Pituitary Gonadotropins, Prolactin and Growth Hormone Differentially Regulate AQP1 Expression in the Porcine Ovarian Follicular Cells
Abstract
:1. Introduction
2. Results
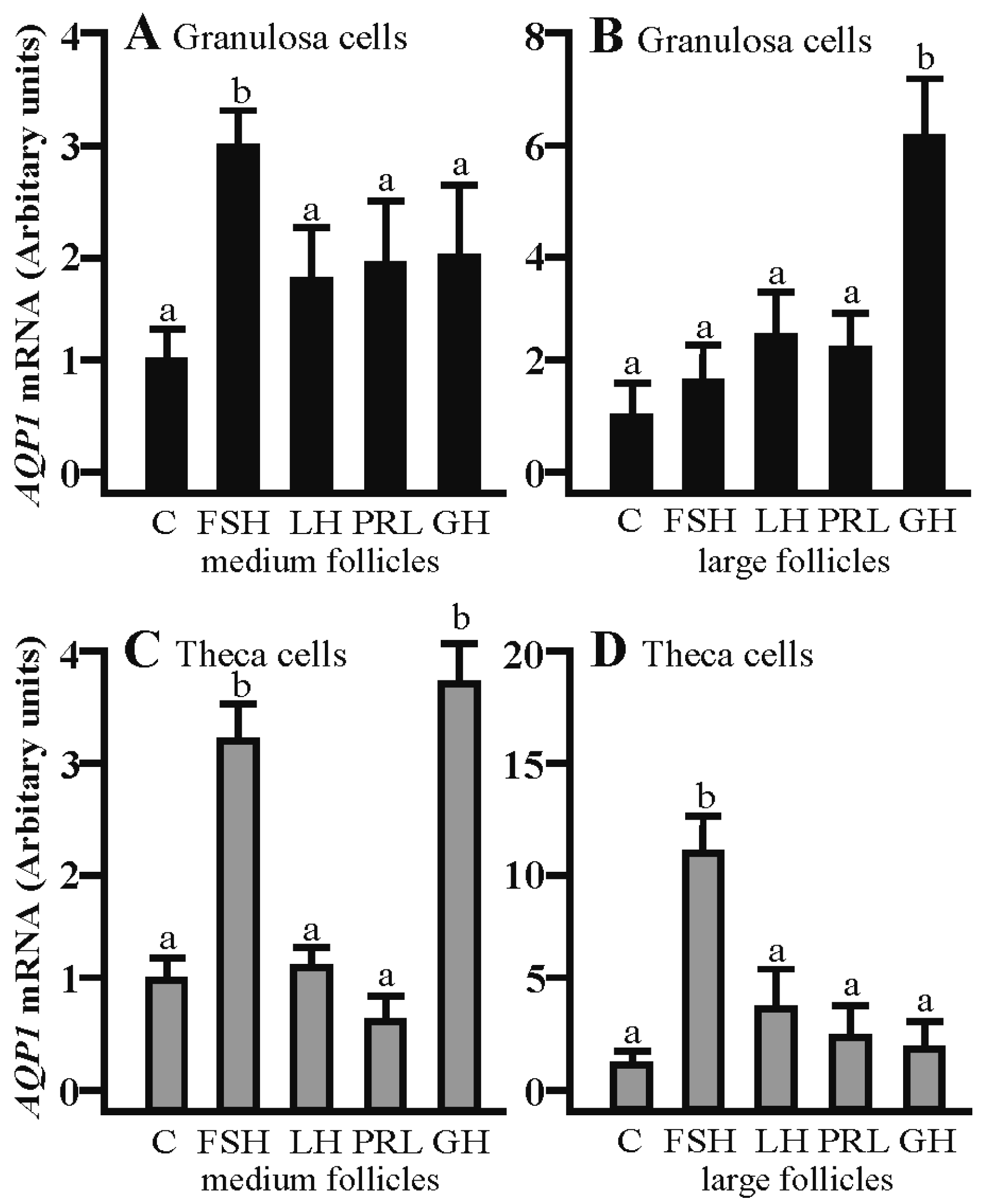
2.1. The Effects of FSH, LH, PRL and GH on Aqp1 mRNA Expression in the Gc and Tc Cells from the Medium and Large Follicles
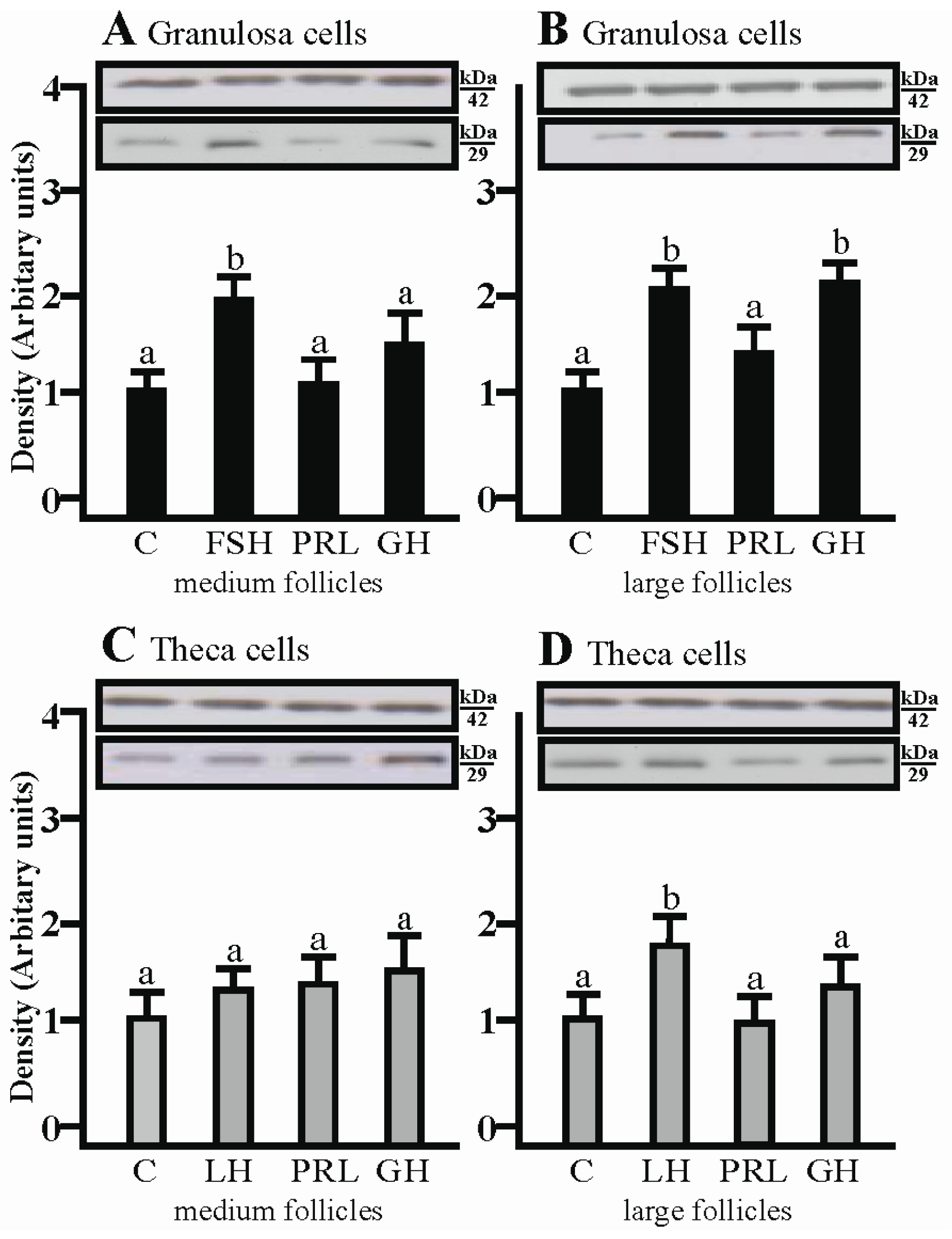
2.2. The Effects of FSH, LH, PRL and GH on AQP1 Protein Expression in the Gc and Tc Cells from Medium and Large Follicles
2.3. The Effects of FSH, LH, PRL and GH on Aqp1 mRNA Expression in the Co-Culture of Gc and Tc Cells from Medium and Large Follicles
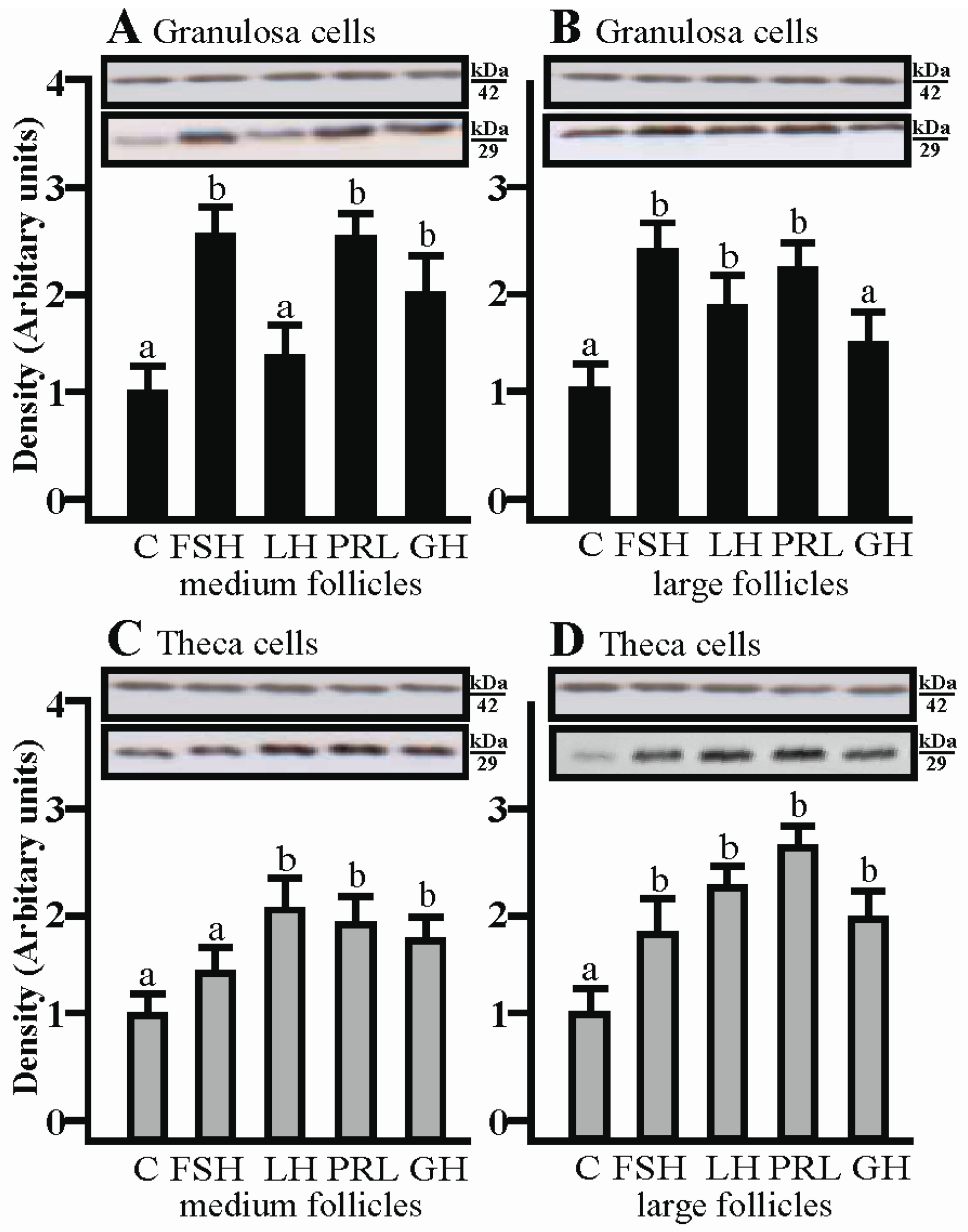
2.4. The Effects of FSH, LH, PRL and GH on AQP1 Protein Expression in the Co-Culture of Gc and Tc Cells from Medium and Large Follicles
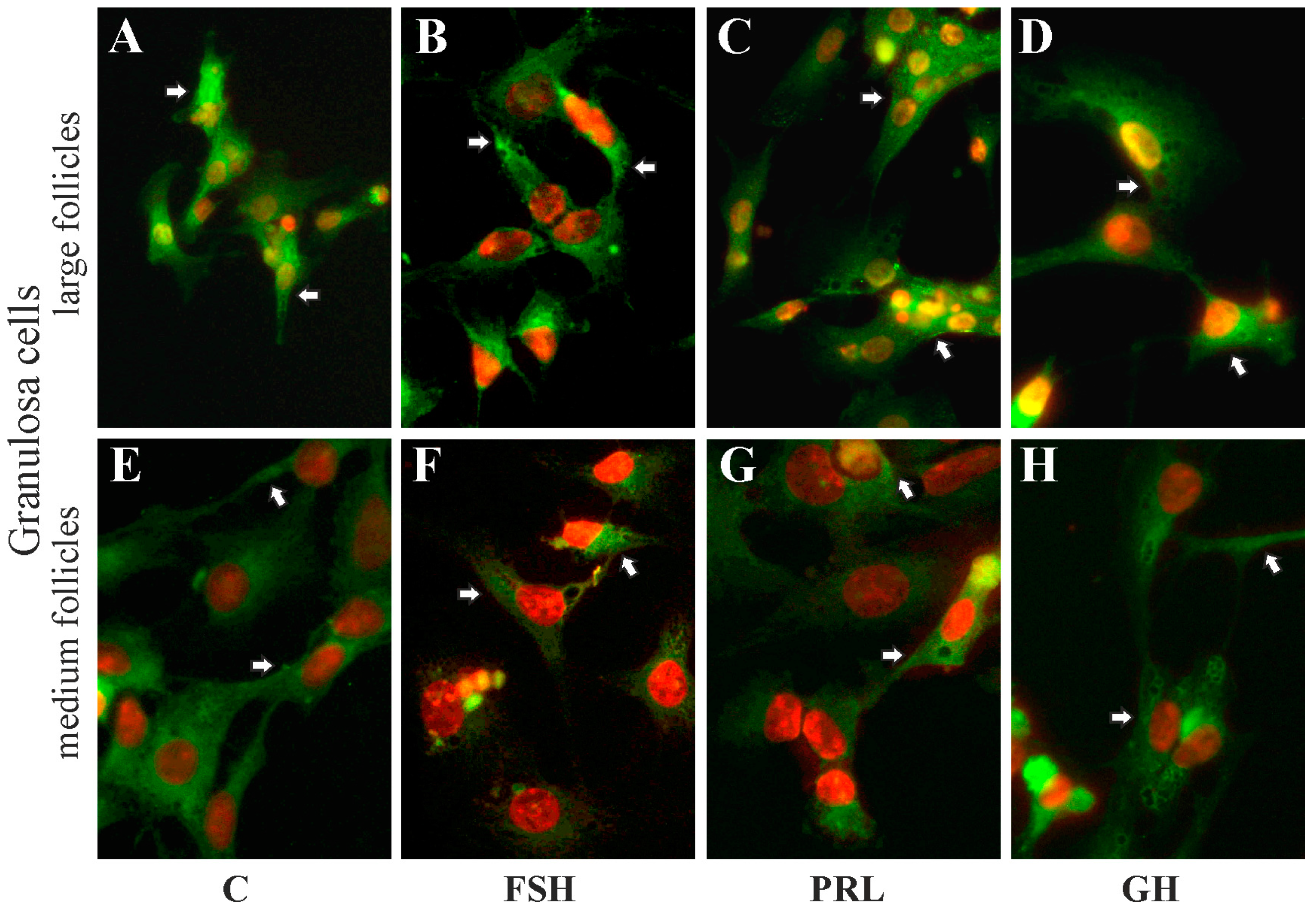
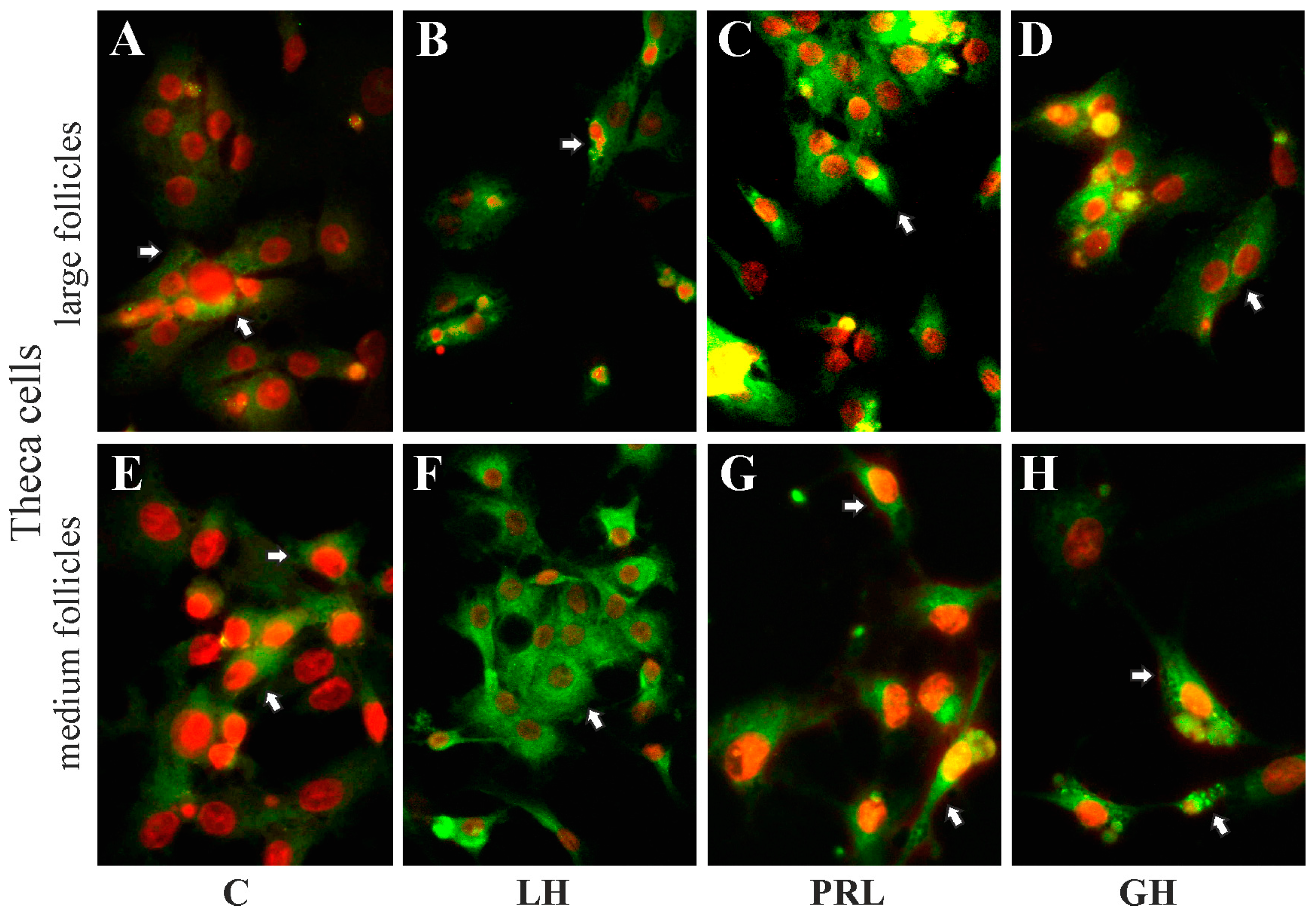
2.5. The Subcellular Expression of AQP1 Protein in Porcine Gc and Tc from the Medium and Large Follicles after In Vitro Treatment with FSH, LH, PRL and GH
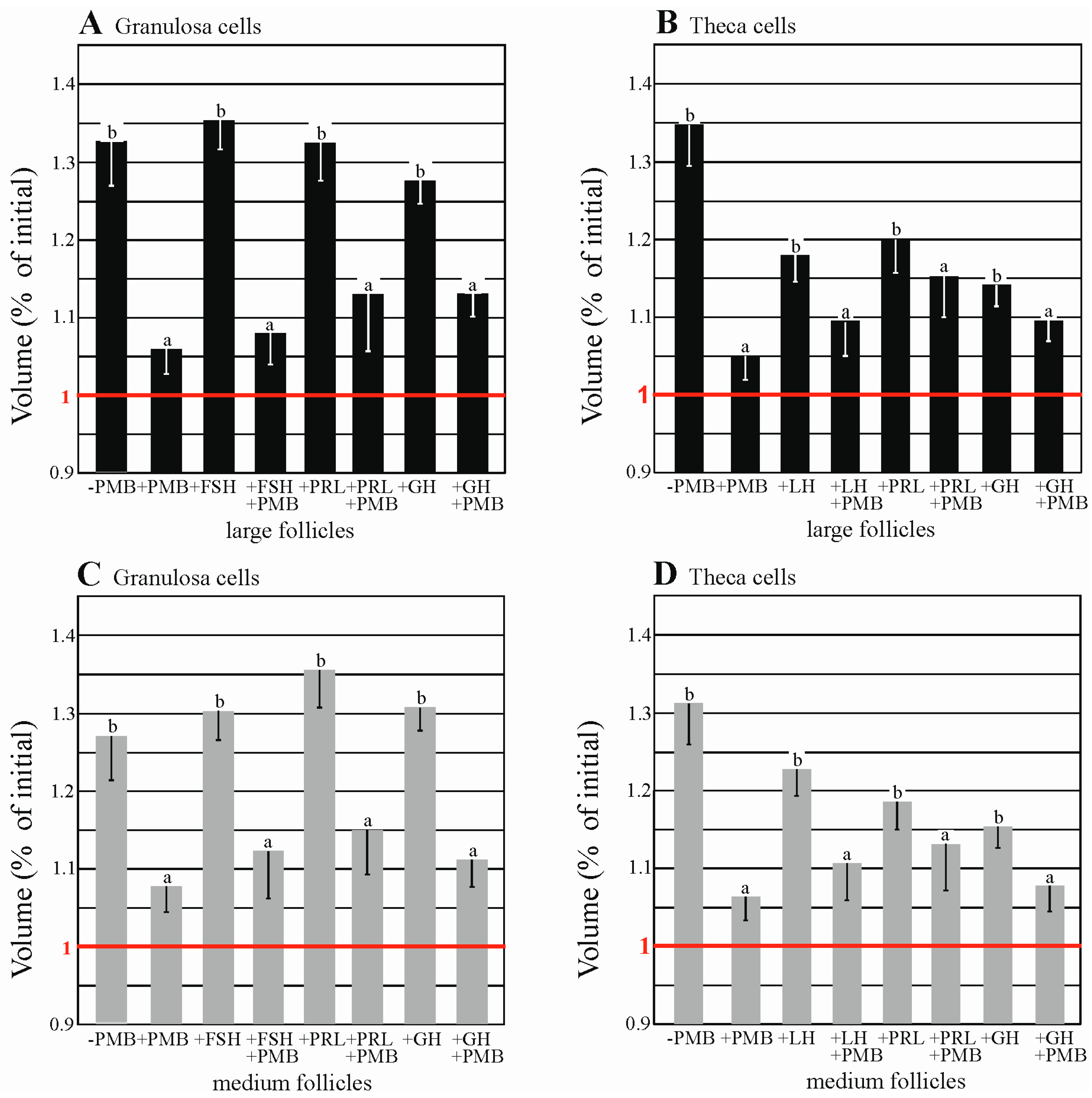
2.6. AQPs Are Functionally Expressed in Porcine Granulosa and thEca Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Cultures and Experimental Design
4.3. RNA Extraction and Real-Time PCR
4.4. SDS-PAGE and Western Blot
4.5. Immunofluorescence for AQP1 in Gc and Tc Cultures
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AQPs | Aquaporins |
| FSH | Follicle-stimulating hormone |
| LH | Luteinising hormone |
| PRL | Prolactin |
| GH | Growth hormone |
| IGF-S | Insulin-like growth factors |
| Gc | Granulosa cells |
| Tc | Theca cells |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| PPIA | Cyclophylin A |
References
- Smitz, J.E.; Cortvindt, R.G. The earliest stages of folliculogenesis in vitro. Reproduction 2002, 123, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Zięcik, A.J.; Shaw, H.J.; Flint, A.P.F. Luteal LH receptors during the estrous cycle and early pregnancy in the pig. J. Reprod. Fertil. 1980, 60, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Tisdall, D.J.; Watanbe, K.; Hudson, N.L.; Smith, P.; McNatty, K.P. FSH receptor gene expression during ovarian follicle development in sheep. J. Mol. Endocrinol. 