Implication of Soluble Forms of Cell Adhesion Molecules in Infectious Disease and Tumor: Insights from Transgenic Animal Models
Abstract
:1. Introduction
2. Soluble Forms of Nectins
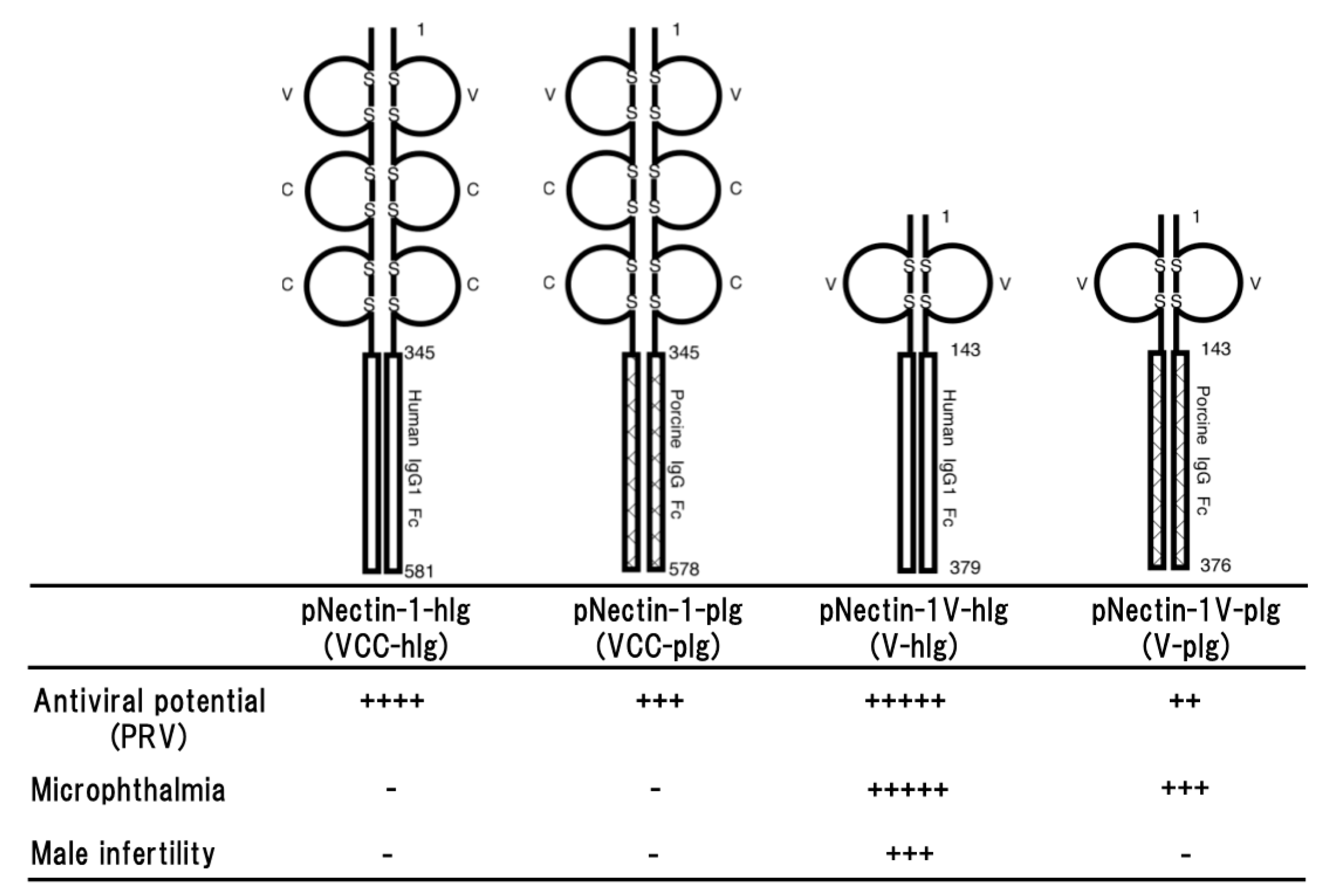
2.1. Porcine Nectin-1
2.2. Comparison of the Antiviral Potentials among Different Types of Soluble Forms of Porcine Nectin-1
2.3. Side Effects Observed in Transgenic Mice
2.4. Possible Mechanism of Resistance against PRV Infection
2.5. Application for Development of Pseudorabies-Resistant Pig
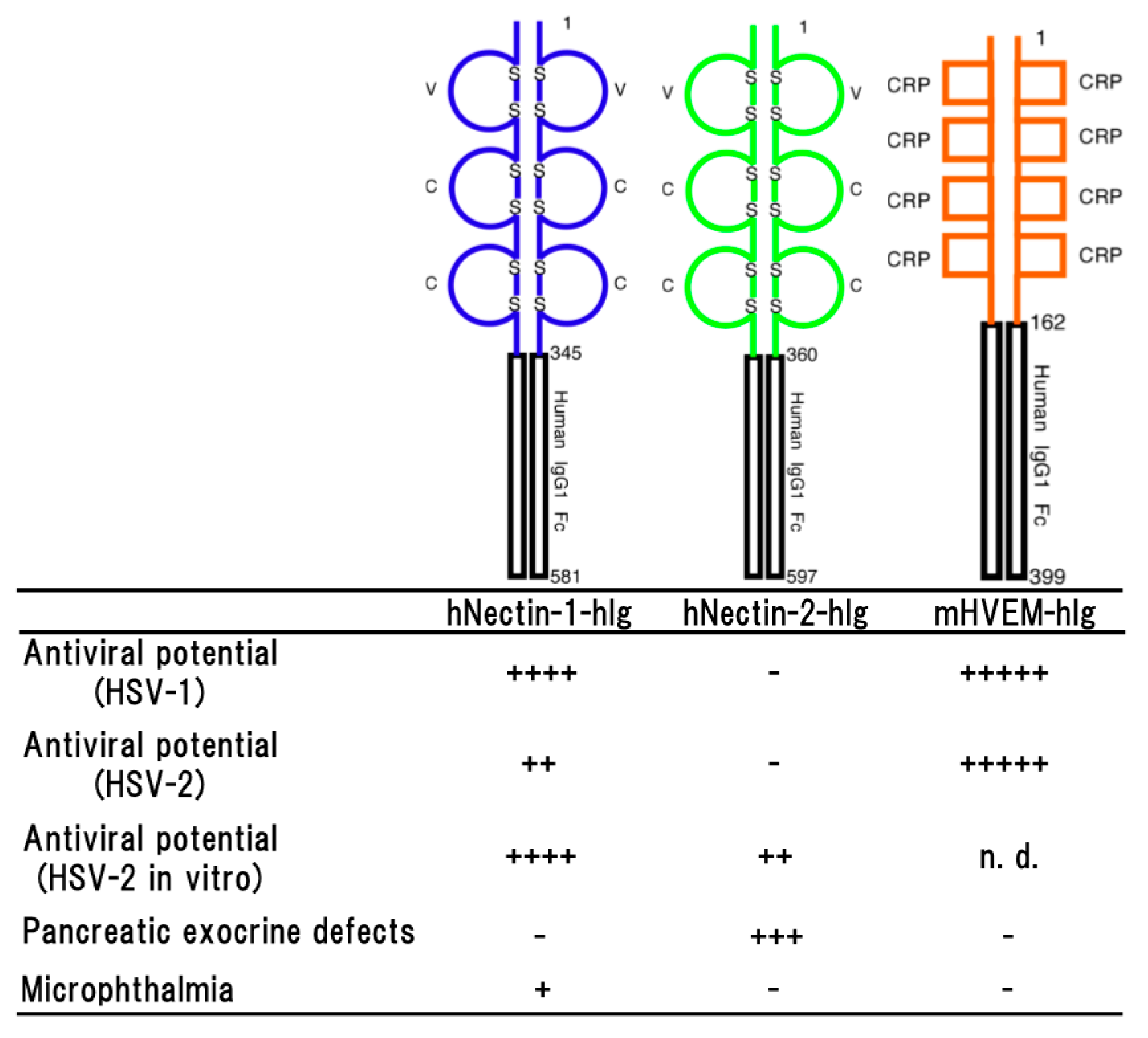
2.6. Human Nectin-1
2.7. Human Nectin-2
3. Herpesvirus Entry Mediator (HVEM)
3.1. HSV-1
3.2. HSV-2
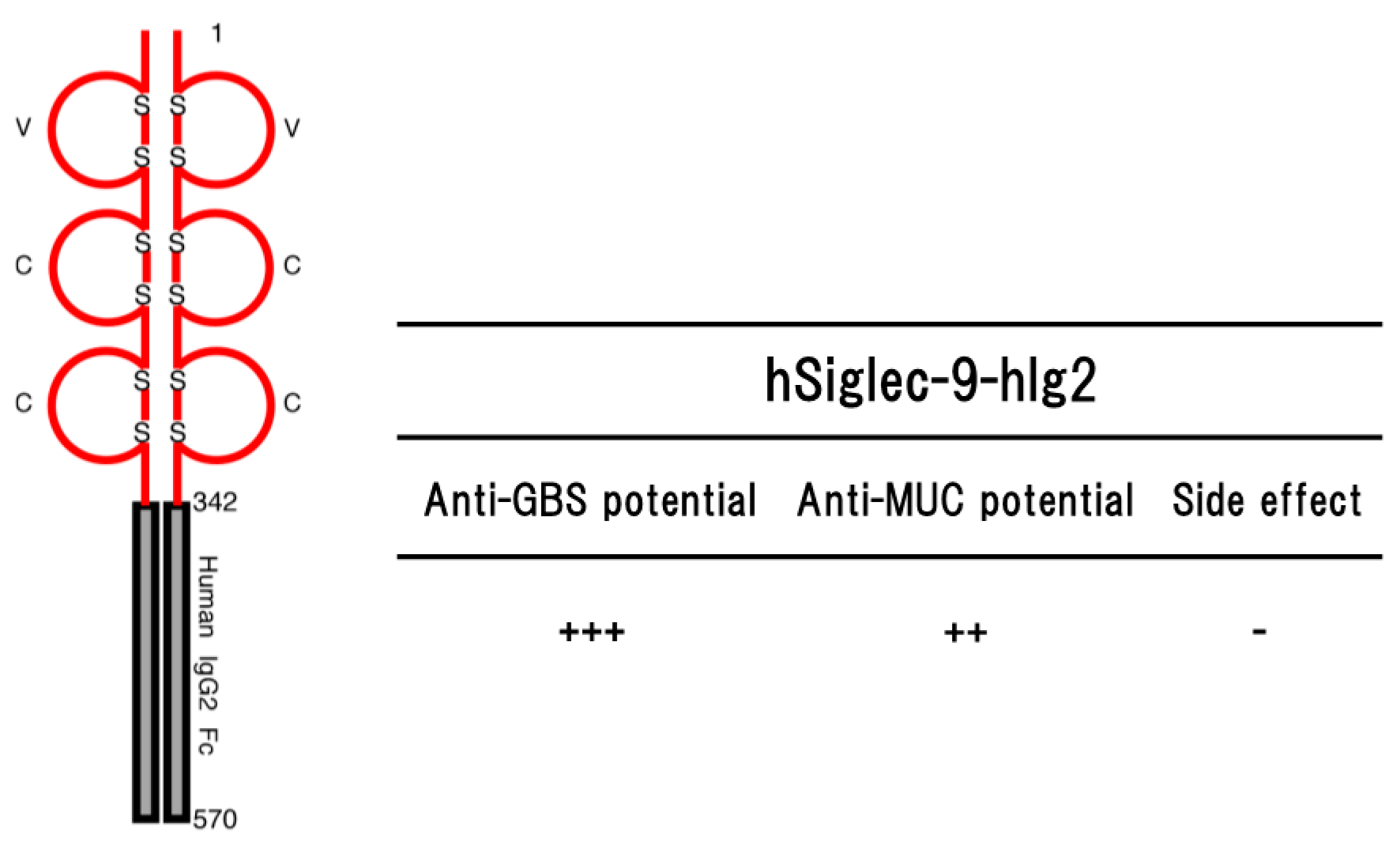
4. Siglec-9
4.1. Group B Streptococcus
4.2. MUC1 Tumor
5. Conclusions and Future Directions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Bhella, D. The role of cellular adhesion molecules in virus attachment and entry. Philos. Trans. R. Soc. Lond. B 2015, 370, 20140035. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Miyoshi, J.; Ikeda, W. Nectins and nectin-like molecules: Roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 2008, 9, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Nakanishi, H. Nectin and afadin: Novel organizers of intercellular junctions. J. Cell Sci. 2003, 116, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Mandai, K.; Nakanishi, H.; Satoh, A.; Obaishi, H.; Wada, M.; Nishioka, H.; Itoh, M.; Mizoguchi, A.; Aoki, T.; Fujimoto, T.; et al. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J. Cell Biol. 1997, 139, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Nakanishi, H.; Sakisaka, T.; Takahashi, K.; Mandai, K.; Nishimura, M.; Sasaki, T.; Takai, Y. Similar and differential behaviour between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells 1999, 4, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nakanishi, H.; Miyahara, M.; Mandai, K.; Satoh, K.; Satoh, A.; Nishioka, H.; Aoki, J.; Nomoto, A.; Mizoguchi, A.; et al. Nectin/PRR: An immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 1999, 145, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, M.; Nakanishi, H.; Takahashi, K.; Satoh-Horikawa, K.; Tachibana, K.; Takai, Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J. Biol. Chem. 2000, 275, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Horikawa, K.; Nakanishi, H.; Takahashi, K.; Miyahara, M.; Nishimura, M.; Tachibana, K.; Mizoguchi, A.; Takai, Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 2000, 275, 10291–10299. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, F.; Menotti, L.; Mirandola, P.; Lopez, M.; Campadelli-Fiume, G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 1998, 72, 9992–10002. [Google Scholar] [PubMed]
- Geraghty, R.J.; Krummenacher, C.; Cohen, G.H.; Eisenberg, R.J.; Spear, P.G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 1998, 280, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.S.; Geraghty, R.J.; Martinez, W.M.; Montgomery, R.I.; Whitbeck, J.C.; Xu, R.; Eisenberg, R.J.; Cohen, G.H.; Spear, P.G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology 1998, 246, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hu, D.; Bustos, T.; Zlotogora, J.; Richieri-Costa, A.; Helms, J.A.; Spritz, R.A. Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat. Genet. 2000, 25, 427–430. [Google Scholar] [PubMed]
- Campadelli-Fiume, G.; Arsenakis, M.; Farabegoli, F.; Roizman, B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J. Virol. 1988, 62, 159–167. [Google Scholar] [PubMed]
