The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Renal and Liver Disease in Western Diet Induced Obesity Mice
Abstract
:1. Introduction
2. Results
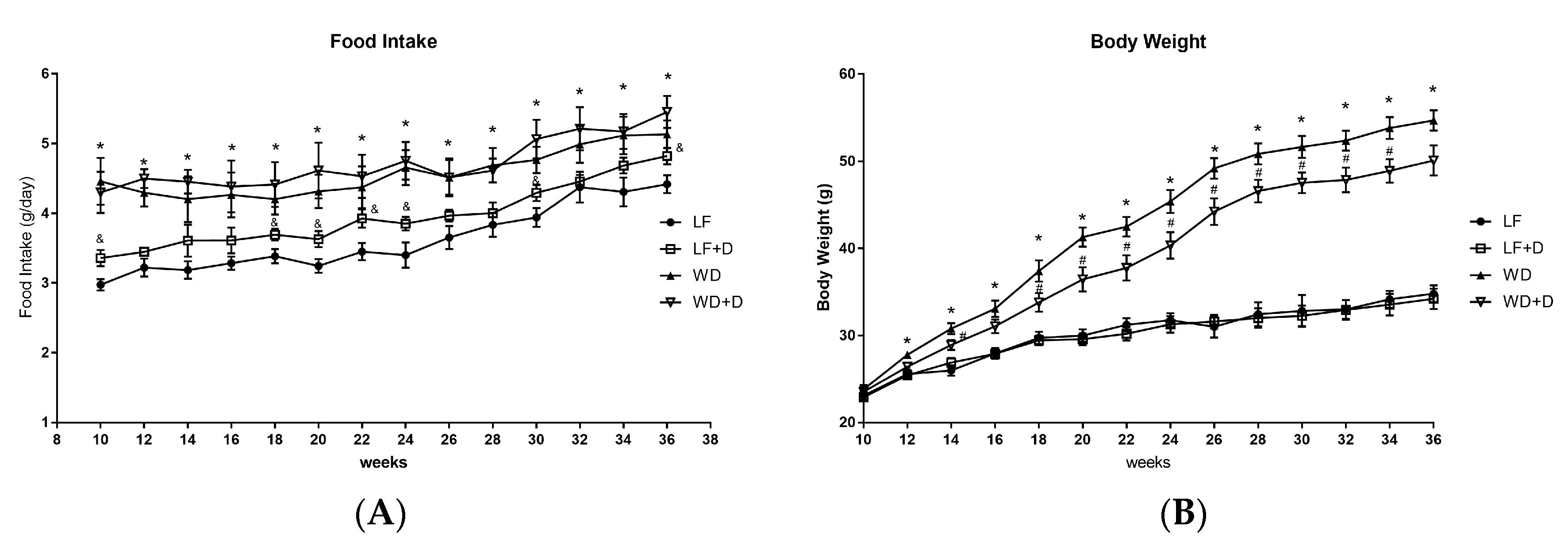
2.1. Dapagliflozin Treatment of WD-Fed C57BL/6J Mice Reduced Body Weight Gain and Improved Plasma Glucose, Insulin Resistance and Plasma Lipids
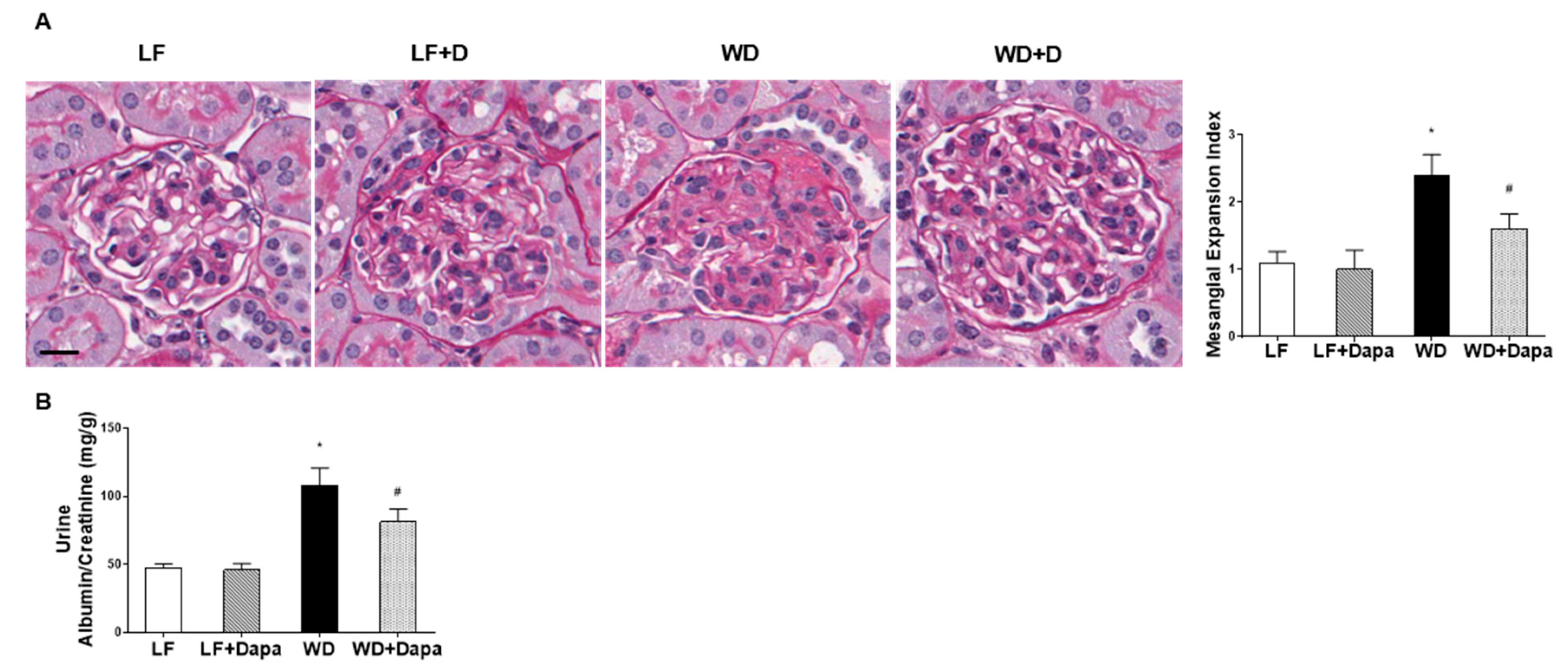
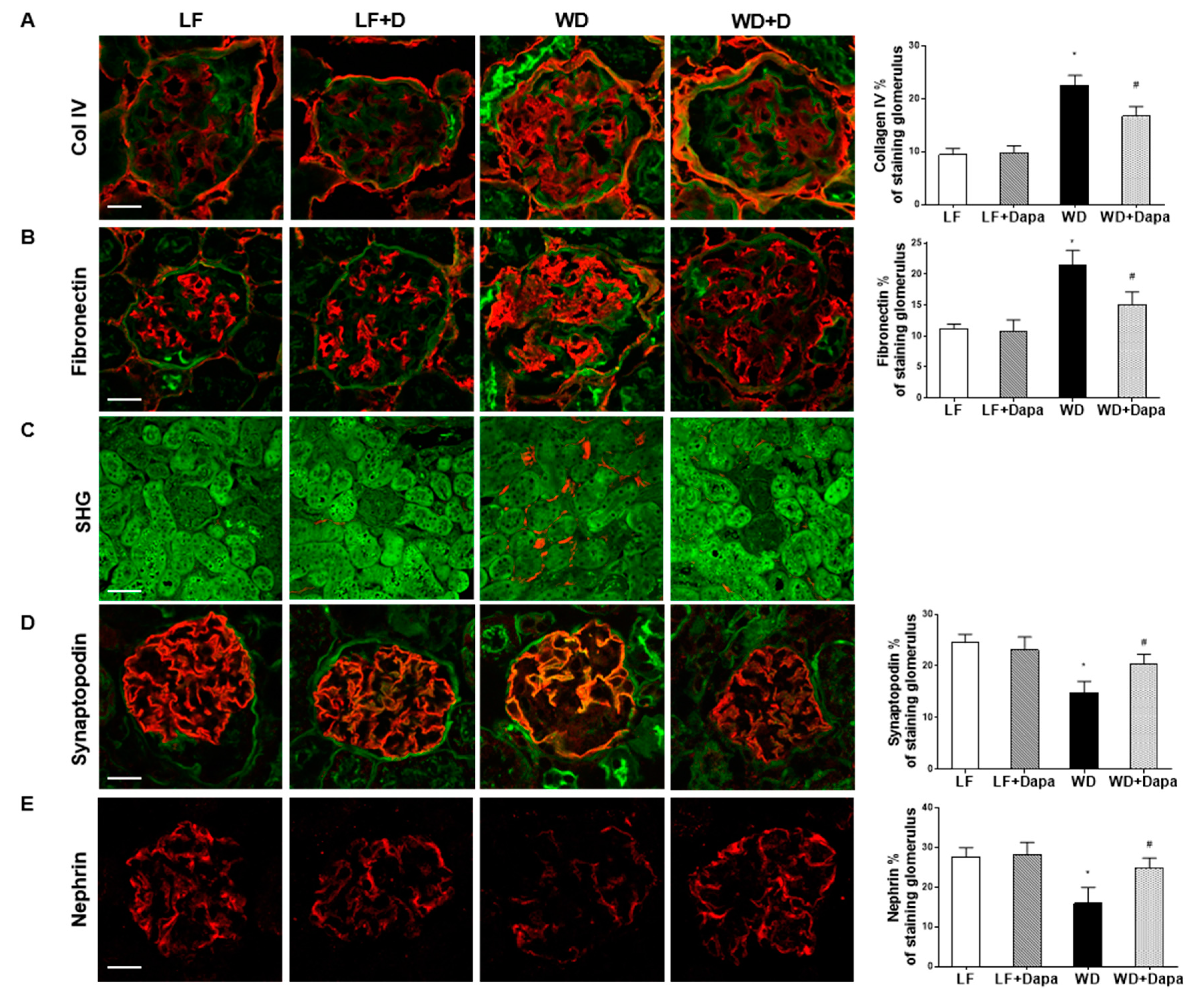
2.2. Dapagliflozin Prevented Glomerular Pathology and Renal Fibrosis in WD-Fed Mice
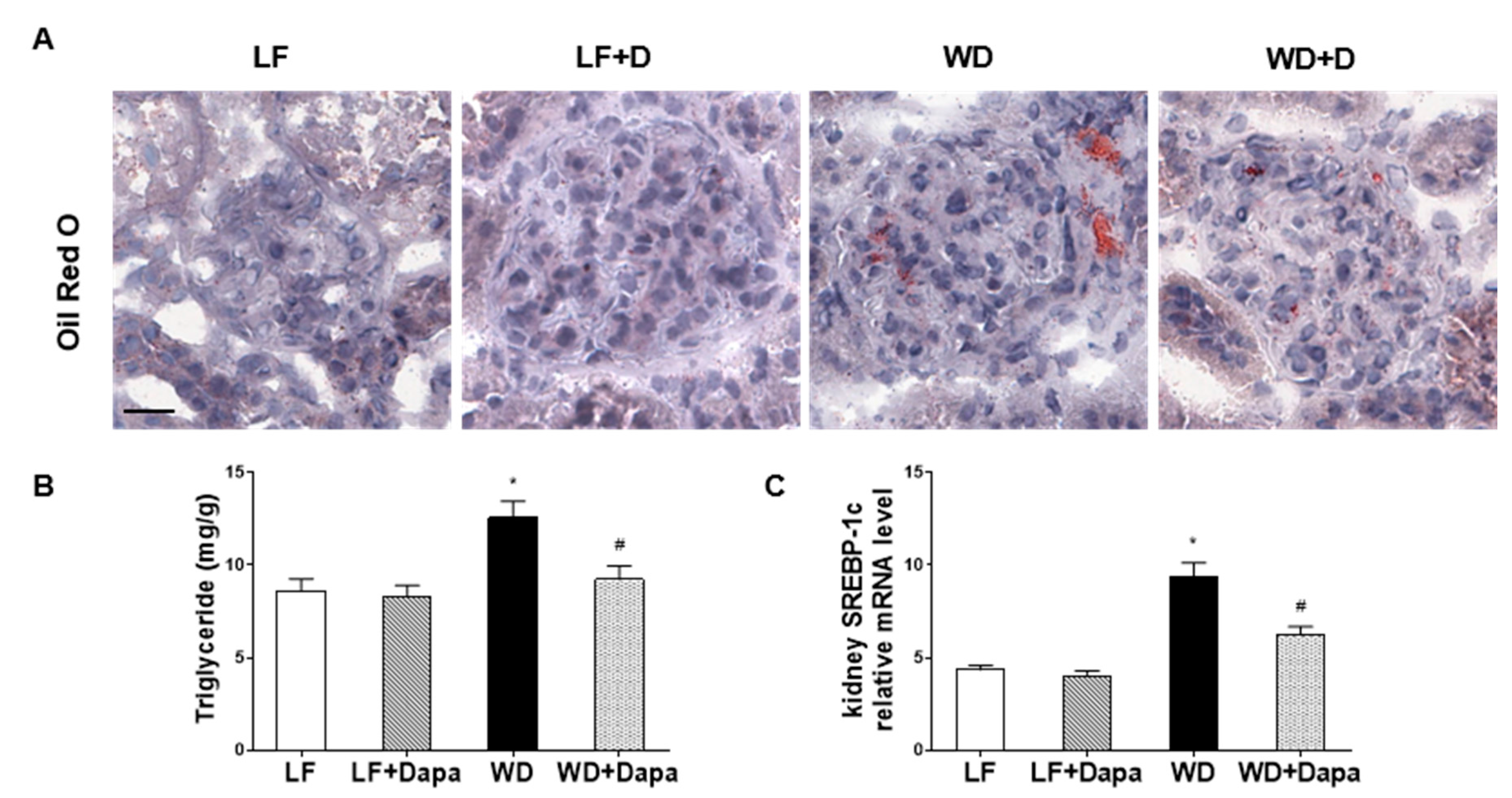
2.3. Dapagliflozin Decreased Lipid Accumulation in Kidney
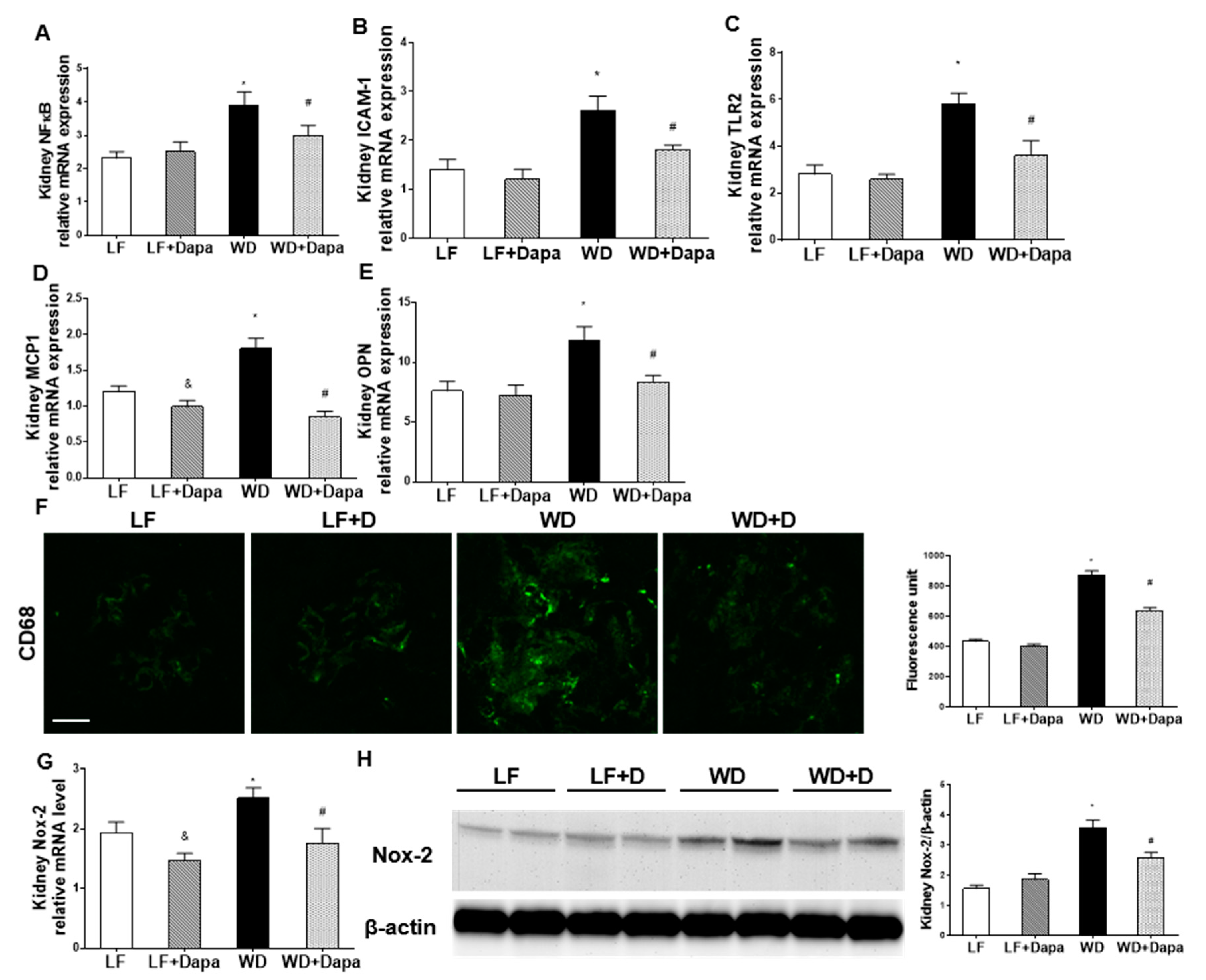
2.4. Dapagliflozin Decreased Renal Inflammation and Oxidative Stress in WD-Fed Mice
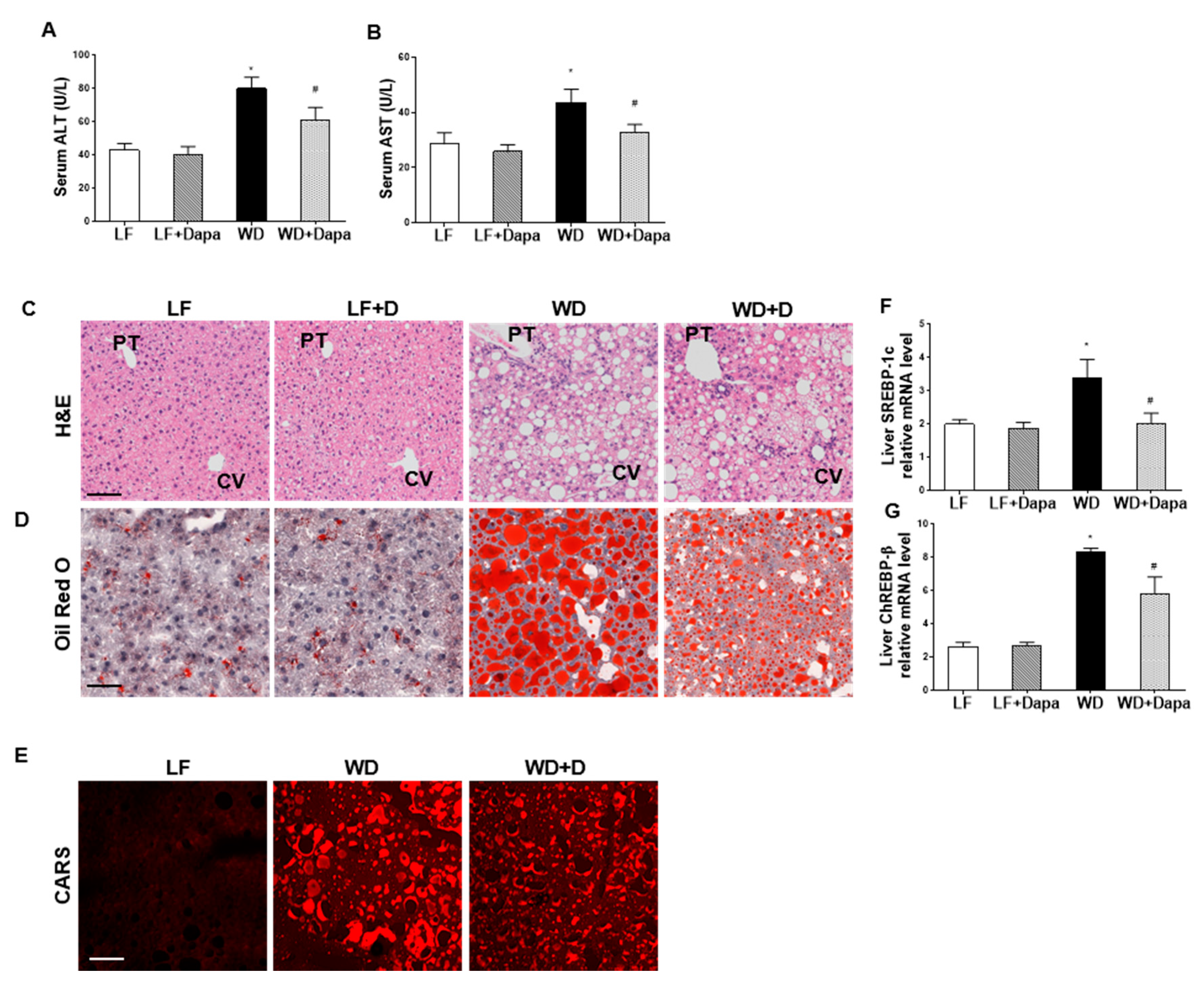
2.5. Dapagliflozin Reduced Hepatic Injury and Lipid Accumulation in Liver
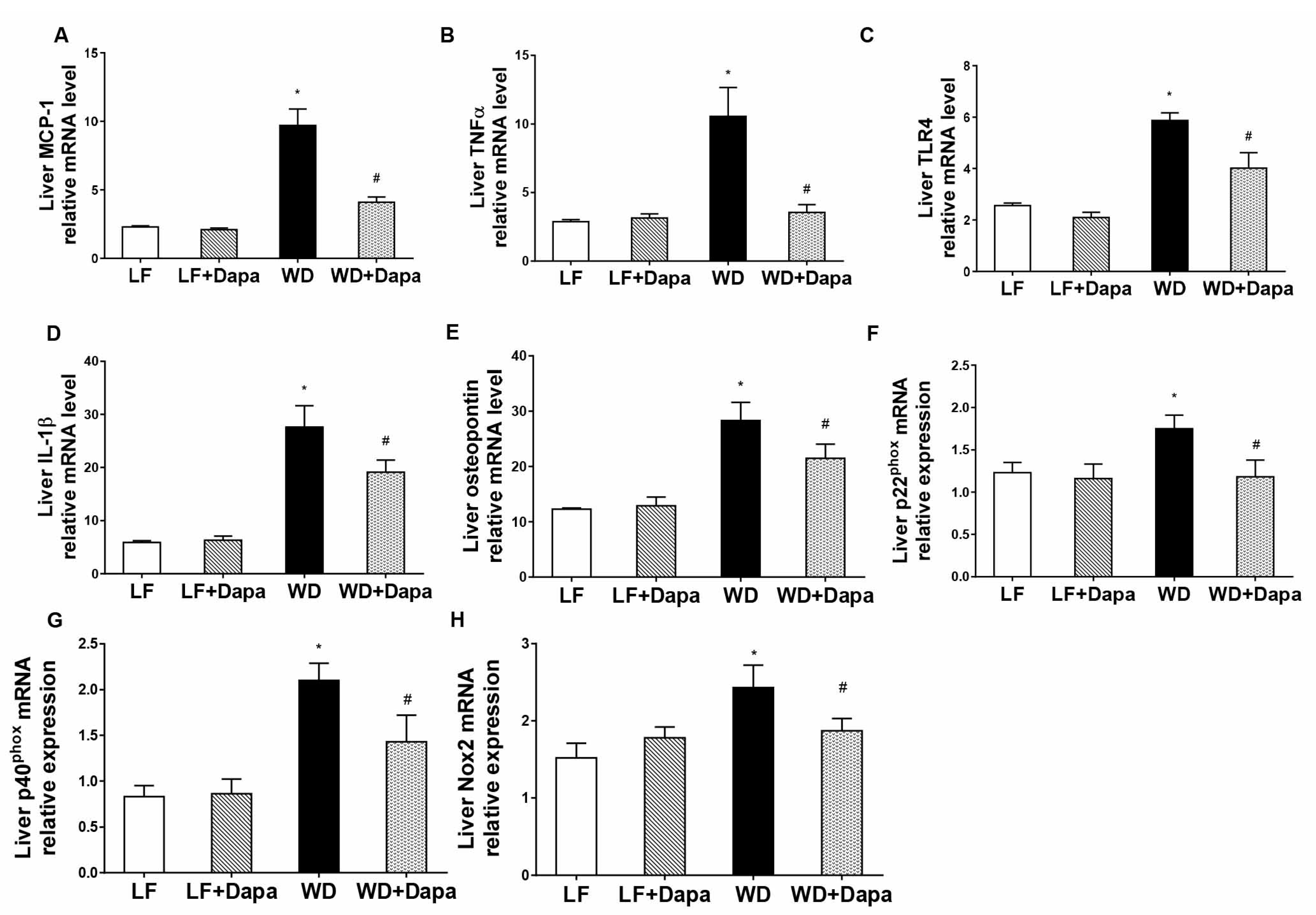
2.6. Dapagliflozin Attenuates Obesity-Induced Inflammation and Oxidative Stress in Liver
2.7. Dapagliflozin Decreases Fibrosis in Liver
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.2. Blood and Urine Chemistry
4.3. RNA Extraction and Quantitative Real-Time PCR
4.4. Western Blotting
4.5. Two Photon Excitation (TPE) and Second Harmonic Generation (SHG) Microscopy
4.6. Coherent Anti-Stokes Raman Scattering (CARS) Microscopy
4.7. Histology Staining and Immunofluorescence Microscopy
