Identification and Comparative Analysis of Premature Senescence Leaf Mutants in Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Results
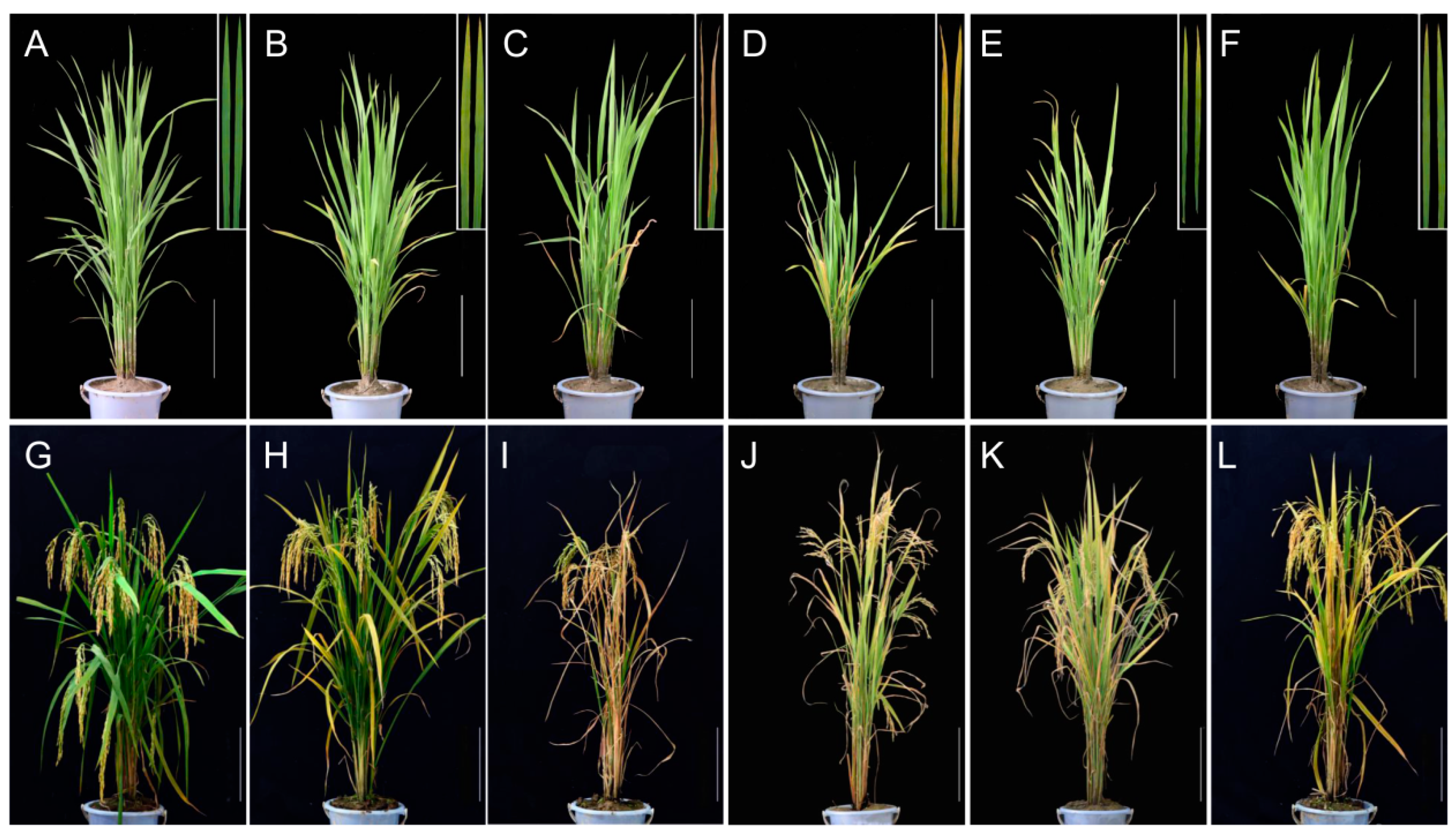
2.1. Phenotype of Premature Senescence Leaf Mutants
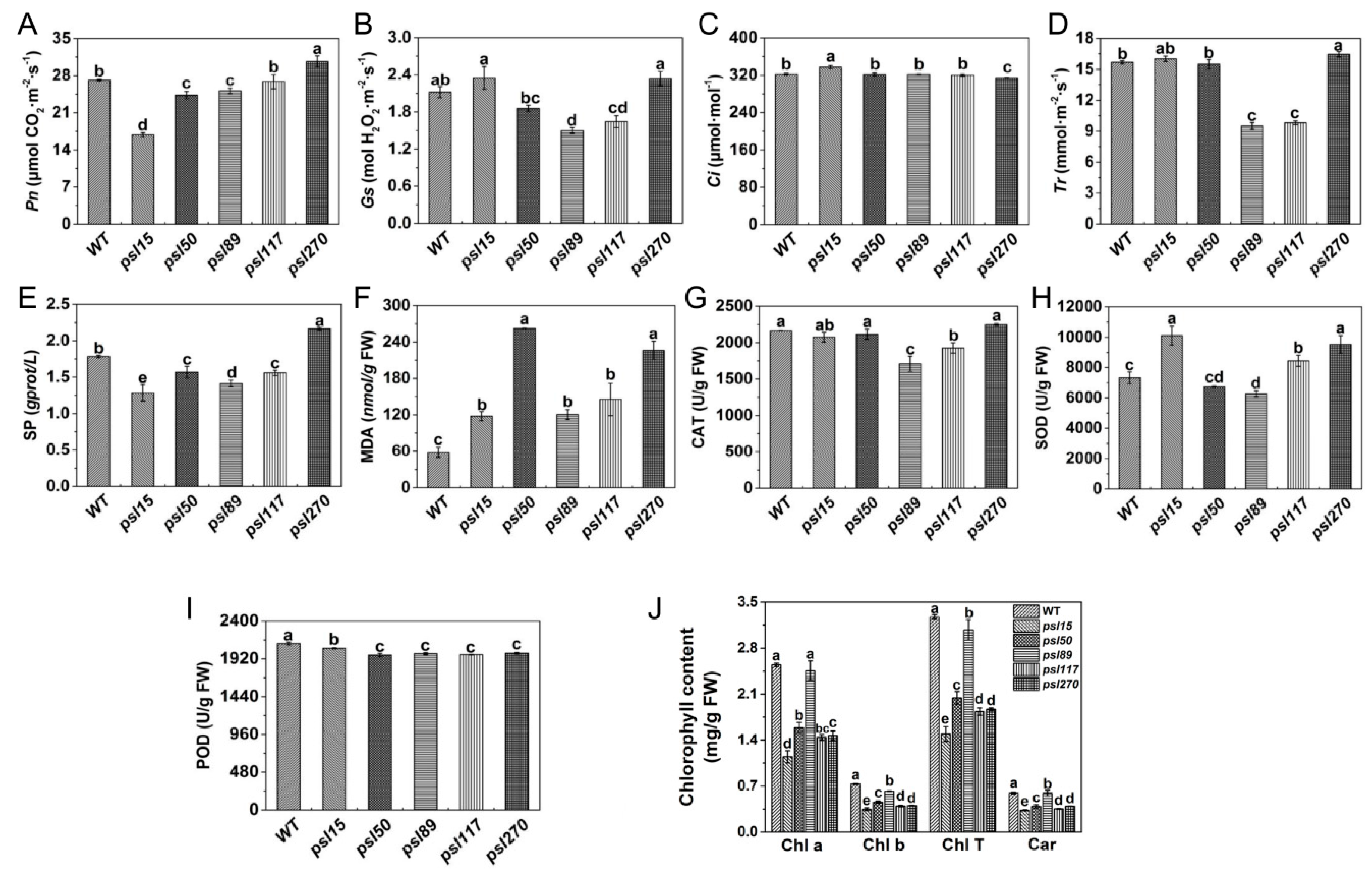
2.2. Alterations of Photosynthetic and Senescence-related Parameters
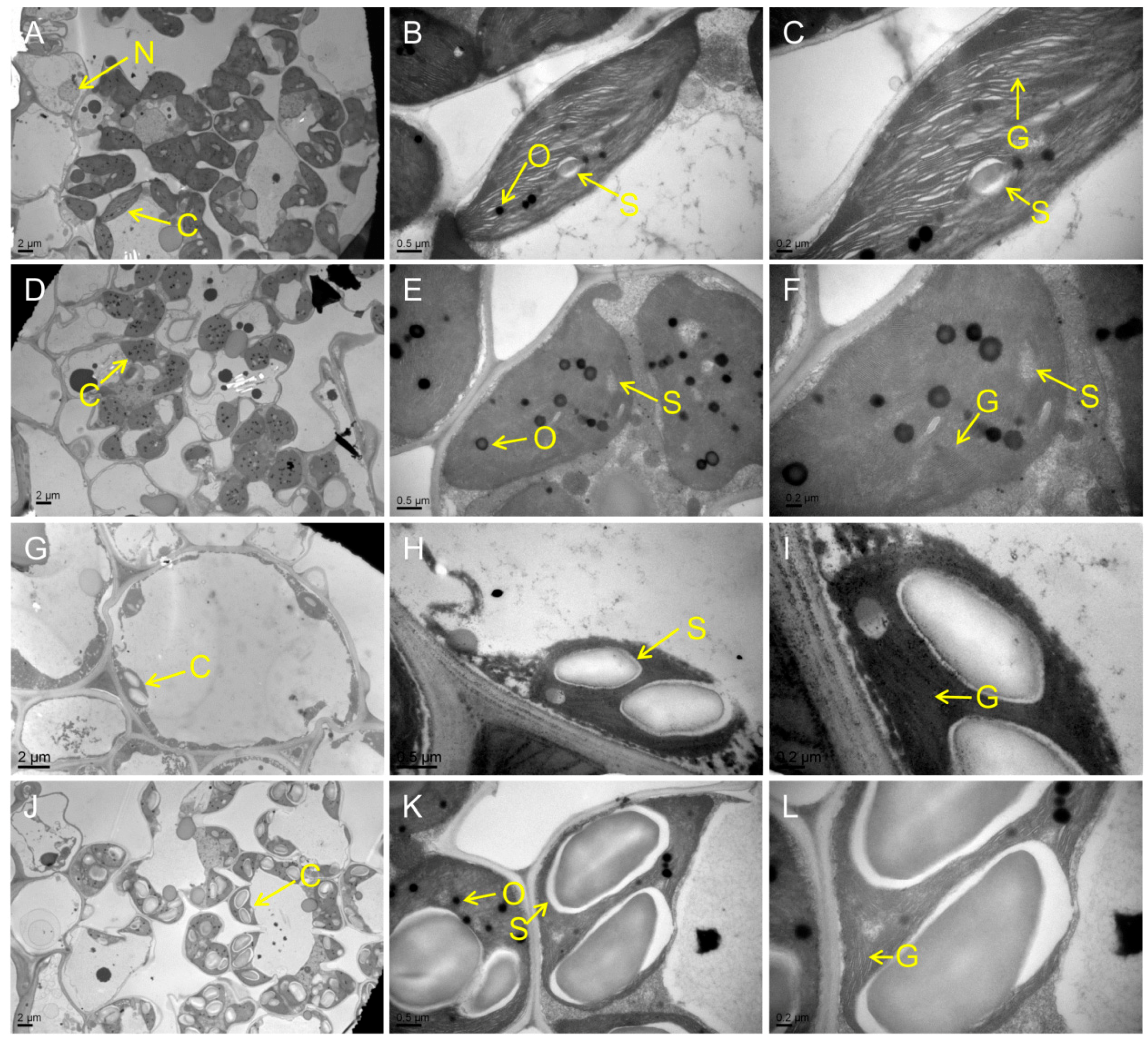
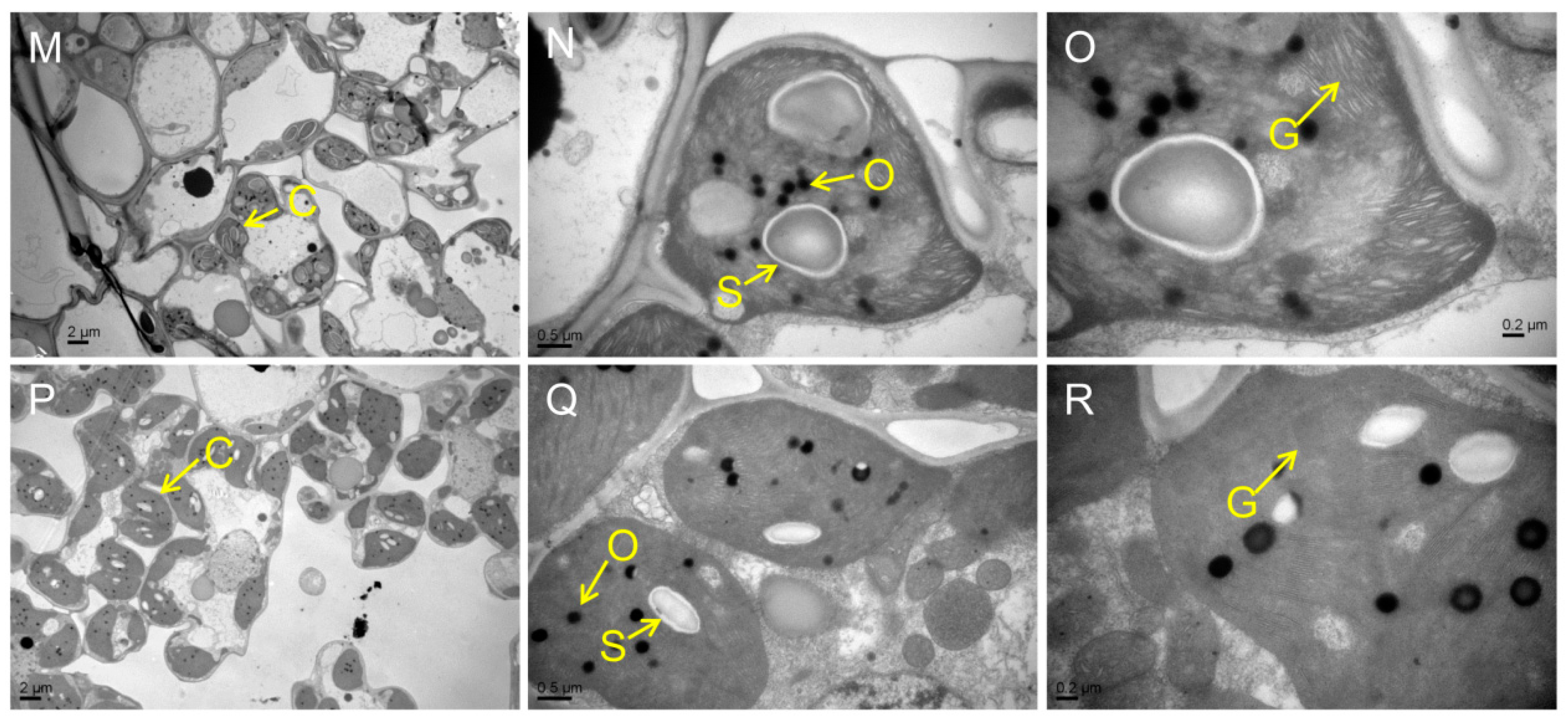
2.3. Impaired Chloroplast Development in the Mutants
2.4. Premature Senescence Leaf phenotypes Are Controlled by Single Recessive Genes
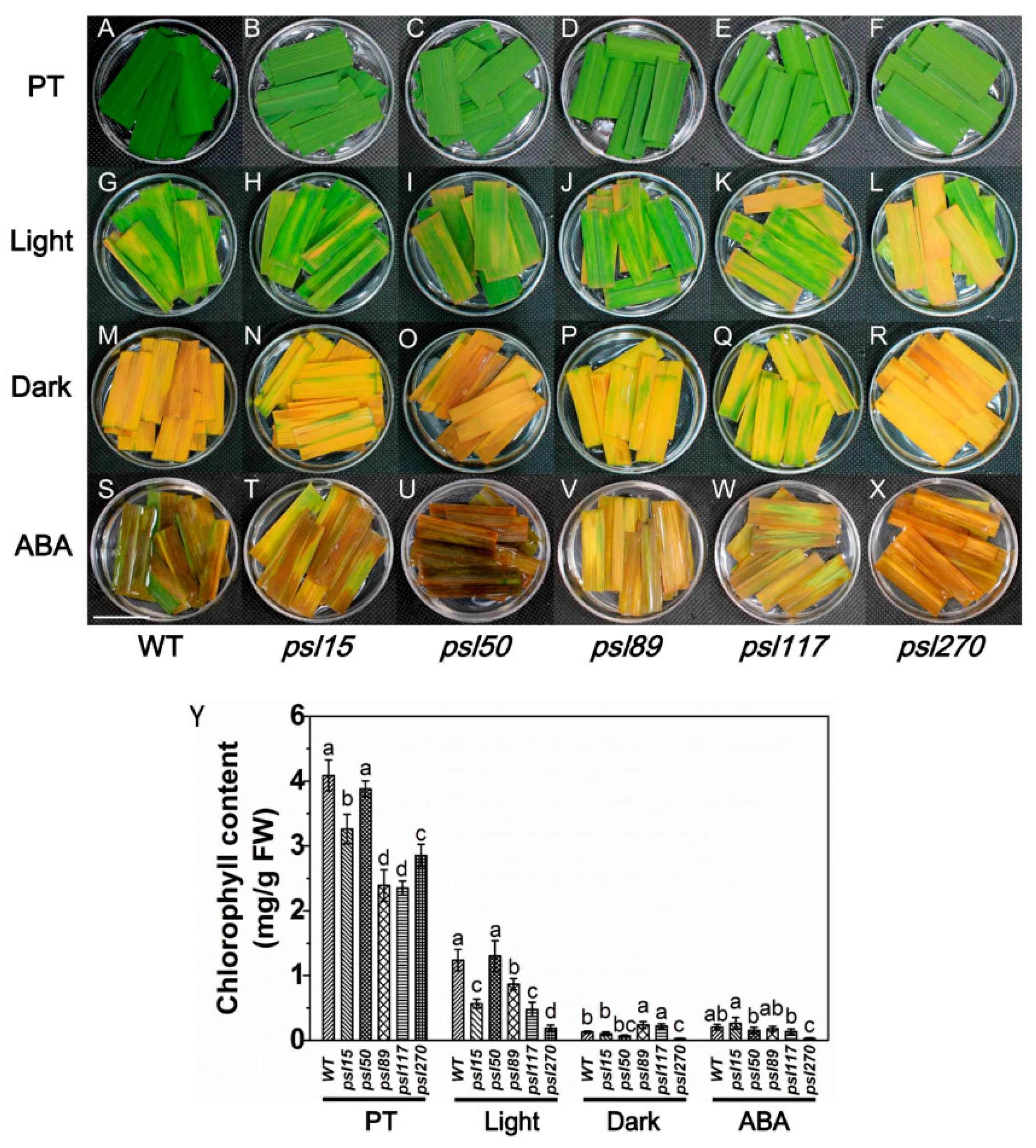
2.5. Darkness and ABA Induce Leaf Senescence
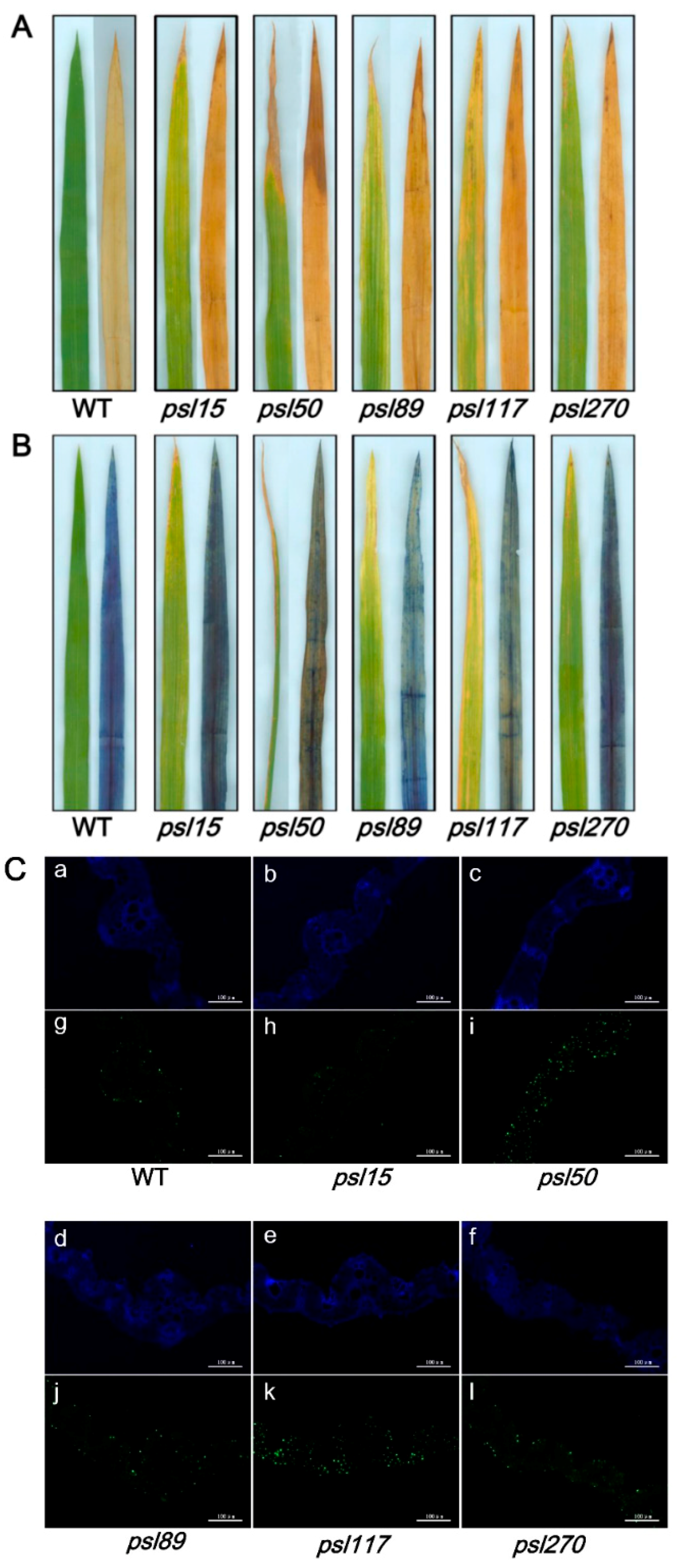
2.6. ROS Accumulation, Cell Death and DNA Fragmentation Occur in the Mutants
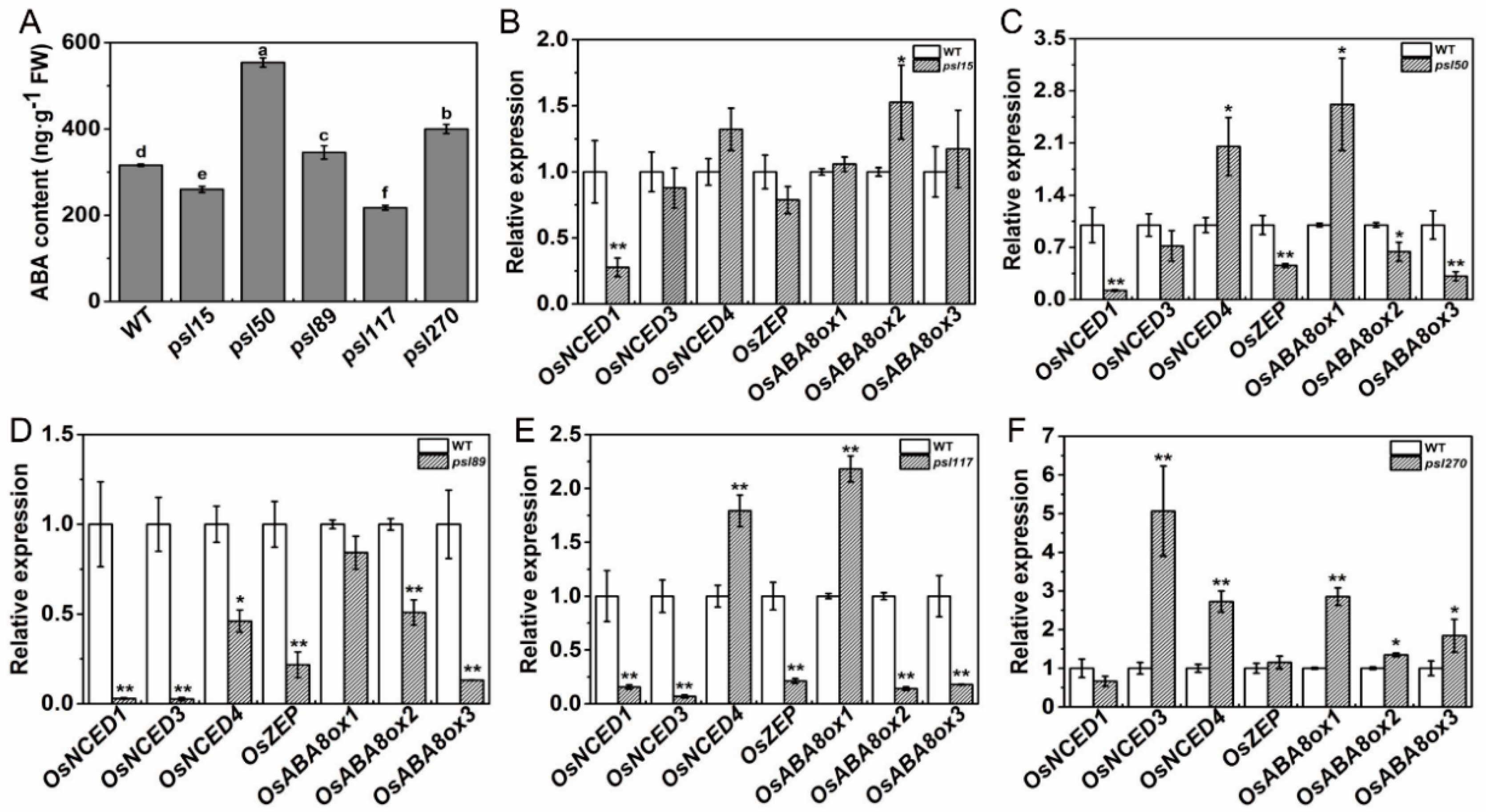
2.7. Alterations of ABA Contents and ABA-Related Gene Expression
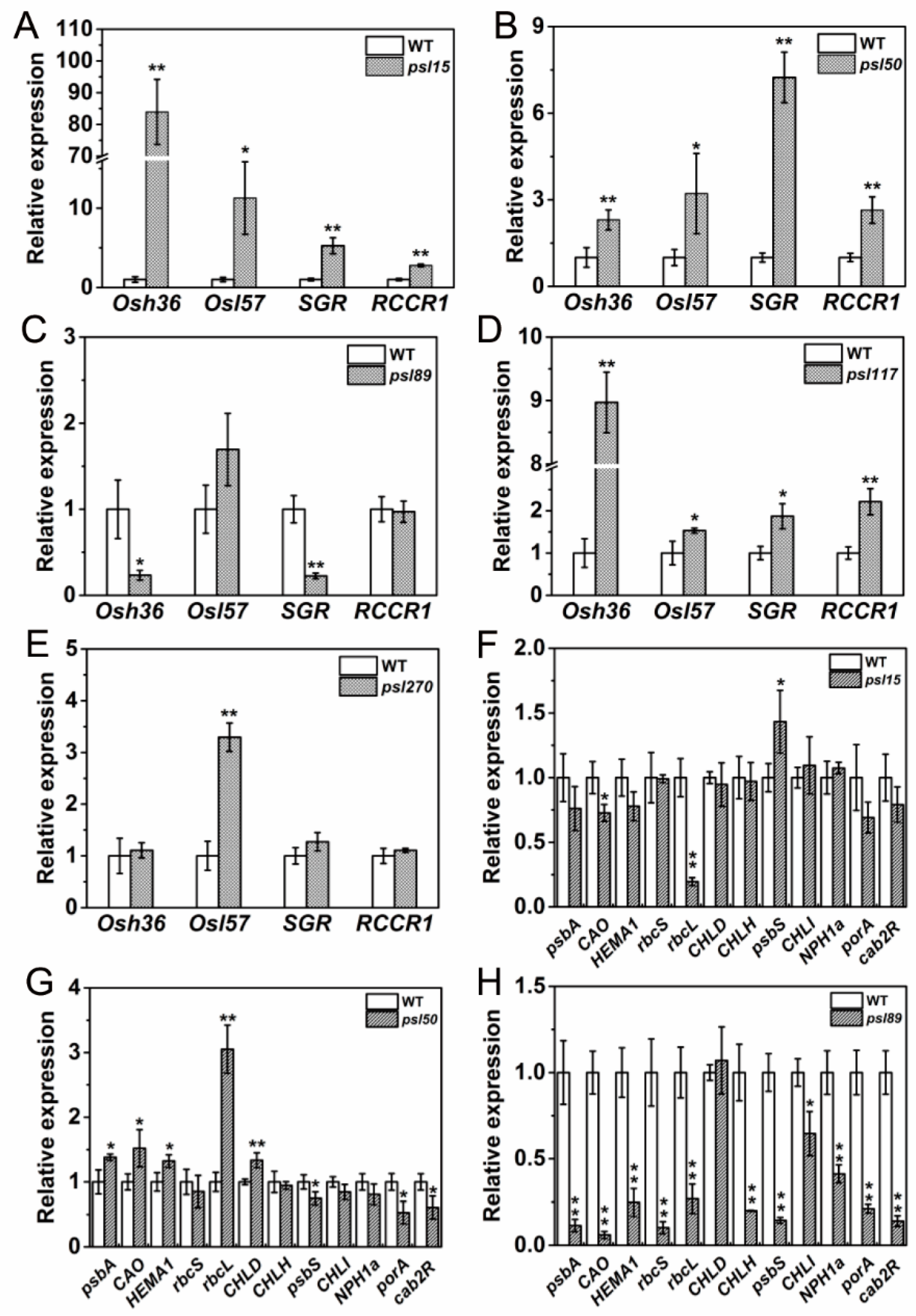
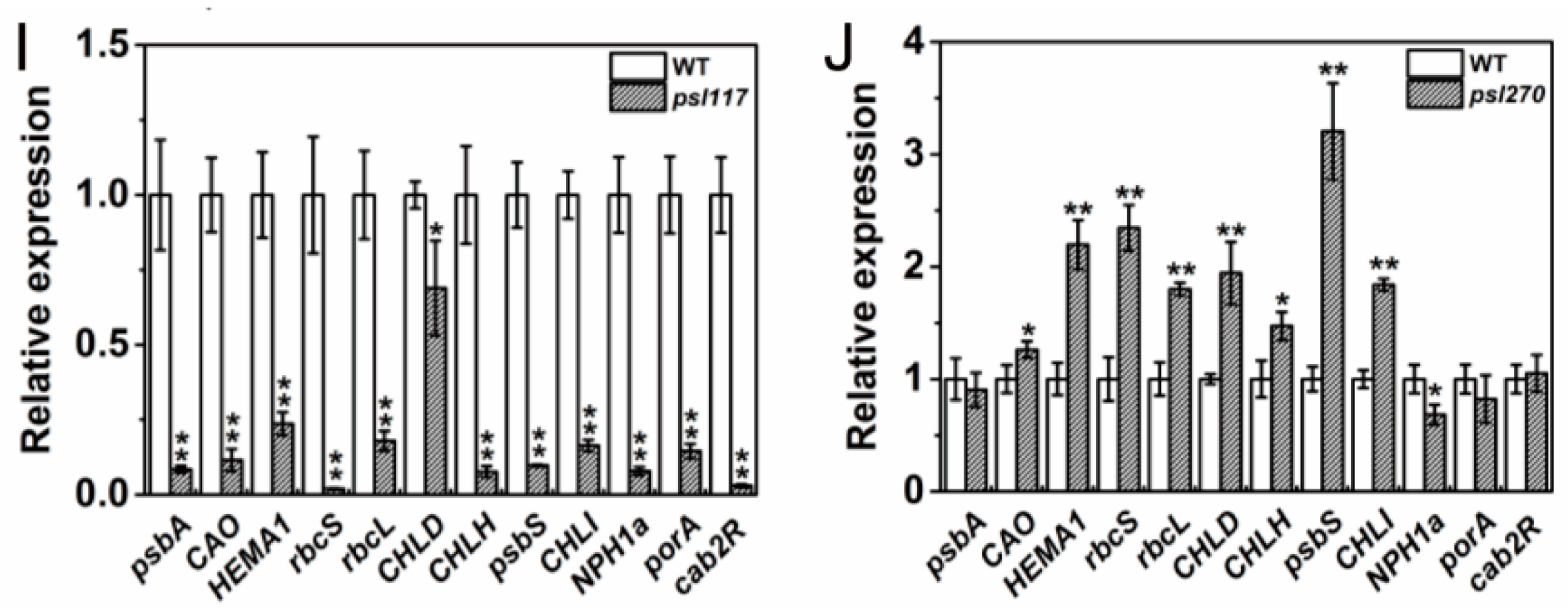
2.8. Differential Expression of Genes Associated with Senescence, Chlorophyll Metabolism and Photosynthesis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Genetic Analysis and Allelism Tests
4.3. Histochemical Analysis
4.4. Measurement of Physio-Biochemical Parameters
4.5. TUNEL Assays
4.6. Transmission Electron Microscopy
4.7. Darkness and ABA treatment
4.8. ABA Content Measurement
4.9. Real-Time Fluorescent Quantitative PCR (qRT-PCR) Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sakuraba, Y.; Han, S.; Yang, H.; Piao, W.; Paek, N. Mutation of Rice Early Flowering3.1 (OsELF3.1) delays leaf senescence in rice. Plant Mol. Biol. 2016, 92, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Chu, C. Towards understanding abscisic acid-mediated leaf senescence. Sci. China Life Sci. 2015, 58, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.; Sato, Y.; Yu, M.; Nishimura, M.; Kusaba, M. Defect in non-yellow coloring 3, an alpha/beta hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J. 2009, 59, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, Y.; Zhu, Y.; Tang, J.; Hu, B.; Liu, L.; Ou, S.; Wu, H.; Sun, X.; Chu, J.; et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Fan, S.; Wei, H.; Tao, C.; Ma, Q.; Zhang, S.; Li, H.; Pang, C.; Yu, S. iTRAQ-based quantitative proteomic analysis reveals cold responsive proteins involved in leaf senescence in upland cotton (Gossypium hirsutum L.). Int. J. Mol. Sci. 2017, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hu, G.; Fu, Y.; Si, H.; Bai, X.; Sun, Z. Genetic analysis and high-resolution mapping of a premature senescence gene Pse(t) in rice (Oryza sativa L.). Genome 2005, 48, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Shi, Y.; Zhang, X.; Song, L.; Feng, B.; Wang, H.; Xu, X.; Li, X.; Guo, D.; Wu, J. Single base substitution in OsCDC48 is responsible for premature senescence and death phenotype in rice. J. Integr. Plant Biol. 2016, 58, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Katoh, S. Two long-term effects of light that control the stability of proteins related to photosynthesis during senescence of rice leaves. Plant Cell Physiol. 1998, 39, 394–404. [Google Scholar] [CrossRef]
- Jiao, B.; Wang, J.; Zhu, X.; Zeng, L.; Li, Q.; He, Z. A novel protein RLS1 with NB-ARM domains is involved in chloroplast degradation during leaf senescence in rice. Mol. Plant 2012, 5, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, M.; Ito, H.; Morita, R.; Iida, S.; Sato, Y.; Fujimoto, M.; Kawasaki, S.; Tanaka, R.; Hirochika, H.; Nishimura, M.; et al. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 2007, 19, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Strecker, V.; Mai, S.; Muster, B.; Beneke, S.; Bürkle, A.; Bereiter-Hahn, J.; Jendrach, M. Aging of different avian cultured cells: Lack of ROS-induced damage and quality control mechanisms. Mech. Ageing Dev. 2010, 131, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yu, Q.; Wang, Z.; Pan, Y.; Lv, W.; Zhu, L.; Chen, R.; He, G. Knockdown of GDCH gene reveals reactive oxygen species-induced leaf senescence in rice. Plant Cell Environ. 2013, 36, 1476–1489. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, Q.; Zhang, S.; Zhao, S. Effect of enhanced UV-B radiation and low-energy N⁺ ion beam radiation on the response of photosynthesis, antioxidant enzymes, and lipid peroxidation in rice (Oryza sativa) seedlings. Appl. Biochem. Biotechnol. 2013, 171, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, D.; Keech, O. Dark-induced leaf senescence: New insights into a complex light-dependent regulatory pathway. New Phytol. 2016, 212, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Lu, S.; Lv, B.; Zhang, B.; Shen, J.; He, J.; Luo, L.; Xi, D.; Chen, X.; Ming, F. A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiol. 2017, 174, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Shinozaki, K. Perception and transduction of abscisic acid signals: Keys to the function of the versatile plant hormone ABA. Trends Plant Sci. 2007, 12, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.; Kao, C. Hydrogen peroxide is necessary for abscisic acid-induced senescence of rice leaves. J. Plant Physiol. 