- Spear, P.G. Entry of alphaherpesviruses into cells. Semin. Virol. 1993, 4, 167–180. [Google Scholar] [CrossRef]
- Kwon, B.S.; Tan, K.B.; Ni, J.; Oh, K.O.; Lee, Z.H.; Kim, K.K.; Kim, Y.J.; Wang, S.; Gentz, R.; Yu, G.L.; et al. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J. Biol. Chem. 1997, 272, 14272–14276. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.I.; Warner, M.S.; Lum, B.J.; Spear, P.G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 1996, 87, 427–436. [Google Scholar] [CrossRef]
- Hsu, H.; Solovyev, I.; Colombero, A.; Elliott, R.; Kelley, M.; Boyle, W.J. ATAR, a novel tumor necrosis factor receptor family member, signal through TRAF2 and TRAF5. J. Biol. Chem. 1997, 272, 13471–13474. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.J.; Eisenberg, R.J.; Cohen, G.H. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 2000, 275, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mauri, D.N.; Ebner, R.; Montgomery, R.I.; Kochel, K.D.; Cheung, T.C.; Yu, G.-L.; Ruben, S.; Murphy, M.; Eisenberg, R.J.; Cohen, G.H.; et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity 1998, 8, 21–30. [Google Scholar] [CrossRef]
- Harrop, J.A.; McDonnell, P.C.; Brigham-Burke, M.; Lyn, S.D.; Minton, L.; Tan, K.B.; Dede, K.; Spampanato, J.; Silverman, C.; Hensley, P.; et al. Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J. Biol. Chem. 1998, 273, 27548–27556. [Google Scholar] [CrossRef] [PubMed]
- Marsters, S.A.; Ayres, T.M.; Skubatch, M.; Gray, C.L.; Rothe, M.; Ashkenazi, A. Herpes virus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-κB and AP-1. J. Biol. Chem. 1997, 272, 14029–14032. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, Y.; Ikehara, S.K.; Paulson, J.C. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J. Biol. Chem. 2004, 279, 43117–43125. [Google Scholar] [CrossRef] [PubMed]
- Avril, T.; Floyd, H.; Lopez, F.; Vivier, E.; Crocker, P.R. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J. Immunol. 2004, 173, 6841–6849. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Ishida, A.; Toda, M.; Akita, K.; Inoue, M.; Yamashita, K.; Watanabe, M.; Murata, T.; Usui, T.; Nakada, H. Immunomodulation of monocyte-derived dendritic cells through ligation of tumor-produced mucins to Siglec-9. Biochem. Biophys. Res. Commun. 2010, 402, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.S.; Connolly, S.A.; Krummenacher, C.; Eisenberg, R.J.; Cohen, G.H. Porcine HveC, a member of the highly conserved HveC/nectin 1 family, is a functional alphaherpesvirus receptor. Virology 2001, 281, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Krummenacher, C.; Nicola, A.V.; Whitbeck, J.C.; Lou, H.; Hou, W.; Lambris, J.D.; Geraghty, R.J.; Spear, P.G.; Cohen, G.H.; Eisenberg, R.J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 1998, 72, 7064–7074. [Google Scholar] [PubMed]
- Ono, E.; Amagai, K.; Taharaguchi, S.; Tomioka, Y.; Yoshino, S.; Watanabe, Y.; Cherel, P.; Houdebine, L.M.; Adam, M.; Eloit, M.; et al. Transgenic mice expressing a soluble form of porcine nectin-1/herpesvirus entry mediator C as a model for pseudorabies-resistant livestock. Proc. Natl. Acad. Sci. USA 2004, 101, 16150–16155. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Tomioka, Y.; Watanabe, Y.; Amagai, K.; Morimatsu, M.; Shinya, K.; Cherel, P. Comparison of the antiviral potentials among the pseudorabies-resistant transgenes encoding different soluble forms of porcine nectin-1 in transgenic mice. J. Gen. Virol. 