1995, 15, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Z.; Garverick, H.A.; Smith, G.W.; Smith, M.F.; Hamilton, S.A.; Young-Quist, R.S. Expression of follicle-stimulating hormone and luteinizing hormone receptor messenger ribonucleic acids in bovine follicles during the first follicular wave. Biol. Reprod. 1995, 53, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Lucy, M.C.; Smith, M.F. Messenger ribonucleic acids for insulin-like growth factors-1 and -II, insulin-like growth factor binding protein-2, gonadotropin receptors, and steroidogenic enzymes in porcine follicles. Reprod. Biol. 1996, 55, 1045–1054. [Google Scholar] [CrossRef]
- Mc Natty, K.P.; Makris, A.; De Grazia, C.; Osathanondh, R.; Ryan, K.J. The production of progesterone, androgens and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro. J. Clin. Endocrinol. Metab. 1979, 49, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Batta, S.K.; Wentz, A.C.; Channing, C.P. Steroidogenesis by human ovarian cell types in culture: Influence of mixing of cell types and effect of added testosterone. J. Clin. Endocrinol. Metab. 1980, 50, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Van De Wiel, D.F.M.; Erkens, J.; Koops, W.; Vos, E.; Van Landeghem, A.A.J. Periestrous and midluteal time courses of circulating LH, FSH, prolactine, estradiol-17β and progesterone in the domestic pig. Biol. Reprod. 1981, 24, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Tilton, J.E.; Foxcroft, G.R.; Zięcik, A.J.; Coombs, S.L.; Williams, G.L. Time of the preovulatory LH surge in the gilt and sow relative to the onset of behavioral estrus. Theriogenology 1982, 18, 227–236. [Google Scholar] [CrossRef]
- Kemp, B.; Soede, N.M.; Hazeleger, W. Control of ovulation. In Progress in Pig Science; Wiseman, J., Varley, M.A., Chadwick, J.P., Eds.; Nottingham University Press: Nottingham, UK, 1998; pp. 285–302. [Google Scholar]
- Knox, R.V. Recruitment and selection of ovarian follicles for determination of ovulation rate in the pig. Domest. Anim. Endocrinol. 2005, 29, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Hirshfield, A.N. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991, 124, 43–101. [Google Scholar] [CrossRef] [PubMed]