2004, 161, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Z.; Jiang, Z.; Zhao, Y.; Peng, J.; Jin, J.; Guo, H.; Luo, J. LSD: A leaf senescence database. Nucl. Acid. Res. 2011, 39, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, Y.; Liu, X.; Peng, J.; Guo, H.; Luo, J. LSD 2.0: An update of the leaf senescence database. Nucl. Acid. Res. 2014, 42, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, B.; Chen, Y.; Liu, Y.; Chen, L. Characterization and fine mapping of the rice premature senescence mutant ospse1. Theor. Appl. Genet. 2013, 126, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, M.; Tanaka, A.; Tanaka, R. Stay-green plants: What do they tell us about the molecular mechanism of leaf senescence. Photosynth. Res. 2013, 117, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, M.; Liang, N.; Yan, H.; Wei, Y.; Xu, X.; Liu, J.; Xu, Z.; Chen, F.; Wu, G. Molecular cloning and function analysis of the stay green gene in rice. Plant J. 2007, 52, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Morita, R.; Katsuma, S.; Nishimura, M.; Tanaka, A.; Kusaba, M. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant J. 2009, 57, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Yang, Y.; Xu, J.; Li, X.; Leng, Y.; Dai, L.; Huang, L.; Shao, G.; Ren, D.; Hu, J.; et al. EARLY SENESCENCE1 encodes a SCAR-LIKE PROTEIN2 that affects water loss in rice. Plant Physiol. 2015, 169, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ren, D.; Hu, S.; Li, G.; Dong, G.; Jiang, L.; Hu, X.; Ye, W.; Cui, Y.; Zhu, L.; et al. Down-regulation of a nicotinate phosphoribosyl transferase gene, OsNaPRT1, leads to withered leaf tips. Plant Physiol. 2016, 171, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Wang, Y.; Tang, J.; Xue, P.; Li, C.; Liu, L.; Hu, B.; Yang, F.; Loake, G.J.; Chu, C. Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 2012, 158, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, G.; Ren, X.; Li, C.; Sun, D. Identification of QTL underlying physiological and morphological traits of flag leaf in barley. BMC Genet. 2015, 16, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Bolaños, E.; Mercado-Silva, E. Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharvest Biol. Technol. 2004, 33, 275–283. [Google Scholar] [CrossRef]
