Minoxidil Induction of VEGF Is Mediated by Inhibition of HIF-Prolyl Hydroxylase
Abstract
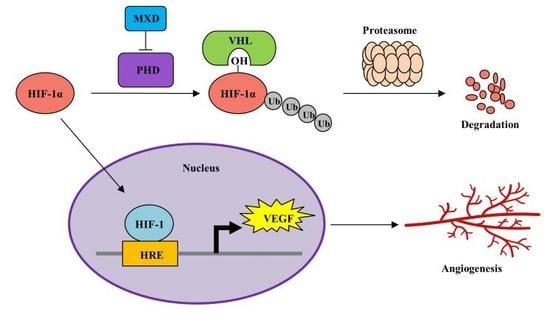
:1. Introduction
2. Results
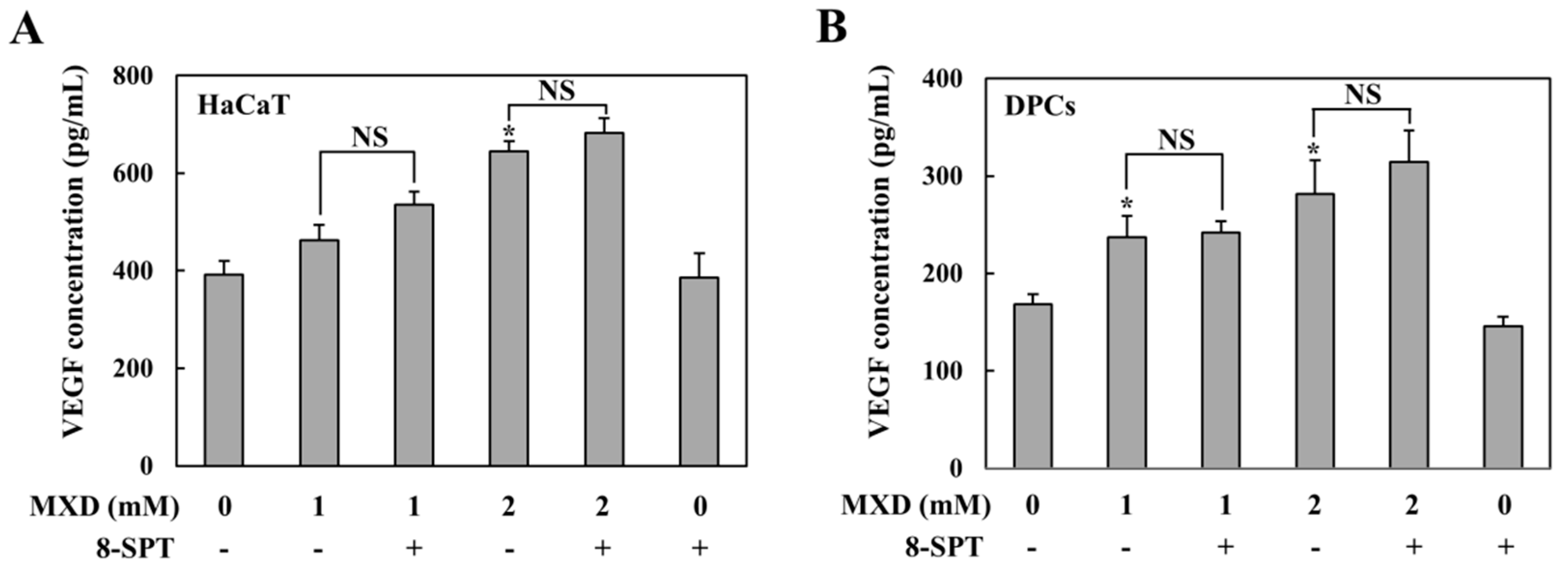
2.1. Millimolar Minoxidil Increases the Secretion of VEGF in Human Skin Cells