2007, 88, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Tomioka, Y.; Watanabe, Y.; Amagai, K.; Taharaguchi, S.; Glenisson, J.; Cherel, P. The first immunoglobulin-like domain of porcine nectin-1 is sufficient to confer resistance to pseudorabies virus infection in transgenic mice. Arch. Virol. 2006, 151, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, Y.; Morimatsu, M.; Amagai, K.; Kuramochi, M.; Watanabe, Y.; Kouda, S.; Wada, T.; Kuboki, N.; Ono, E. A fusion protein consisting of the first immunoglobulin-like domain of porcine nectin-1 and Fc portion of human IgG1 provides a marked resistance against pseudorabies virus infection to the transgenic mice. Microbiol. Immunol. 2009, 53, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, F.; Lopez, M.; Dubreuil, P.; Campadelli-Fiume, G.; Menotti, L. Chimeric Nectin 1-poliovirus receptor molecules identify a Nectin 1 region functional in herpes simplex virus entry. J. Virol. 2001, 75, 7987–7994. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Yoshino, S.; Amagai, K.; Taharaguchi, S.; Kimura, C.; Morimoto, J.; Inobe, M.; Uenishi, T.; Uede, T. Enhanced resistance to herpes simplex virus type 1 infection in transgenic mice expressing a soluble form of herpesvirus entry mediator. Virology 2004, 320, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Tomioka, Y.; Kase, S.; Amagai, K.; Kuramochi, M.; Watanabe, Y.; Shinya, K.; Ohno, S.; Kouda, S.; Ono, E. Microphthalmia and lack of vitreous body in transgenic mice expressing the first immunoglobulin-like domain of nectin-1. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Irie, K.; Ishizaki, H.; Tanaka-Okamoto, M.; Morimoto, K.; Inoue, E.; Ohtsuka, T.; Miyoshi, J.; Takai, Y. Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development 2005, 132, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Ozaki-Kuroda, K.; Nakanishi, H.; Ohta, H.; Tanaka, H.; Kurihara, H.; Mueller, S.; Irie, K.; Ikeda, W.; Sakai, T.; Wimmer, E.; et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr. Biol. 2002, 12, 1145–1150. [Google Scholar] [CrossRef]
- Fabre, S.; Reymond, N.; Cocchi, F.; Menotti, L.; Dubreuil, P.; Campadelli-Fiume, G.; Lopez, M. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C′-C″-D β-strands of the nectin1 V domain. J. Biol. Chem. 2002, 277, 27006–27013. [Google Scholar] [CrossRef] [PubMed]
- Marasco, W.A.; Haseltine, W.A.; Chen, S.-Y. Design, intracellular expression, and activity of a human anti-human immunodeficiency virus type 1 gp120 single-chain antibody. Proc. Natl. Acad. Sci. USA 1993, 90, 7889–7893. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Khouri, Y.; Bagley, J.; Marasco, W.A. Combined intra- and extracellular immunization against human immunodeficiency virus type 1 infection with a human anti-gp120 antibody. Proc. Natl. Acad. Sci. USA 1994, 91, 5932–5936. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Amagai, K.; Yoshino, S.; Taharaguchi, S.; Inobe, M.; Uede, T. Resistance to pseudorabies virus infection in transformed cell lines expressing a soluble form of porcine herpesvirus entry mediator C. J. Gen. Virol. 2004, 85, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Krummenacher, C.; Baribaud, I.; Eisenberg, R.J.; Cohen, G.H. Cellular localization of nectin-1 and glycoprotein D during herpes simplex virus infection. J. Virol. 2003, 77, 8985–8999. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Cocchi, F.; Menotti, L.; Avitabile, E.; Dubreuil, P.; Campadelli-Fiume, G. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J. Virol. 2000, 74, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Martinez, W.M.; Spear, P.G. Structural features of nectin-2 (HveB) required for herpes simplex virus entry. J. Virol. 2001, 75, 11185–11195. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Tomioka, Y.; Ozaki, K.; Takeda, K.; Suyama, H.; Yamamoto, S.; Takakuwa, H.; Morimatsu, M.; Uede, T.; Ono, E. Comparison of the antiviral potential among soluble forms of herpes simplex virus type-2 glycoprotein D receptors, herpes virus entry mediator A, nectin-1 and nectin-2, in transgenic mice. J. Gen. Virol. 2017, 98, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Linehan, M.M.; Richman, S.; Krummenacher, C.; Eisenberg, R.J.; Cohen, G.H.; Iwasaki, A. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J. Virol. 2004, 78, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Grau, D.R.; Visalli, R.J.; Brandt, C.R. Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Investig. Ophthalmol. Vis. Sci. 1989, 30, 2474–2480. [Google Scholar] [PubMed]
- Smith, T.J.; Ackland-Berglund, C.E.; Leib, D.A. Herpes simplex virus virion host shutoff (vhs) activity alters periocular disease in mice. J. Virol. 2000, 74, 3598–3604. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, Y.; Fujimoto, Y.; Nakai, K.; Ozaki, K.; Yamamoto, S.; Suyama, H.; Morimatsu, M.; Ito, T.; Ono, E. A soluble form of human nectin-2 impairs exocrine secretion of pancreas and formation of zymogen granules in transgenic mice. Biochem. Biophys. Rep. 2016, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Ozaki, K.; Iwamori, N.; Takakuwa, H.; Ono, E. Accumulation of a soluble form of human nectin-2 is required for exerting the resistance against herpes simplex virus type 2 infection in transfected cells. Acta Virol. 2016, 60, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Nicola, A.V.; Ponce de Leon, M.; Xu, R.; Hou, W.; Whitbeck, J.C.; Krummenacher, C.; Montgomery, R.I.; Spear, P.G.; Eisenberg, R.J.; Cohen, G.H. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J. Virol. 1998, 72, 3595–3601. [Google Scholar] [PubMed]
- Whitbeck, J.C.; Peng, C.; Lou, H.R.; Xu, R.; Willis, S.H.; Ponce de Leon, M.; Peng, T.; Nicola, A.V.; Montgomery, R.I.; Warner, M.S.; et al. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the TNFR superfamily and a mediator of HSV entry. J. Virol. 1997, 71, 6083–6093. [Google Scholar] [PubMed]
- Yoon, M.; Zago, A.; Shukla, D.; Spear, P.G. Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1. J. Virol. 2003, 77, 9221–9231. [Google Scholar] [CrossRef] [PubMed]
- Bossen, C.; Ingold, K.; Tardivel, A.; Bodmer, J.L.; Gaide, O.; Hertig, S.; Ambrose, C.; Tschopp, J.; Schneider, P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J. Biol. Chem. 2006, 281, 13964–13971. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M.; Lin, E.; Susmarski, N.; Yoon, M.; Zago, A.; Ware, C.F.; Pfeffer, K.; Miyoshi, J.; Takai, Y.; Spear, P.G. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe 2007, 2, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.J.; Banisadr, G.; Glajch, K.; Maurer, U.E.; Grünewald, K.; Miller, R.J.; Osten, P.; Spear, P.G. Infection of neurons and encephalitis after intracranial inoculation of herpes simplex virus requires the entry receptor nectin-1. Proc. Natl. Acad. Sci. USA 2009, 106, 17916–17920. [Google Scholar] [CrossRef] [PubMed]