- Kim, H.; Ryu, H.; Hong, S.; Woo, H.; Lim, P.; Lee, I.; Sheen, J.; Nam, H.G.; Hwang, I. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Li, M.; Yang, W.; Xu, W.; Xue, Y. A novel nuclear-localized CCCH-type zinc finger protein, OsDOS, is involved in delaying leaf senescence in rice. Plant Physiol. 2006, 141, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.; Kim, H.; Nam, H. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hu, S.; Zhang, B.; Ye, W.; Niu, Y.; Guo, L.; Qian, Q. Characterization and fine mapping of a new early leaf senescence mutant es3(t) in rice. Plant Growth Regul. 2017, 81, 419–431. [Google Scholar] [CrossRef]
- Lee, R.; Wang, C.; Huang, L.; Chen, S. Leaf senescence in rice plants: Cloning and characterization of senescence up-regulated genes. J. Exp. Bot. 2001, 52, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, M.; Chen, Y.; Wu, P.; Wu, G.; Jiang, H. Knockdown of OsPAO and OsRCCR1 cause different plant death phenotypes in rice. J. Plant Physiol. 2011, 168, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Shi, Y.; Xu, X.; Wei, Y.; Wang, H.; Zhang, X.; Wu, J. Oryza sativa chloroplast signal recognition particle 43 (OscpSRP43) is required for chloroplast development and photosynthesis. PLoS ONE 2015, 10, e0143249. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, Y.; Qu, M.; Ort, D.; Zhu, X. The impact of modifying photosystem antenna size on canopy photosynthetic efficiency-Development of a new canopy photosynthesis model scaling from metabolism to canopy level processes. Plant Cell Environ. 