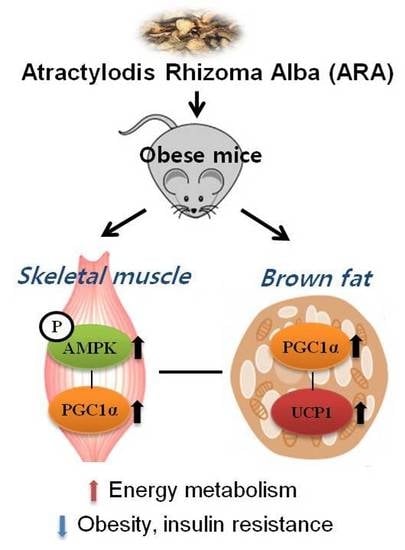
The Root of Atractylodes macrocephala Koidzumi Prevents Obesity and Glucose Intolerance and Increases Energy Metabolism in Mice
Abstract
:1. Introduction
2. Results
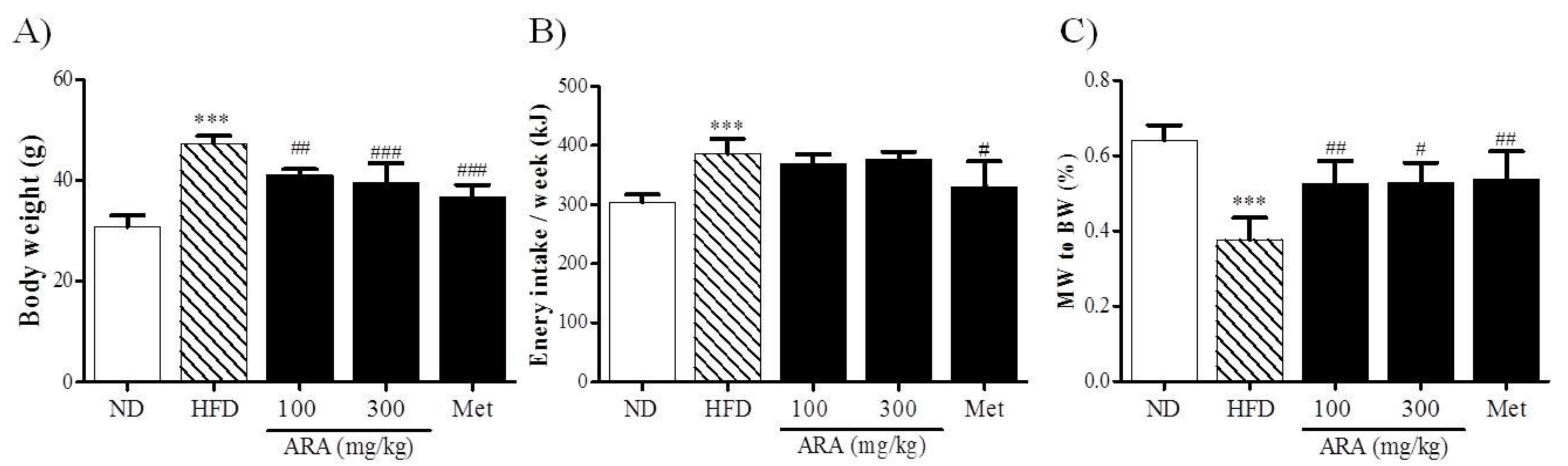
2.1. ARA Reduced Body Weight Gain
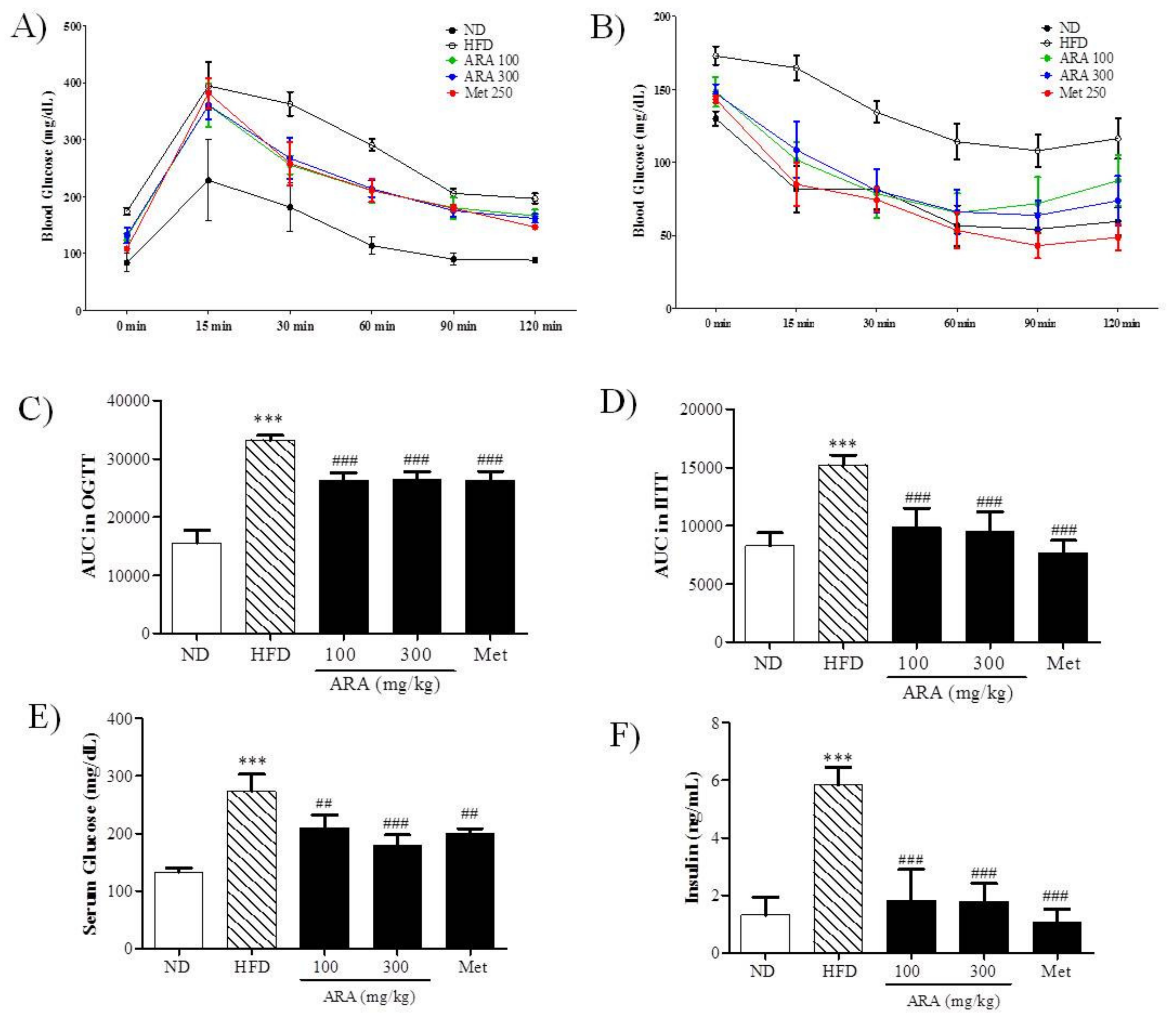
2.2. ARA Ameliorated Obesity-Induced Glucose Intolerance
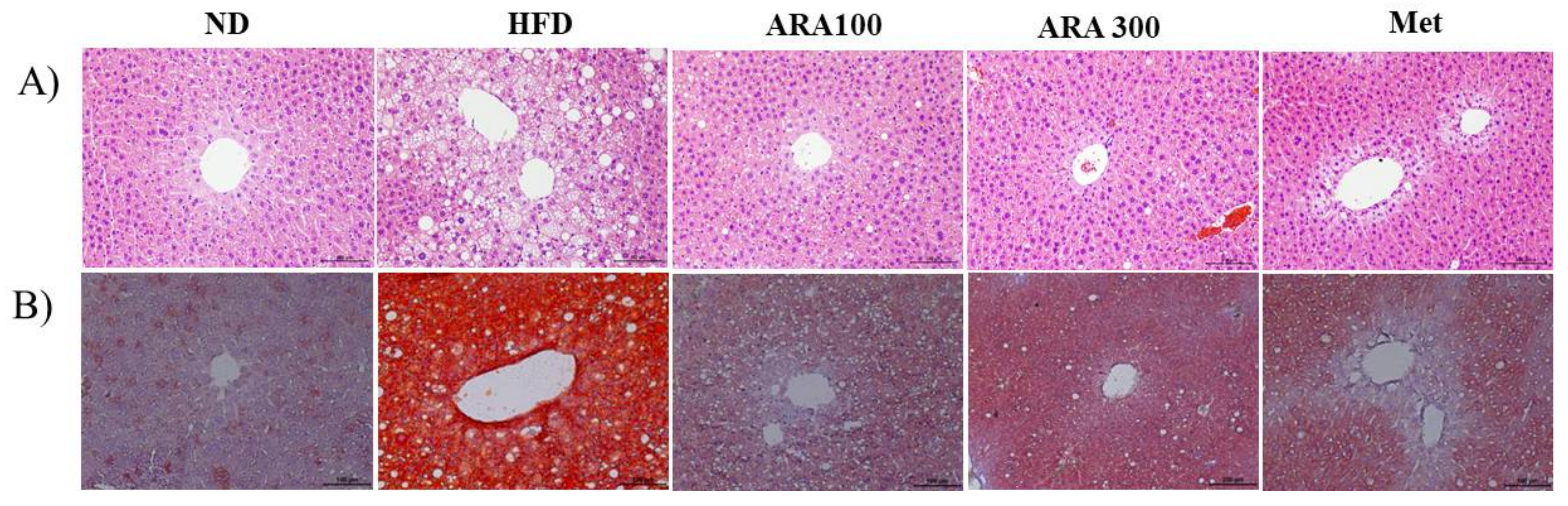
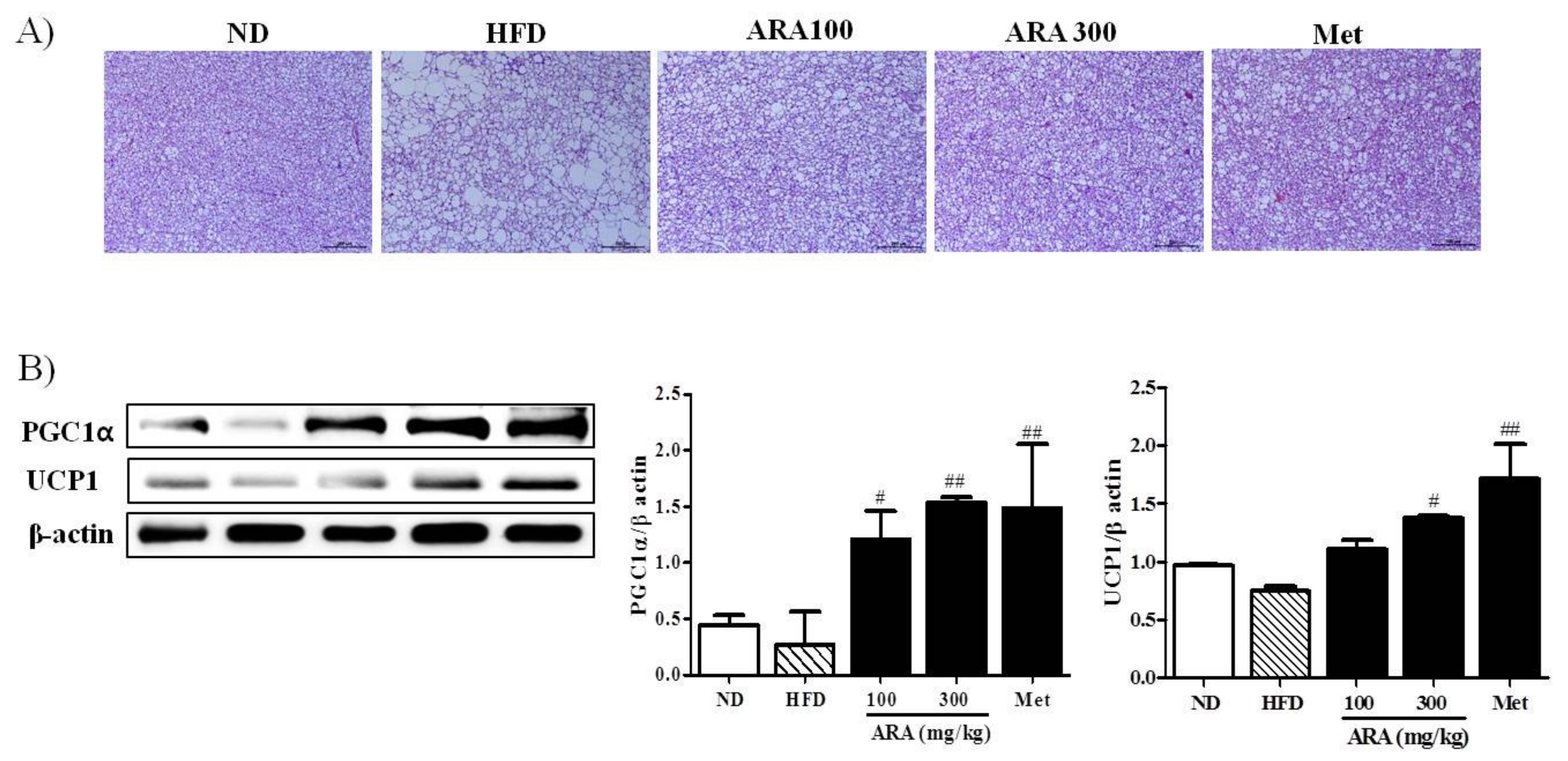
2.3. ARA Reduced Obesity-Induced Lipid Accumulation and Improved Serum Parameters
2.4. ARA Regulated Energy Metabolism in Skeletal Muscle
2.5. ARA Increased Energy Metabolism in Brown Adipose Tissue
3. Discussion
4. Materials and Methods
4.1. Preparation of ARA Extract
4.2. Animals and Experimental Design
4.3. Oral Glucose Tolerance Testing
4.4. Intraperitoneal Insulin Tolerance Testing
4.5. Serum Analysis
4.6. Histology Analysis
4.7. Lipid Accumulation Analysis in Liver
4.8. Western Blot
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AMPK | Adenosine monophosphate-activated protein kinase |
| ARA | Atractylodis rhizoma alba |
| AST | Aspartate aminotransferase |
| AUC | Area under the curve |
| BAT | Brown adipose tissue |
| BW | Body weight |
| CAT | Catalase |
| H&E | Hematoxylin and eosin |
| HDL-C | High-density lipoprotein cholesterol |
| HFD | High-fat diet |
| HO-1 | Heme oxygenase-1 |
| IITT | Intraperitoneal insulin tolerance test |
| Met | Metformin |
| MW | Muscle weight |
| ND | Normal diet |
| NRF1 | Nuclear respiratory factor 1 |