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Eckel, R.H.; Kahn, S.E.; Ferrannini, E.; Goldfine, A.B.; Nathan, D.M.; Schwartz, M.W.; Smith, R.J.; Smith, S.R. Obesity and type 2 diabetes: What can be unified and what needs to be individualized? J. Clin. Endocrinol. Metab. 2011, 96, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Van Gaal, L.F. Combating the dual burden: Therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol. 2014, 2, 911–922. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef] [PubMed]
- Re, R.N. Obesity-related hypertension. Ochsner J. 2009, 9, 133–136. [Google Scholar] [PubMed]
- Marchesini, G.; Moscatiello, S.; Di Domizio, S.; Forlani, G. Obesity-associated liver disease. J. Clin. Endocrinol. Metab. 2008, 93, S74–S80. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou-Marketou, N.; Paschou, S.A.; Marketos, N.; Adamidi, S.; Adamidis, S.; Kanaka-Gantenbein, C. Diabetic nephropathy in type 1 diabetes. Minerva Med. 2017. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, T.M.; Rajneesh, A.H.R.; Zacharia, G.S.; Adarsh, R.P. Cirrhosis of Liver and Diabetes Mellitus: The Diabolic Duo? J. Clin. Diagn. Res. 2017, 11, OC01–OC05. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.H.; Shackel, N.A.; Gorrell, M.D.; McLennan, S.V.; Twigg, S.M. Diabetes and Nonalcoholic Fatty Liver Disease: A Pathogenic Duo. Endocr. Rev. 2013, 34, 84–129. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Matsui, T.; Ishibashi, Y.; Yamagishi, S. Insulin stimulates SGLT2-mediated tubular glucose absorption via oxidative stress generation. Diabetol. Metab. Syndr. 