- Ciereszko, R.; Opałka, M.; Kamińska, B.; Kamiński, T.; Dusza, L. Prolactin involvement in the regulation of hypothalamic-pituitary-ovarian axis during the early luteal phase of the porcine estrous cycle. Anim. Reprod. Sci. 2002, 69, 99–115. [Google Scholar] [CrossRef]
- Bole-Feysot, C.; Goffin, V.; Edery, M.; Binart, N.; Kelly, P.A. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 1998, 19, 225–268. [Google Scholar] [CrossRef] [PubMed]
- Słomczyńska, M.; Gregoraszczuk, E.; Kochman, K.; Stokłosowa, S. Prolactin binding analysis and immunohistochemical localization of prolactin receptor in porcine ovarian cells. Endocr. J. 2001, 48, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ciereszko, R.; Opałka, M.; Kamińska, B.; Górska, T.; Dusza, L. Prolactin signalling in porcine theca cells: The involvement of protein kinases and phosphatases. Reprod. Fertil. Dev. 2003, 15, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gregoraszczuk, E.L.; Gertler, A.; Bylica, A. Response of porcine theca and granulosa cells to GH during short-term in vitro culture. Anim. Reprod. 2000, 58, 113–125. [Google Scholar] [CrossRef]
- Benga, G. The first discovered water channel protein, later called aquaporin 1: Molecular characteristics, functions and medical implications. Mol. Asp. Med. 2012, 33, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; Bryla, A.; Perret, J. Aquaporins in Salivary Glands: From Basic Research to Clinical Applications. Int. J. Mol. Sci. 2016, 17, E166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chen, Z.; Jiang, Z. Expression, Distribution and Role of Aquaporin Water Channels in Human and Animal Stomach and Intestines. Int. J. Mol. Sci. 2016, 17, 1399. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Squillacioti, C.; Mirabella, N.; Meli, R. Aquaporins in Health and Disease: An Overview Focusing on the Gut of Different Species. Int. J. Mol. Sci. 2016, 17, E1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oklinski, M.K.; Skowronski, M.T.; Skowronska, A.; Rützler, M.; Nørgaard, K.; Nieland, J.D.; Kwon, T.H.; Nielsen, S. Aquaporins in the Spinal Cord. Int. J. Mol. Sci. 2016, 17, 2050. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Dorward, H.; Yool, A.J.; Smith, E.; Townsend, A.R.; Price, T.J.; Hardingham, J.E. Role of Aquaporin 1 Signalling in Cancer Development and Progression. Int. J. Mol. Sci. 2017, 18, 299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jiang, Z.; Bazer, F.W.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Aquaporins in the female reproductive system of mammals. Front. Biosci. 2015, 20, 838–871. [Google Scholar] [CrossRef]
- Thoroddsen, A.; Dahm-Kähler, P.; Lind, A.K.; Weijdegård, B.; Lindenthal, B.; Müller, J.; Brännström, M. The water permeability channels aquaporins 1–4 are differentially expressed in granulosa and theca cells of the preovulatory follicle during precise stages of human ovulation. J. Clin. Endocrinol. Metab. 2011, 96, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.X.; Gao, H.J.; Xu, C.M.; Dong, M.Y.; Sheng, X.; Sheng, J.Z.; Huang, H.F. Reduced Expression and Function of Aquaporin-3 in Mouse Metaphase-II Oocytes Induced by Controlled Ovarian Hyperstimulation were Associated with Subsequent Low Fertilization Rate. Cell Physiol. Biochem. 2008, 21, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Kwon, T.H.; Nielsen, S. Immunolocalization of aquaporin 1, 5 and 9 in the female pig reproductive system. J. Histochem. Cytochem. 2009, 57, 61–67. [Google Scholar] [CrossRef] [PubMed]
- McConnell, N.A.; Yunus, R.S.; Gross, S.A.; Bost, K.L.; Clemens, M.G.; Hughes, F.M., Jr. Water permeability of an ovarian antral follicle is predominantly transcellular and mediated by aquaporins. Endocrinology 2002, 143, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, A.; Grzesiak, M.; Mobasheri, A.; Szoltys, M. Immunolocalization of aquaporin 5 during rat ovarian follicle development and expansion of the preovulatory cumulus oophorus. Acta Histochem. 2014, 116, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Wang, F.F.; Lu, X.E.; Dong, M.Y.; Sheng, J.Z.; Lv, P.P.; Ding, G.L.; Shi, B.W.; Zhang, D.; Huang, H.F. Altered aquaporin expression in women with polycystic ovary syndrome: Hyperandrogenism in follicular fluid inhibits aquaporin-9 in granulosa cells through the phosphatidylinositosol 3-kinase pathway. Hum. Reprod. 