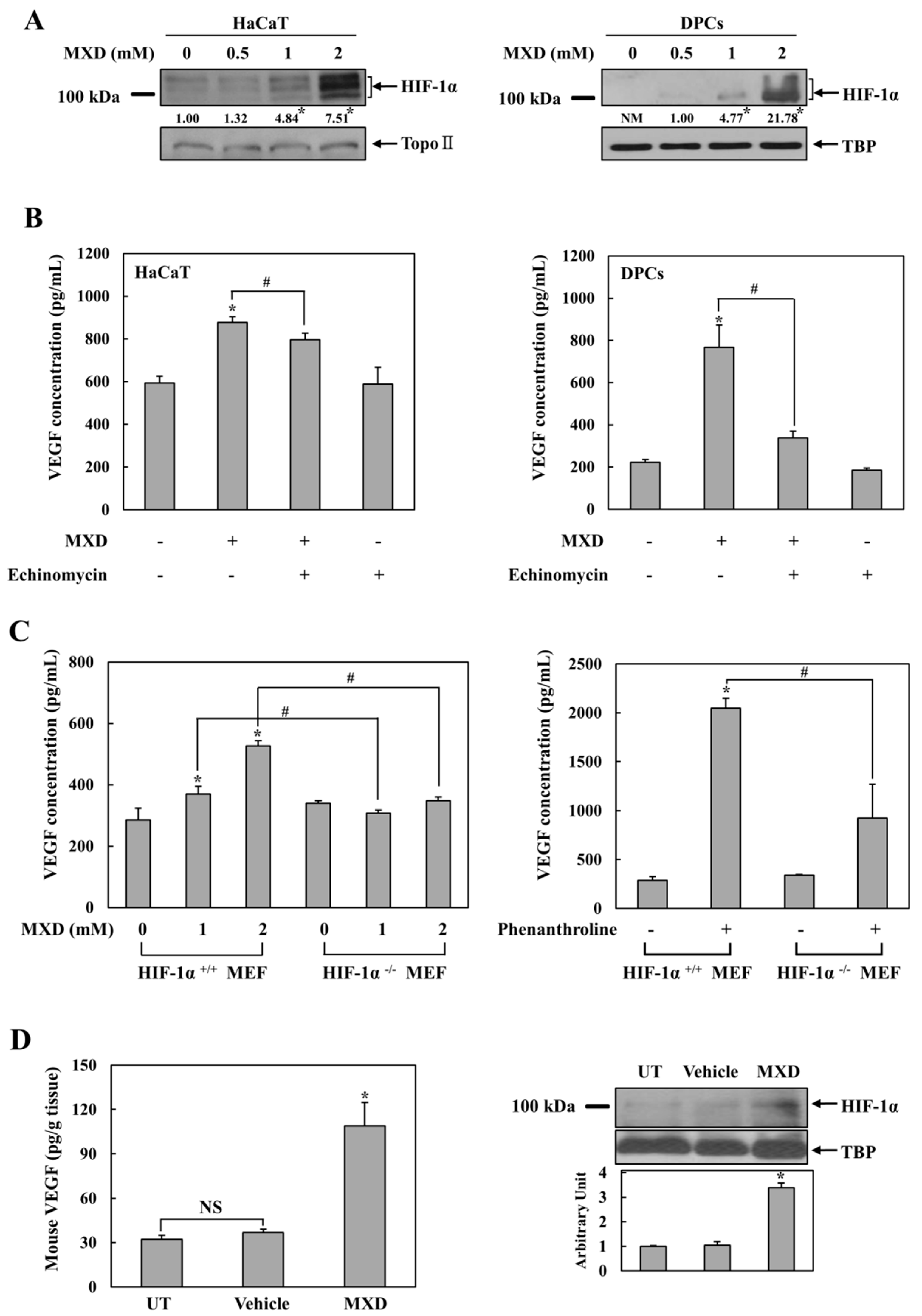
2.2. Millimolar Minoxidil Induction of VEGF Is Dependent on HIF-1