2015, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Skrtic, M.; Cherney, D.Z. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 2015, 24, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G.; Mohandas, T.K.; Hediger, M.A. Localization of the Na+/glucose cotransporter gene SGLT2 to human chromosome 16 close to the centromere. Genomics 1993, 17, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Hirayama, B.A.; Loo, D.F. Active sugar transport in health and disease. J. Intern. Med. 2007, 261, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. Renal Na+-glucose cotransporters. Am. J. Physiol. Ren. Physiol. 2001, 280, F10–F18. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Platt, K.A.; Cunard, R.; Schroth, J.; Whaley, J.; Thomson, S.C.; Koepsell, H.; Rieg, T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J. Am. Soc. Nephrol. 2011, 22, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Gorboulev, V.; Schurmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na+-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.B.; Patel, M.C.; Mishra, B. SGLT2 inhibition: A novel prospective strategy in treatment of diabetes mellitus. Ren. Fail. 2013, 35, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Chao, E.C.; Henry, R.R. SGLT2 inhibition—A novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Rose, M.; Gerasimova, M.; Satriano, J.; Platt, K.A.; Koepsell, H.; Cunard, R.; Sharma, K.; Thomson, S.C.; Rieg, T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am. J. Physiol. Ren. Physiol. 2013, 304, F156–F167. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of SGLT2 inhibitors: Part 3. Effects on diabetic complications in type 2 diabetic mice. Eur. J. Pharmacol. 2017, 809, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Antidiabetic and antiobesity effects of SGLT2 inhibitor ipragliflozin in type 2 diabetic mice fed sugar solution. Eur. J. Pharmacol. 2017, 818, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, T.; Zhu, S.; Guo, G.; Matsumoto, A.; Zhao, J.; Endo, M.; Horiguchi, H.; Morinaga, J.; Tian, Z.; Kadomatsu, T.; et al. Treatment of diabetic mice with the SGLT2 inhibitor TA-1887 antagonizes diabetic cachexia and decreases mortality. NPJ Aging Mech. Dis. 2017, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Vivian, E.M. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: A growing class of antidiabetic agents. Drugs Context 2014, 3, 212264. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; de Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet. Med. 2010, 27, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Terami, N.; Ogawa, D.; Tachibana, H.; Hatanaka, T.; Wada, J.; Nakatsuka, A.; Eguchi, J.; Horiguchi, C.S.; Nishii, N.; Yamada, H.; et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 2014, 9, e100777. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L. Dapagliflozin efficacy and safety: A perspective review. Ther. Adv. Drug Saf. 2014, 5, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Ouyang, X.; Lei, X.; Wu, M.; Chen, L.; Wu, Q.; Deng, W.; Liang, Z. The SGLT-2 Inhibitor Dapagliflozin Has a Therapeutic Effect on Atherosclerosis in Diabetic ApoE−/− Mice. Mediat. Inflamm. 2016, 2016, 6305735. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wu, Y.; Tian, M.; Sjostrom, C.D.; Johansson, U.; Peng, X.R.; Smith, D.M.; Huang, Y. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E563–E576. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Levi, J.; Luo, Y.; Myakala, K.; Herman-Edelstein, M.; Qiu, L.; Wang, D.; Peng, Y.; Grenz, A.; Lucia, S.; et al. SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J. Biol. Chem. 2017, 292, 5335–5348. [Google Scholar] [CrossRef] [PubMed]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H. Sodium glucose co-transporter 2 (SGLT2) inhibitors: New among antidiabetic drugs. Cardiovasc. Drugs Ther. 2014, 28, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Gupta, N.; Aronson, R. Effect of Dapagliflozin on Glycemic Control, Weight, and Blood Pressure in Patients with Type 2 Diabetes Attending a Specialist Endocrinology Practice in Canada: A Retrospective Cohort Analysis. Diabetes Technol. Ther. 2017, 19, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Shigiyama, F.; Kumashiro, N.; Miyagi, M.; Ikehara, K.; Kanda, E.; Uchino, H.; Hirose, T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc. Diabetol. 