2010, 25, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Skowronska, A.; Mlotkowska, P.; Eliszewski, M.; Nielsen, S.; Skowronski, M.T. Expression of aquaporin 1, 5 and 9 in the ovarian follicles of cycling and early pregnant pigs. Physiol. Res. 2015, 64, 237–245. [Google Scholar] [PubMed]
- Cho, G.; Bragiel, A.M.; Wang, D.; Pieczonka, T.D.; Skowronski, M.T.; Shono, M.; Nielsen, S.; Ishikawa, Y. Activation of muscarinic receptors in rat parotid acinar cells induces AQP5 trafficking to nuclei and apical plasma membrane. Biochim. Biophys. Acta 2015, 1850, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, E.M.; McConnell, N.A.; Hughes, F.M., Jr.; Huet-Hudson, Y.M. Estrogen Regulation of Aquaporins in the Mouse Uterus: Potential Roles in Uterine Water Movement. Biol. Reprod. 2003, 69, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Carnes, K.; França, L.R.; Hermo, L.; Hess, R.A. Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol. Cell 2005, 97, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Takahashi, E.; Miyagawa, S.; Watanabe, H.; Iguchi, T. Chromatin immunoprecipitation-mediated target identification proved aquaporin 5 is regulated directly by estrogen in the uterus. Genes Cells 2006, 11, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.B.; Zhang, R.J.; Tan, Y.J.; Ding, G.L.; Shi, S.; Zhang, D.; He, R.H.; Liu, A.X.; Wang, T.T.; Leung, P.C.K.; et al. Identification of Estrogen Response Element in the Aquaporin-2 Gene That Mediates Estrogen-Induced Cell Migration and Invasion in Human Endometrial Carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, E1399–E1408. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.L.; Findlay, J.K. Estrogen actions in the ovary revisited. J. Endocrinol. 2002, 175, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Filicori, M.; Cognigni, G.E.; Gamberini, E.; Parmegiani, L.; Troilo, E.; Roset, B. Efficacy of low-dose human chorionic gonadotropin alone to complete controlled ovarian stimulation. Fertil. Steril. 