2017, 40, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Pruzinská, A.; Anders, I.; Aubry, S.; Schenk, N.; Tapernoux-Luthi, E.; Muller, T.; Kräutler, B.; Hörtensteiner, S. In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. Plant Cell 2007, 19, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, Y.; Ito, H.; Tanaka, A. Arabidopsis STAY-GREEN, Mendel’s green cotyledon gene, encodes magnesium-dechelatase. Plant Cell 2016, 28, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hörtensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Pruzinská, A.; Tanner, G.; Anders, I.; Roca, M.; Hörtensteiner, S. Chlorophyll breakdown: Pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA 2003, 100, 15259–15264. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Ito, H.; Kusaba, M.; Tanaka, R.; Tanaka, A. Participation of chlorophyll b reductase in the initial step of the degradation of light-harvesting chlorophyll a/b-protein complexes in Arabidopsis. J. Biol. Chem. 2009, 284, 17449–17456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yuan, B.; Leng, P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 2009, 60, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Ye, N.; Zhang, J. Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol. 2009, 50, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Saika, H.; Okamoto, M.; Miyoshi, K.; Kushiro, T.; Shinoda, S.; Nakazono, M. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8′-hydroxylase in rice. Plant Cell Physiol. 2007, 47, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.; Noctor, G. Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant 2003, 119, 355–364. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Maurino, V.; Peterhansel, C. Photorespiration: Current status and approaches for metabolic engineering. Curr. Opin. Plant Biol. 2010, 13, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Orf, I.; Timm, S.; Bauwe, H.; Fernie, A.; Hagemann, M.; Kopka, J.; Nikoloski, Z. Can cyanobacteria serve as a model of plant photorespiration?—A comparative meta-analysis of metabolite profiles. J. Exp. Bot. 2016, 67, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Sairam, R.; Srivastava, G.; Singh, D. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Alscher, R.; Erturk, N.; Heath, L. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, S.; Lu, Q.; Yang, Z.; Wen, X.; Zhang, L.; Lu, C. Characterization of photosystem II in transgenic tobacco plants with decreased iron superoxide dismutase. Biochim. Biophys. Acta 2011, 1807, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, J.; Zeng, L.; Goh, M.; Leung, H.; Khush, G.; Wang, G. Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight. Mol. Plant Microbe Interact. 2000, 13, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Huang, L.; Sun, Q.; Qin, F.; Li, C.; Zhao, Y.; Zhou, D. Down-regulation of a SILENT INFORMATION REGULATOR2-related histone deacetylase gene, OsSRT1, induces DNA fragmentation and cell death in rice. Plant Physiol. 2007, 144, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
| Material | Plant Height (cm) | Panicle Length (cm) | No. Panicle | 1000-Grain Weight (g) | Seed-Setting Rate (%) |
|---|---|---|---|---|---|
| ZJ100 | 101.66 ± 2.89a | 26.60 ± 0.53a | 14.67 ± 0.58b | 22.01 ± 0.10a | 85.99 ± 0.86a |
| M15 | 97.50 ± 1.82ab | 24.00 ± 0.50bc | 11.00 ± 1.00de | 21.48 ± 0.12b | 76.33 ± 0.75c |
| M50 | 97.83 ± 1.76ab | 23.73 ± 0.72bc | 10.33 ± 0.58e | 19.53 ± 0.09d | 74.88 ± 0.46c |
| M89 | 95.17 ± 2.25b | 19.63 ± 1.23d | 13.33 ± 1.15bc | 18.80 ± 0.07e | 81.14 ± 2.08b |