- Krummenacher, C.; Baribaud, F.; Ponce de Leon, M.; Baribaud, I.; Whitbeck, J.C.; Xu, R.; Cohen, G.H.; Eisenberg, R.J. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology 2004, 322, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Nelson, C.A.; Sedý, J.R. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat. Rev. Immunol. 2006, 6, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chun, T.; Lo, J.C.; Wu, Q.; Wang, Y.; Foster, A.; Roca, K.; Chen, M.; Tamada, K.; Chen, L.; et al. The critical role of LIGHT, a TNF family member, in T cell development. J. Immunol. 2001, 167, 5099–5105. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J. Herpes simplex viruses. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; pp. 2461–2510. [Google Scholar]
- Corey, L.; Wald, A.; Celum, C.L.; Quinn, T.C. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: A review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 2004, 35, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Serwadda, D.; Gray, R.H.; Sewankambo, N.K.; Wabwire-Mangen, F.; Chen, M.Z.; Quinn, T.C.; Lutalo, T.; Kiwanuka, N.; Kigozi, G.; Nalugoda, F.; et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: A nested case-control study in Rakai, Uganda. J. Infect. Dis. 2003, 188, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Link, K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: A meta-analysis. J. Infect. Dis. 2002, 185, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; Pfister, J.R.; Spear, S.J. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex. Transm. Dis. 2003, 30, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Vyse, A.J.; Gay, N.J.; Slomka, M.J.; Gopal, R.; Gibbs, T.; Morgan-Capner, P.; Brown, D.W. The burden of infection with HSV-1 and HSV-2 in England and Wales: Implications for the changing epidemiology of genital herpes. Sex. Transm. Infect. 2000, 76, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Wald, A. Genital HSV-1 infections. Sex. Transm. Infect. 2006, 82, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Carlin, A.F.; Uchiyama, S.; Chang, Y.C.; Lewis, A.L.; Nizet, V.; Varki, A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 2009, 113, 3333–3336. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S. Issues of antimicrobial resistance in group B streptococcus in the era of intrapartum antibiotic prophylaxis. Semin. Pediatr. Infect. Dis. 2006, 17, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Schuchat, A. Perinatal group B streptococcal disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, M.C.; Whitney, C.G.; Messonnier, N.E.; Zell, E.R.; Lynfield, R.; Hadler, J.L.; Harrison, L.H.; Farley, M.M.; Reingold, A.; Bennett, N.M.; et al. Bacterial meningitis in the United States, 1998–2007. N. Engl. J. Med. 2011, 364, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Ferrieri, P.; Cleary, P.P.; Seeds, A.E. Epidemiology of group-B streptococcal carriage in pregnant women and newborn infants. J. Med. Microbiol. 1977, 10, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Galask, R.P.; Varner, M.W.; Petzold, C.R.; Wilbur, S.L. Bacterial attachment to the chorioamniotic membranes. Am. J. Obstet. Gynecol. 1984, 148, 915–928. [Google Scholar] [CrossRef]