2005, 84, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Skowronska, A.; Mlotkowska, P.; Nielsen, S.; Skowronski, M.T. Difference in expression between AQP1 and AQP5 in porcine endometrium and myometrium in response to steroid hormones, oxytocin, arachidonic acid, forskolin and cAMP during the mid-luteal phase of the estrous cycle and luteolysis. Reprod. Biol. Endocrinol. 2015, 13, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Skowronska, A.; Mlotkowska, P.; Okrasa, S.; Nielsen, S.; Skowronski, M.T. Modulatory effects of steroid hormones, oxytocin, arachidonic acid, forskolin and cyclic AMP on the expression of aquaporin 1 and aquaporin 5 in the porcine uterus during placentation. J. Physiol. Pharmacol. 2016, 67, 311–319. [Google Scholar] [PubMed]
- Skowronska, A.; Mlotkowska, P.; Majewski, M.; Nielsen, S.; Skowronski, M.T. Expression of aquaporin 1 and 5 and their regulation by ovarian hormones, arachidonic acid, forskolin and cAMP during implantation in pigs. Physiol. Res. 2016, 65, 637–650. [Google Scholar] [PubMed]
- Chen, Q.; Peng, H.; Lei, L.; Zhang, Y.; Kuang, H.; Cao, Y.; Shi, Q.X.; Ma, T.; Duan, E. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011, 21, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Zhang, J.; Fan, Y.; Ding, J.H.; Sha, J.H.; Hu, G. Aquaporin-4 deficiency induces subfertility in female mice. Fertil. Steril. 2008, 92, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Qiao, Y.; Yi, F.; Guan, X.; Zhang, D.; Zhang, S.; Hao, F.; Xiao, Y.; Zhang, H.; Guo, L.; et al. Increased female fertility in aquaporin 8-deficient mice. IUBMB Life 2010, 62, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.Y.; Xiong, Z.F.; Liu, H.S.; Zheng, Z.; Ma, T.H. Pregnant phenotype in aquaporin 8-deficient mice. Acta Pharmacol. Sin. 2011, 32, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Eur. J. Physiol. 2008, 456, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Watanabe, H.; Yan, D.; Manley, G.T.; Verkman, A.S. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J. Cell Sci. 2005, 118, 5691–5698. [Google Scholar] [CrossRef] [PubMed]