2017, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Lee, Y.H. Non-Alcoholic Fatty Liver Disease: The Emerging Burden in Cardiometabolic and Renal Diseases. Diabetes Metab. J. 2017, 41, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Touros, A.; Kim, W.R. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin. Liver Dis. 2018, 22, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Imajo, K.; Kato, T.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Kato, S.; Mawatari, H.; Fujita, K.; Yoneda, M.; et al. The Selective SGLT2 Inhibitor Ipragliflozin Has a Therapeutic Effect on Nonalcoholic Steatohepatitis in Mice. PLoS ONE 2016, 11, e0146337. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Kubota, N.; Kubota, T.; Iwamoto, M.; Sato, H.; Sakurai, Y.; Takamoto, I.; Katsuyama, H.; Suzuki, Y.; Fukazawa, M.; et al. Tofogliflozin Improves Insulin Resistance in Skeletal Muscle and Accelerates Lipolysis in Adipose Tissue in Male Mice. Endocrinology 2016, 157, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Katsuno, K.; Isaji, M.; Nagasawa, T.; Buehrer, B.; Walker, S.; Wilkison, W.O.; Cheatham, B. Remogliflozin Etabonate Improves Fatty Liver Disease in Diet-Induced Obese Male Mice. J. Clin. Exp. Hepatol. 2015, 5, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, A.A.; Luck, N.H.; Majid, Z. Factors predicting non-alcoholic steatohepatitis (NASH) and advanced fibrosis in patients with non-alcoholic fatty liver disease (NAFLD). Trop. Dr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bobulescu, I.A. Renal lipid metabolism and lipotoxicity. Curr. Opin. Nephrol. Hypertens. 