol. Vitro 2008, 22, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Gottesman, R.T.; Liebling, E.J.; Schuck, A.G. Theaflavin-3-Gallate and Theaflavin-3′-Gallate, Polyphenols in Black Tea with Prooxidant Properties. Basic Clin. Pharm. Toxicol. 2008, 103, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Pinsky, S.; Muskin, E.; Zuckerbraun, H. In vitro cytotoxicity of a theaflavin mixture from black tea to malignant, immortalized, and normal cells from the human oral cavity. Toxicol. Vitro 2006, 20, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Ciarimboli, G. Membrane transporters as mediators of cisplatin effects and side effects. Scientifica 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Holzer, A.K.; Manorek, G.H.; Howell, S.B. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol. Pharm. 2006, 70, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Song, I.-S.; Savaraj, N.; Siddik, Z.H.; Liu, P.; Wei, Y.; Wu, C.J.; Kuo, M.T. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol. Cancer Ther. 2004, 3, 1543–1549. [Google Scholar] [PubMed]
- Lee, Y.-Y.; Choi, C.H.; Do, I.-G.; Song, S.Y.; Lee, W.; Park, H.S.; Song, T.J.; Kim, M.K.; Kim, T.-J.; Lee, J.-W. Prognostic value of the copper transporters, CTR1 and CTR2, in patients with ovarian carcinoma receiving platinum-based chemotherapy. Gynecol. Oncol. 2011, 122, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jin, Y.; Wu, Y.; Tu, Y. Isolation and purification of four individual theaflavins using semi-preparative high performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1791–1801. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Kim, E.; Rankin, G.O.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Theaflavin-3,3′-Digallate Enhances the Inhibitory Effect of Cisplatin by Regulating the Copper Transporter 1 and Glutathione in Human Ovarian Cancer Cells. Int. J. Mol. Sci. 2018, 19, 117. https://doi.org/10.3390/ijms19010117
Pan H, Kim E, Rankin GO, Rojanasakul Y, Tu Y, Chen YC. Theaflavin-3,3′-Digallate Enhances the Inhibitory Effect of Cisplatin by Regulating the Copper Transporter 1 and Glutathione in Human Ovarian Cancer Cells. International Journal of Molecular Sciences. 2018; 19(1):117. https://doi.org/10.3390/ijms19010117
Chicago/Turabian StylePan, Haibo, Eunhye Kim, Gary O. Rankin, Yon Rojanasakul, Youying Tu, and Yi Charlie Chen. 2018. "Theaflavin-3,3′-Digallate Enhances the Inhibitory Effect of Cisplatin by Regulating the Copper Transporter 1 and Glutathione in Human Ovarian Cancer Cells" International Journal of Molecular Sciences 19, no. 1: 117. https://doi.org/10.3390/ijms19010117