- McCoy, E.; Sontheimer, H. MAPK induces AQP1 expression in astrocytes following injury. Glia 2010, 58, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Galán-Cobo, A.; Ramírez-Lorca, R.; Toledo-Aral, J.J.; Echevarría, M. Aquaporin-1 plays important role in proliferation by affecting cell cycle progression. J. Cell. Physiol. 2016, 231, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Yu, Y.Q.; Yan, C.X. Localisation and expression of aquaporin subtypes in epithelial ovarian tumours. Histol. Histopathol. 2011, 26, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Ritter, M.; Gamper, N.; Huber, S.; Fillon, S.; Tanneur, V.; Lepple-Wienhues, A.; Szabo, I.; Gulbins, E. Cell Volume in the Regulation of Cell Proliferation and Apoptotic Cell Death. Cell. Physiol. Biochem. 2000, 10, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Rouzaire-Dubois, B.; Dubois, J.M. K+ channel block induced mammalian neuroblastoma cell swelling: A possible mechanism to influence proliferation. J. Physiol. 1998, 510, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Bachtell, N.E.; Conaghan, J.; Turek, P.J. The relative viability of human spermatozoa from the vas deferens, epididymis and testis before and after cryopreservation. Hum. Reprod. 1999, 14, 3048–3051. [Google Scholar] [CrossRef] [PubMed]
- Edashige, K.; Sakamoto, M.; Kasai, M. Expression of mRNAs of the aquaporin family in mouse oocytes and embryos. Cryobiology 2000, 40, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Madej, A.; Lang, A.; Brandt, Y.; Kindhal, H.; Madens, M.T.; Einarsson, S. Factors regulating ovarian function in pigs. Domest. Anim. Endocrinol. 2005, 29, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.; Figueiredo, J.R.; Van Den Hurk, R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009, 71, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, J.; Gertler, A.; Leibovich, H.; Rzasa, J.; Gregoraszczuk, E.L. Synergistic action of growth hormone and insulin-like growth factor I (IGF-I) on proliferation and estradiol secretion in porcine granulosa and theca cells cultured alone or in coculture. Theriogenology 2003, 60, 559–570. [Google Scholar] [CrossRef]
- Breves, J.; Inokuchi, M.; Yamaguchi, Y.; Seale, A.; Watanabe, S.; Lerner, D.; Kaneko, T.; Grau, E. Hormonal regulation of aquaporin 3: Opposing actions of prolactin and cortisol in tilapia gill. J. Endocrinol. 2016, 230, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Akins, E.L.; Morrissette, M.C. Gross ovarian changes during estrous cycle of swine. Am. J. Vet. Res. 1968, 10, 1953–1957. [Google Scholar]