| OGTT | Oral glucose tolerance test |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
| SOD | Superoxide dismutase |
| TC | Total cholesterol |
| TCM | Traditional Chinese medicine |
| TFAM | Mitochondrial transcription factor |
| TG | Triglycerides |
| TKM | Traditional Korean medicine |
| UCP1 | Uncoupling protein 1 |
References
- Moller, D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 2001, 414, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-H.; Cypess, A.M.; Kahn, C.R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Duchamp, C.; Barre, H. Skeletal muscle as the major site of nonshivering thermogenesis in cold-acclimated ducklings. Am. J. Physiol. 1993, 265, R1076–R1083. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Kahn, C.R. Brown fat as a therapy for obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Krssak, M.; Petersen, K.F.; Dresner, A.; DiPietro, L.; Vogel, S.; Rothman, D.; Shulman, G.; Roden, M. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1H NMR spectroscopy study. Diabetologia 1999, 42, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-T.; Kuang, H.-X.; Zhao, J. Why Is Qi-Invigorating Therapy in Chinese Medicine Suitable for Mitochondrial Diseases? A Bioenergetic Perspective; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Wallace, D.C. Mitochondria as chi. Genetics 2008, 179, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Shin, S.W.; Kim, H.J. Obesity from the viewpoint of metabolic rate. J. Korean Med. Obes. Res. 2003, 3, 95–105. [Google Scholar]