| M117 | 83.33 ± 4.72c | 22.33 ± 0.86c | 16.67 ± 1.15a | 20.84 ± 0.20c | 74.27 ± 1.63c |
| M270 | 93.67 ± 4.62b | 24.73 ± 1.46b | 12.33 ± 1.15cd | 21.74 ± 0.32ab | 86.32 ± 2.41a |
| Cross | F1 | No. F2 Individual | χ2 (3:1) | |
|---|---|---|---|---|
| Wild-Type | Mutant-Type | |||
| psl15/ZJ100 | Normal | 292 | 88 | 0.69 |
| psl50/ZJ100 | Normal | 495 | 170 | 0.11 |
| psl89/IR64 | Normal | 457 | 137 | 1.19 |
| psl117/ZJ100 | Normal | 501 | 154 | 0.77 |
| psl270/80A90YR72 | Normal | 225 | 63 | 1.50 |
| Cross (Female Parent × Male Parent) | Phenotype of F1 Plants | Allelism |
|---|---|---|
| psl15 × psl50 | Wild-type | Non-allelic |
| psl89 × psl15 | Non-survival | Not determined |
| psl117 × psl15 | Wild-type | Non-allelic |
| psl270 × psl15 | Wild-type | Non-allelic |
| psl50 × psl89 | Wild-type | Non-allelic |
| psl117 × psl50 | Non-survival | Not determined |
| psl270 × psl50 | Wild-type | Non-allelic |
| psl89 × psl117 | Wild-type | Non-allelic |
| psl89 × psl270 | Wild-type | Non-allelic |
| psl117 × psl270 | Wild-type | Non-allelic |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Li, L.; Zhang, Z.; Wu, J.-L. Identification and Comparative Analysis of Premature Senescence Leaf Mutants in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2018, 19, 140. https://doi.org/10.3390/ijms19010140
He Y, Li L, Zhang Z, Wu J-L. Identification and Comparative Analysis of Premature Senescence Leaf Mutants in Rice (Oryza sativa L.). International Journal of Molecular Sciences. 2018; 19(1):140. https://doi.org/10.3390/ijms19010140
Chicago/Turabian StyleHe, Yan, Liangjian Li, Zhihong Zhang, and Jian-Li Wu. 2018. "Identification and Comparative Analysis of Premature Senescence Leaf Mutants in Rice (Oryza sativa L.)" International Journal of Molecular Sciences 19, no. 1: 140. https://doi.org/10.3390/ijms19010140