- Rubens, C.E.; Wessels, M.R.; Heggen, L.M.; Kasper, D.L. Transposon mutagenesis of type III group B Streptococcus: Correlation of capsule expression with virulence. Proc. Natl. Acad. Sci. USA 1987, 84, 7208–7212. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, A.O.; Rote, N.S.; Santos, J.I.; Hill, H.R. Assessment of the virulence factors of group B streptococci: Correlation with sialic acid content. J. Infect. Dis. 1983, 147, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.R.; Rubens, C.E.; Benedi, V.J.; Kasper, D.L. Definition of a bacterial virulence factor: Sialylation of the group B streptococcal capsule. Proc. Natl. Acad. Sci. USA 1989, 86, 8983–8987. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.B.; Kasper, D.L.; Pangburn, M.K.; Wessels, M.R. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect. Immun. 1992, 60, 3986–3993. [Google Scholar] [PubMed]
- Takahashi, S.; Aoyagi, Y.; Adderson, E.E.; Okuwaki, Y.; Bohnsack, J.F. Capsular sialic acid limits C5a production on type III group B streptococci. Infect. Immun. 1999, 67, 1866–1870. [Google Scholar] [PubMed]
- Slotved, H.-C.; Kong, F.; Lambertsen, L.; Sauer, S.; Gilbert, G.L. Serotype IX, a proposed new Streptococcus agalactiae serotype. J. Clin. Microbiol. 2007, 45, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Olson, J.; Beasley, F.C.; Tung, C.; Zhang, J.; Crocker, P.R.; Varki, A.; Nizet, V. Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo. PLoS Pathog. 2014, 10, e1003846. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yamamoto, S.; Ozaki, K.; Tomioka, Y.; Suyama, H.; Morimatsu, M.; Nishijima, K.; Yoshida, S.; Ono, E. A soluble form of Siglec-9 provides a resistance against Group B Streptococcus (GBS) infection in transgenic mice. Microb. Pathog. 2016, 99, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Winslow, G.M.; Yager, E.; Li, J.S. Mechanisms of humoral immunity during Ehrlichia chaffeensis infection. Ann. N. Y. Acad. Sci. 2003, 990, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Overdijk, M.B.; Verploegen, S.; Buijsse, A.O.; Vink, T.; Leusen, J.H.; Bleeker, W.K.; Parren, P.W. Crosstalk between human IgG isotypes and murine effector cells. J. Immunol. 2012, 189, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- Garred, P.; Michaelsen, T.E.; Aase, A. The IgG subclass pattern of complement activation depends on epitope density and antibody and complement concentration. Scand. J. Immunol. 1989, 30, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, T.E.; Garred, P.; Aase, A. Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur. J. Immunol. 1991, 21, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Weisman, L.; Troendle, J.; Adams, K. Prematurity is the major risk factor for late-onset group B Streptococcus disease. J. Infect. Dis. 2003, 188, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Phares, C.R.; Lynfield, R.; Farley, M.M.; Mohle-Boetani, J.; Harrison, L.H.; Petit, S.; Craig, A.S.; Schaffner, W.; Zansky, S.M.; Gershman, K.; et al. Active Bacterial Core surveillance/Emerging Infections Program Network. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 2008, 299, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Schuchat, A. Group B streptococcus. Lancet 1999, 353, 51–56. [Google Scholar] [CrossRef]
- Nuccitelli, A.; Rinaudo, C.D.; Maione, D. Group B Streptococcus vaccine: State of the art. Ther. Adv. Vaccines 2015, 3, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, Y.; Morimatsu, M.; Nishijima, K.; Usui, T.; Yamamoto, S.; Suyama, H.; Ozaki, K.; Ito, T.; Ono, E. A soluble form of Siglec-9 provides an antitumor benefit against mammary tumor cells expressing MUC1 in transgenic mice. Biochem. Biophys. Res. Commun. 2014, 450, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, M.J.; Kruijshaar, L.; Buijs, F.; van Meijer, M.; Litvinov, S.V.; Hilkens, J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J. Biol. Chem. 1992, 267, 6171–6177. [Google Scholar] [PubMed]