- Li, X.-T. Investigation on the Mechanism of Qi-Invigoration from a Perspective of Effects of Sijunzi Decoction on Mitochondrial Energy Metabolism; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Lee, S.H.; Lee, H.J.; Lee, Y.H.; Lee, B.W.; Cha, B.S.; Kang, E.S.; Ahn, C.W.; Park, J.S.; Kim, H.J.; Lee, E.Y. Korean red ginseng (Panax ginseng) improves insulin sensitivity in high fat fed Sprague-Dawley rats. Phytother. Res. 2012, 26, 142–147. [Google Scholar] [CrossRef] [PubMed]
- The Korea Association of Herbology. Herbology; Younglimsa: Seoul, Korea, 1991; p. 536. [Google Scholar]
- Huang, Y.; Wang, L.; Wang, S.; Cai, F.; Zheng, G.; Lu, A.; Yu, X.; Jiang, M. Treatment principles of obesity with chinese herbal medicine: Literature analysis by text mining. Engineering 2013, 5, 7–11. [Google Scholar] [CrossRef]
- Wang, J.-H.; Bose, S.; Kim, H.-G.; Han, K.-S.; Kim, H. Fermented rhizoma Atractylodis macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats. Sci. Rep. 2015, 5, 8391. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Kim, M.; Oh, S.D.; Lee, S.-M.; Sun, B.; Choi, G.S.; Kim, S.-K.; Bae, H.; Kang, C.; Min, B.-I. Effects of Atractylodes macrocephala Koidzumi rhizome on 3T3-L1 adipogenesis and an animal model of obesity. J. Ethnopharmacol. 2011, 137, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Kang, S.Y.; Oh, T.W.; Kumar, R.V.; Jung, H.W.; Park, Y.-K. The roots of Atractylodes macrocephala Koidzumi enhanced glucose and lipid metabolism in C2C12 myotubes via mitochondrial regulation. Evid. Based Complement Alternat. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Jung, H.W.; Kang, S.Y.; Park, Y.-K. Atractylenolide III enhances energy metabolism by increasing the SIRT-1 and PGC1α expression with AMPK phosphorylation in C2C12 mouse skeletal muscle cells. Biol. Pharm. Bull. 2017, 40, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Li Ji, L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Plutzky, J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab. J. 2016, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. The global epidemic of obesity: An overview. Epidemiol. Rev. 2007, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: A systematic review. BMC Med. 2016, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Daneschvar, H.L.; Aronson, M.D.; Smetana, G.W. FDA-approved anti-obesity drugs in the United States. Am. J. Med. 2016, 129, 879.e1–879.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 421–433. [Google Scholar] [CrossRef]
- Jung, H.W.; Kang, A.N.; Kang, S.Y.; Park, Y.-K.; Song, M.Y. The root extract of Pueraria lobata and its main compound, puerarin, prevent obesity by increasing the energy metabolism in skeletal muscle. Nutrients 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Rouru, J.; Huupponen, R.; Pesonen, U.; Koulu, M. Subchronic treatment with metformin produces anorectic effect and reduces hyperinsulinemia in genetically obese Zucker rats. Life Sci. 1992, 50, 1813–1820. [Google Scholar] [CrossRef]
- Rouru, J.; Pesonen, U.; Koulu, M.; Huupponen, R.; Santti, E.; Virtanen, K.; Rouvari, T.; Jhanwar-Uniyal, M. Anorectic effect of metformin in obese Zucker rats: Lack of evidence for the involvement of neuropeptide Y. Eur. J. Pharmacol. 1995, 273, 99–106. [Google Scholar] [CrossRef]
- Lv, W.-S.; Wen, J.-P.; Li, L.; Sun, R.-X.; Wang, J.; Xian, Y.-X.; Cao, C.-X.; Wang, Y.-L.; Gao, Y.-Y. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res. 2012, 1444, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.-S.; Lindberg, R.A. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. PGC-1α: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, J.D. PGC-1 coactivators in the control of energy metabolism. Acta Biochim. Biophys. Sin. 2011, 43, 248–257. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.M.; Holloway, G.P.; Steinberg, G.R. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: Implications for obesity. Mol. Cell. Endocrinol. 2013, 366, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of Pgc-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. Pgc-1α protects skeletal muscle from atrophy by suppressing foxo3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014, 537, 93–122. [Google Scholar] [CrossRef] [PubMed]

| Serum | ND | HFD | ARA 100 | ARA 300 | Met |
|---|---|---|---|---|---|
| ALT (IU/L) | 8.96 ± 4.95 | 54.84 ± 16.73 *** | 12.88 ± 7.76 ### | 10.53 ± 4.74 ### | 11.95 ± 1.88 ### |
| AST (IU/L) | 44.06 ± 1.40 | 58.03 ± 7.45 * | 47.16 ± 4.72 * | 49.02 ± 7.88 | 61.76 ± 12.87 |