2010, 19, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, S.; Dobrinskikh, E.; Montford, J.; Dvornikov, A.; Lehman, A.; Orlicky, D.J.; Nemenoff, R.; Gratton, E.; Levi, M.; Furgeson, S. Label-free fluorescence lifetime and second harmonic generation imaging microscopy improves quantification of experimental renal fibrosis. Kidney Int. 2016, 90, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.S.; Kratzer, A.; Barry, N.P.; Miyazaki-Anzai, S.; Miyazaki, M.; Mantulin, W.W.; Levi, M.; Potma, E.O.; Tromberg, B.J. Multimodal CARS microscopy determination of the impact of diet on macrophage infiltration and lipid accumulation on plaque formation in ApoE-deficient mice. J. Lipid Res. 2010, 51, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Luo, Y.; Myakala, K.; Orlicky, D.J.; Dobrinskikh, E.; Wang, X.; Levi, M. Serelaxin improves cardiac and renal function in DOCA-salt hypertensive rats. Sci. Rep. 2017, 7, 9793. [Google Scholar] [CrossRef] [PubMed]

| LF | LF + Dapagliflozin | WD | WD + Dapagliflozin | |
|---|---|---|---|---|
| Body Weight (g) | 34.81 ± 0.97 | 34.21 ± 1.18 | 54.69 ± 1.16 * | 50.09 ± 1.73 # |
| Kidney weight (g) | 0.28 ± 0.01 | 0.29 ± 0.02 | 0.47 ± 0.03 * | 0.42 ± 0.01 |
| Liver weight (g) | 1.25 ± 0.02 | 1.24 ± 0.01 | 2.24 ± 0.09 * | 1.98 ± 0.06 # |