- Levitin, F.; Baruch, A.; Weiss, M.; Stiegman, K.; Hartmann, M.L.; Yoeli-Lerner, M.; Ziv, R.; Zrihan-Licht, S.; Shina, S.; Gat, A.; et al. A novel protein derived from the MUC1 gene by alternative splicing and frameshifting. J. Biol. Chem. 2005, 280, 10655–10663. [Google Scholar] [CrossRef] [PubMed]
- Macao, B.; Johansson, D.G.A.; Hansson, G.C.; Härd, T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat. Struct. Mol. Biol. 2006, 13, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.; Inghirami, G.; Abe, M.; Hayes, D.; Justi-Wheeler, H.; Schlom, J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma 1984, 3, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, J.; van der Valk, S.W.; Vos, H.L.; Sonnenberg, A.; Hilkens, J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J. Cell Biol. 1995, 129, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Boyce, R.W.G.; Abd El-Rehim, D.; Kurien, T.; Green, A.R.; Paish, E.C.; Robertson, J.F.; Ellis, I.O. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod. Pathol. 2005, 18, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Rahn, J.J.; Dabbagh, L.; Pasdar, M.; Hugh, J.C. The importance of MUC1 cellular localization in patients with breast carcinoma: An immunohistologic study of 71 patients and review of the literature. Cancer 2001, 91, 1973–1982. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Walsh, M.D.; Hohn, B.G.; Ward, B.G.; Wright, R.G. Prognostic significance of MUC1 epithelial mucin expression in breast cancer. Hum. Pathol. 1995, 26, 432–439. [Google Scholar] [CrossRef]
- Kufe, D.W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, S.; Goto, M.; Yamada, N.; Higashi, M.; Nomoto, M. Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics 2008, 8, 3329–3341. [Google Scholar] [CrossRef] [PubMed]
- Raina, D.; Kharbanda, S.; Kufe, D. The MUC1 oncoprotein activates the anti-apoptotic phosphoinositide 3-kinase/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J. Biol. Chem. 2004, 279, 20607–20612. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Huang, L.; Kufe, D. MUC1 oncoprotein activates the FOXO3a transcription factor in a survival response to oxidative stress. J. Biol. Chem. 2004, 279, 45721–45727. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xu, H.; Kufe, D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell 2005, 7, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W. MUC1-C oncoprotein as a target in breast cancer: Activation of signaling pathways and therapeutic approaches. Oncogene 2013, 32, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, D.; Liu, D.; Yin, L.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein blocks glycogen synthase kinase 3β-mediated phosphorylation and degradation of β-catenin. Cancer Res. 2005, 65, 10413–10422. [Google Scholar] [CrossRef] [PubMed]