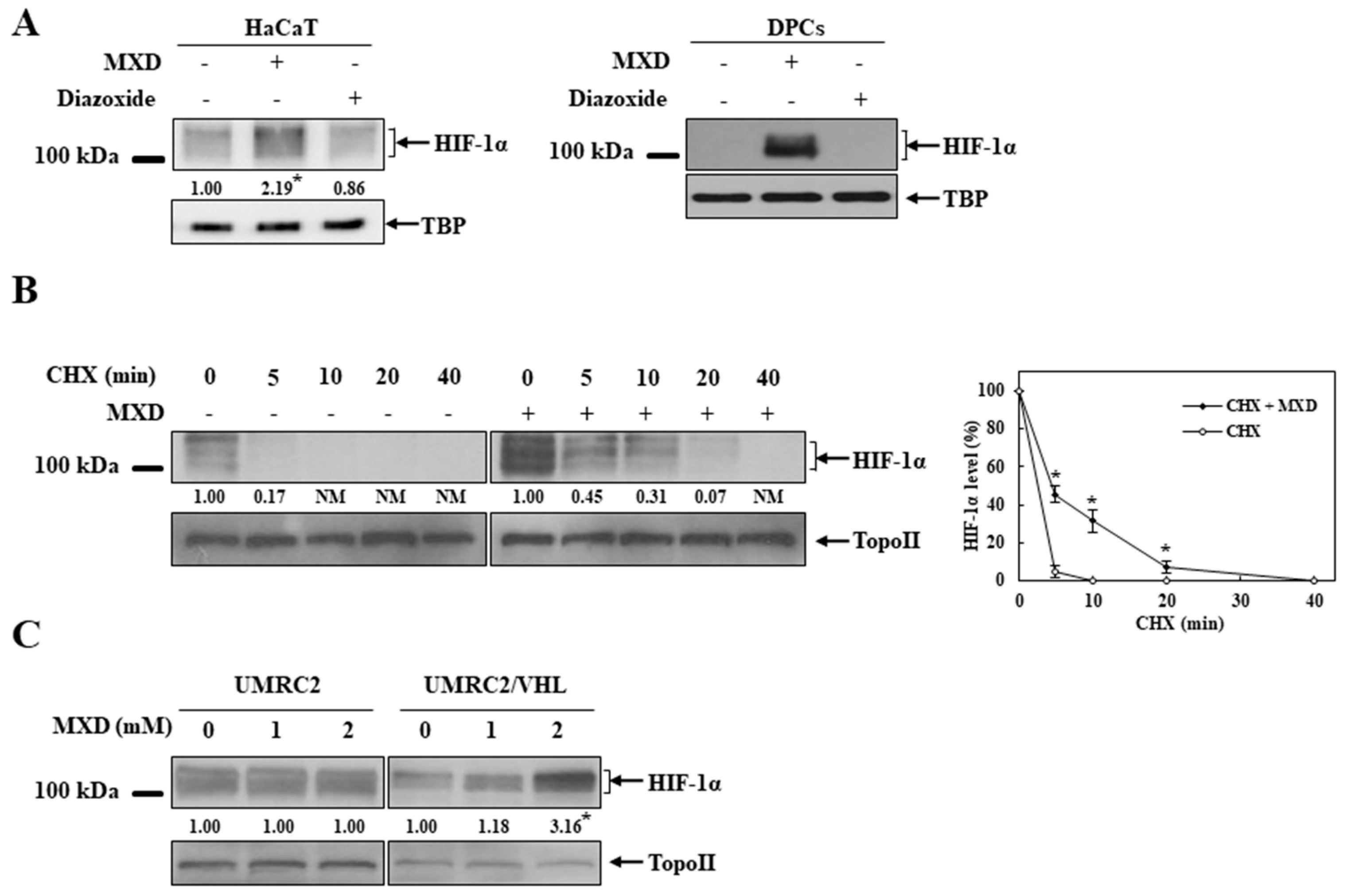
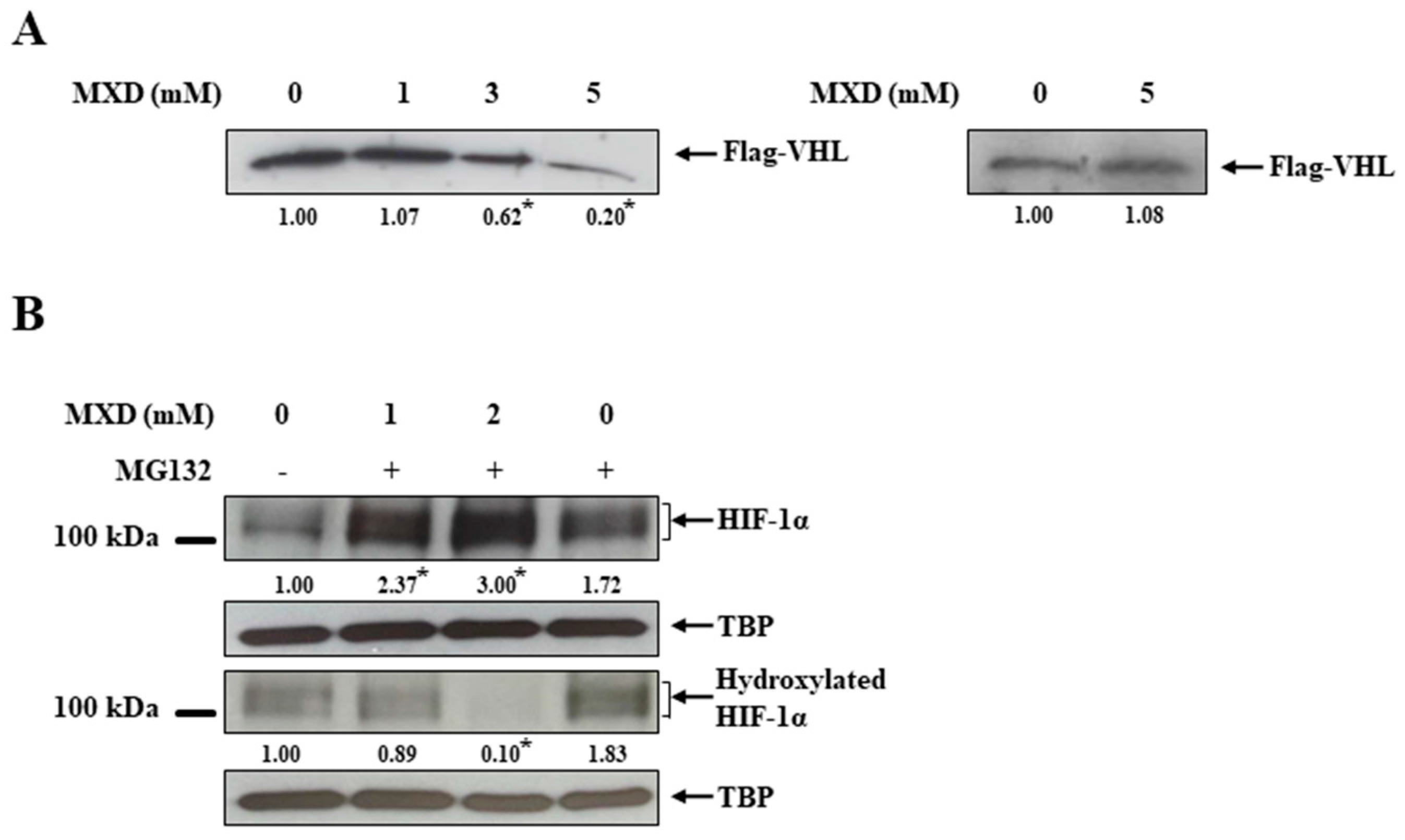
2.3. Minoxidil Activation of HIF-1 Occurs by Inhibiting HIF-Prolyl Hdroxylase