| Plasma Glucose (mg/dL) | 121.7 ± 1.5 | 119.8 ± 1.2 | 206.9 ± 3.1 * | 183.8 ± 2.7 # |
| Plasma Cholesterol (mg/dL) | 137.9 ± 1.2 | 128.8 ± 1.5 | 343.7 ± 4.2 * | 260.1 ± 3.8 # |
| Plasma Triglycerides (mg/dL) | 45.7 ± 3.2 | 44.2 ± 2.1 | 59.1 ± 6.4 * | 46.2 ± 5.3 # |
| Plasma insulin (ng/mL) | 2.8 ± 0.2 | 2.5 ± 0.2 | 5.7 ± 0.7 * | 4.2 ± 0.5 # |
| Systolic blood pressure (mmHg) | 121.8 ± 7.9 | 118.9 ± 1.5 | 131.2 ± 3.2 | 128.5 ± 3.6 |
| Diastolic blood pressure (mmHg) | 78.5 ± 5.8 | 83.5 ± 7.3 | 72.5 ± 5.8 | 86.5 ± 3.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Luo, Y.; Wang, X.; Orlicky, D.J.; Myakala, K.; Yang, P.; Levi, M. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Renal and Liver Disease in Western Diet Induced Obesity Mice. Int. J. Mol. Sci. 2018, 19, 137. https://doi.org/10.3390/ijms19010137
Wang D, Luo Y, Wang X, Orlicky DJ, Myakala K, Yang P, Levi M. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Renal and Liver Disease in Western Diet Induced Obesity Mice. International Journal of Molecular Sciences. 2018; 19(1):137. https://doi.org/10.3390/ijms19010137
Chicago/Turabian StyleWang, Dong, Yuhuan Luo, Xiaoxin Wang, David J. Orlicky, Komuraiah Myakala, Pengyuan Yang, and Moshe Levi. 2018. "The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Renal and Liver Disease in Western Diet Induced Obesity Mice" International Journal of Molecular Sciences 19, no. 1: 137. https://doi.org/10.3390/ijms19010137