- Stokłosowa, S.; Bahr, J.; Gregoraszczuk, E.L. Some morphological and characteristics of cells of the porcine theca interna in tissue culture. Biol. Reprod. 1978, 19, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Nynca, A.; Jablonska, O.; Slomczynska, M.; Petroff, B.K.; Ciereszko, R.E. Effects of phytoestrogen daidzein and estradiol on steroidogenesis and expression of estrogen receptors in porcine luteinized granulosa cells from large follicles. J. Physiol. Pharmacol. 2009, 60, 95–105. [Google Scholar] [PubMed]
- Skowronski, M.T.; Skowronska, A.; Rojek, A.; Oklinski, M.K.; Nielsen, S. Prolonged Starvation Causes Up-Regulation of AQP1 in Adipose Tissue Capillaries of AQP7 Knock-Out Mice. Int. J. Mol. Sci. 2016, 17, 1101. [Google Scholar] [CrossRef] [PubMed]
- Terris, J.; Ecelbarger, C.A.; Nielsen, S.; Knepper, M.A. Long-term regulation of four renal aquaporins in rats. Am. J. Physiol. 1996, 271 Pt 2, F414–F422. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowronski, M.T.; Mlotkowska, P.; Tanski, D.; Lepiarczyk, E.; Oklinski, M.K.; Nielsen, S.; Skowronska, A. Pituitary Gonadotropins, Prolactin and Growth Hormone Differentially Regulate AQP1 Expression in the Porcine Ovarian Follicular Cells. Int. J. Mol. Sci. 2018, 19, 5. https://doi.org/10.3390/ijms19010005
Skowronski MT, Mlotkowska P, Tanski D, Lepiarczyk E, Oklinski MK, Nielsen S, Skowronska A. Pituitary Gonadotropins, Prolactin and Growth Hormone Differentially Regulate AQP1 Expression in the Porcine Ovarian Follicular Cells. International Journal of Molecular Sciences. 2018; 19(1):5. https://doi.org/10.3390/ijms19010005
Chicago/Turabian StyleSkowronski, Mariusz T., Patrycja Mlotkowska, Damian Tanski, Ewa Lepiarczyk, Michal K. Oklinski, Soren Nielsen, and Agnieszka Skowronska. 2018. "Pituitary Gonadotropins, Prolactin and Growth Hormone Differentially Regulate AQP1 Expression in the Porcine Ovarian Follicular Cells" International Journal of Molecular Sciences 19, no. 1: 5. https://doi.org/10.3390/ijms19010005