| ALP (IU/L) | 8.42 ± 0.25 | 8.63 ± 0.86 | 7.54 ± 0.45 | 7.32 ± 0.77 | 7.99 ± 0.62 |
| TG (mg/dL) | 42.75 ± 0.63 | 42.95 ± 0.39 | 41.75 ± 0.76 | 40.38 ± 0.88 ### | 40.70 ± 0.31 ### |
| TC (mg/dL) | 225.86 ± 9.84 | 276.61 ± 27.01 * | 223.59 ± 11.09 ## | 204.46 ± 26.11 ### | 182.55 ± 7.85 ### |
| HDL-C (mg/dL) | 73.26 ± 8.25 | 107.52 ± 12.68 ** | 82.87 ± 5.31 | 94.48 ± 18.75 | 69.96 ± 5.36 ### |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.Y.; Lim, S.-K.; Wang, J.-H.; Kim, H. The Root of Atractylodes macrocephala Koidzumi Prevents Obesity and Glucose Intolerance and Increases Energy Metabolism in Mice. Int. J. Mol. Sci. 2018, 19, 278. https://doi.org/10.3390/ijms19010278
Song MY, Lim S-K, Wang J-H, Kim H. The Root of Atractylodes macrocephala Koidzumi Prevents Obesity and Glucose Intolerance and Increases Energy Metabolism in Mice. International Journal of Molecular Sciences. 2018; 19(1):278. https://doi.org/10.3390/ijms19010278
Chicago/Turabian StyleSong, Mi Young, Soo-Kyoung Lim, Jing-Hua Wang, and Hojun Kim. 2018. "The Root of Atractylodes macrocephala Koidzumi Prevents Obesity and Glucose Intolerance and Increases Energy Metabolism in Mice" International Journal of Molecular Sciences 19, no. 1: 278. https://doi.org/10.3390/ijms19010278
APA StyleSong, M. Y., Lim, S. -K., Wang, J. -H., & Kim, H. (2018). The Root of Atractylodes macrocephala Koidzumi Prevents Obesity and Glucose Intolerance and Increases Energy Metabolism in Mice. International Journal of Molecular Sciences, 19(1), 278. https://doi.org/10.3390/ijms19010278