- Pegram, M.D.; Borges, V.F.; Ibrahim, N.; Fuloria, J.; Shapiro, C.; Perez, S.; Wang, K.; Schaedli Stark, F.; Courtenay Luck, N. Phase I dose escalation pharmacokinetic assessment of intravenous humanized anti-MUC1 antibody AS1402 in patients with advanced breast cancer. Breast Cancer Res. 2009, 11, R73. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K.; Yariz, K.O.; Bondarenko, I.; Manikhas, A.; Semiglazov, V.; Alyasova, A.; Komisarenko, V.; Shparyk, Y.; Murray, J.L.; Jones, D.; et al. Randomized phase II trial of letrozole plus anti-MUC1 antibody AS1402 in hormone receptor-positive locally advanced or metastatic breast cancer. Clin. Cancer Res. 2011, 17, 6822–6830. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Arlen, P.M.; Tsang, K.-Y.; Yokokawa, J.; Palena, C.; Poole, D.J.; Remondo, C.; Cereda, V.; Jones, J.L.; Pazdur, M.P.; et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin. Cancer Res. 2008, 14, 3060–3069. [Google Scholar] [CrossRef] [PubMed]
- Mohebtash, M.; Tsang, K.-Y.; Madan, R.A.; Huen, N.Y.; Poole, D.J.; Jochems, C.; Jones, J.; Ferrara, T.; Heery, C.R.; Arlen, P.M.; et al. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin. Cancer Res. 2011, 17, 7164–7173. [Google Scholar] [CrossRef] [PubMed]
- Raina, D.; Ahmad, R.; Joshi, M.D.; Yin, L.; Wu, Z.; Kawano, T.; Vasir, B.; Avigan, D.; Kharbanda, S.; Kufe, D. Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009, 69, 5133–5141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Rajabi, H.; Kufe, D. Mucin 1 C-terminal subunit oncoprotein is a target for small-molecule inhibitors. Mol. Pharmacol. 2011, 79, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.D.; Ahmad, R.; Yin, L.; Raina, D.; Rajabi, H.; Bubley, G.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein is a druggable target in human prostate cancer cells. Mol. Cancer Ther. 2009, 8, 3056–3065. [Google Scholar] [CrossRef] [PubMed]
- Tanida, S.; Akita, K.; Ishida, A.; Mori, Y.; Toda, M.; Inoue, M.; Ohta, M.; Yashiro, M.; Sawada, T.; Hirakawa, K.; et al. Binding of the Sialic Acid-binding Lectin, Siglec-9, to the Membrane Mucin, MUC1, Induces Recruitment of β-Catenin and Subsequent Cell Growth. J. Biol. Chem. 2013, 288, 31842–31852. [Google Scholar] [CrossRef] [PubMed]
- Yurugi, H.; Tanida, S.; Akita, K.; Ishida, A.; Toda, M.; Nakada, H. Prohibitins function as endogenous ligands for Siglec-9 and negatively regulate TCR signaling upon ligation. Biochem. Biophys. Res. Commun. 2013, 434, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, B.; Krantz, M.J.; Reddish, M.A.; Longenecker, B.M. Cancer-associated MUC1 mucin inhibits human T-cell proliferation, which is reversible by IL-2. Nat. Med. 1998, 4, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Gimmi, C.D.; Morrison, B.W.; Mainprice, B.A.; Gribben, J.G.; Boussiotis, V.A.; Freeman, G.J.; Park, S.Y.; Watanabe, M.; Gong, J.; Hayes, D.F.; et al. Breast cancer-associated antigen, DF3/MUC1, induces apoptosis of activated human T cells. Nat. Med. 1996, 2, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, E.; Uede, T. Implication of Soluble Forms of Cell Adhesion Molecules in Infectious Disease and Tumor: Insights from Transgenic Animal Models. Int. J. Mol. Sci. 2018, 19, 239. https://doi.org/10.3390/ijms19010239
Ono E, Uede T. Implication of Soluble Forms of Cell Adhesion Molecules in Infectious Disease and Tumor: Insights from Transgenic Animal Models. International Journal of Molecular Sciences. 2018; 19(1):239. https://doi.org/10.3390/ijms19010239
Chicago/Turabian StyleOno, Etsuro, and Toshimitsu Uede. 2018. "Implication of Soluble Forms of Cell Adhesion Molecules in Infectious Disease and Tumor: Insights from Transgenic Animal Models" International Journal of Molecular Sciences 19, no. 1: 239. https://doi.org/10.3390/ijms19010239