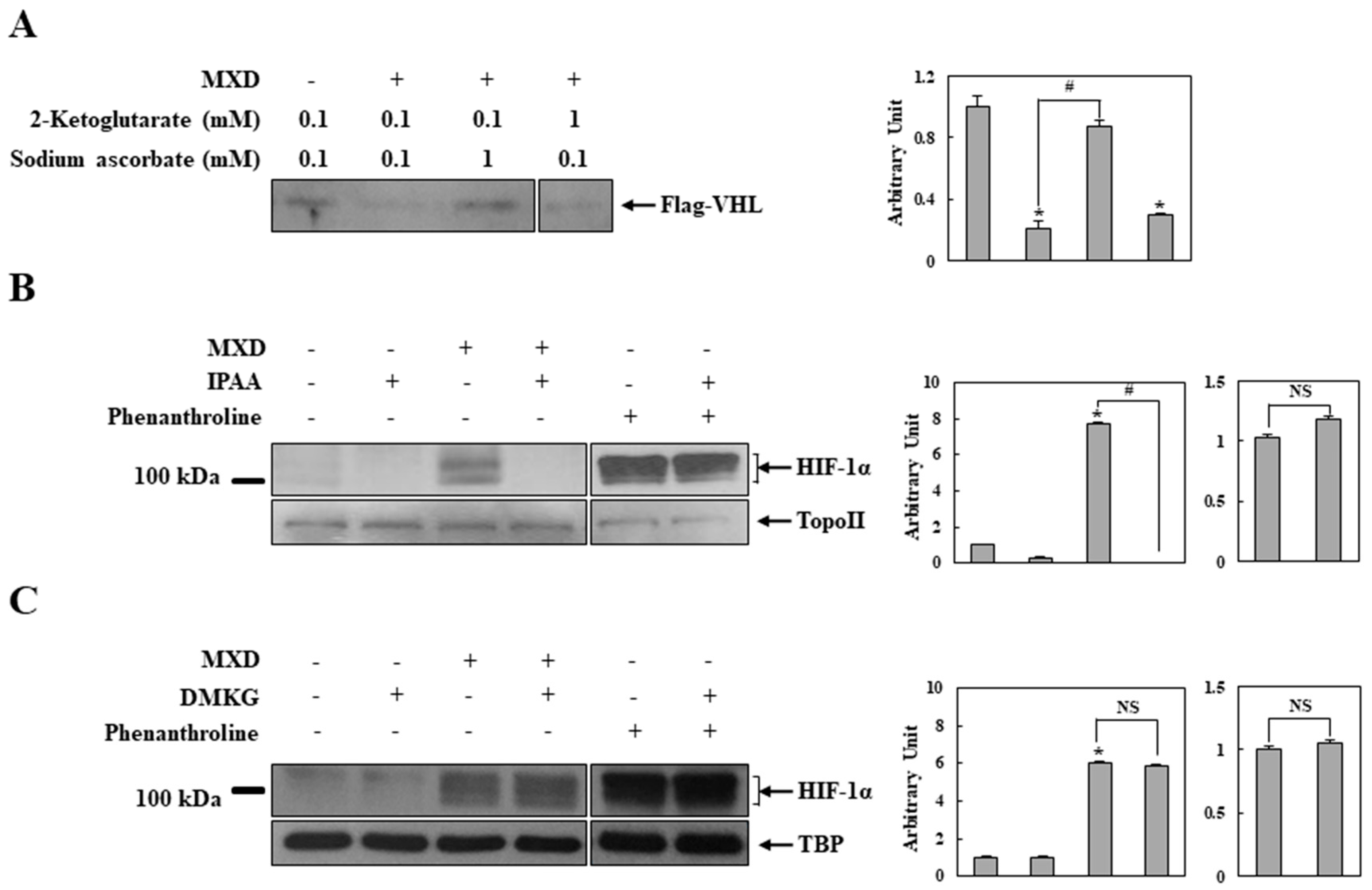
2.4. Millimolar Minoxidil Inhibition of PHD Is Reversed by Ascorbate, a Cofactor of PHD
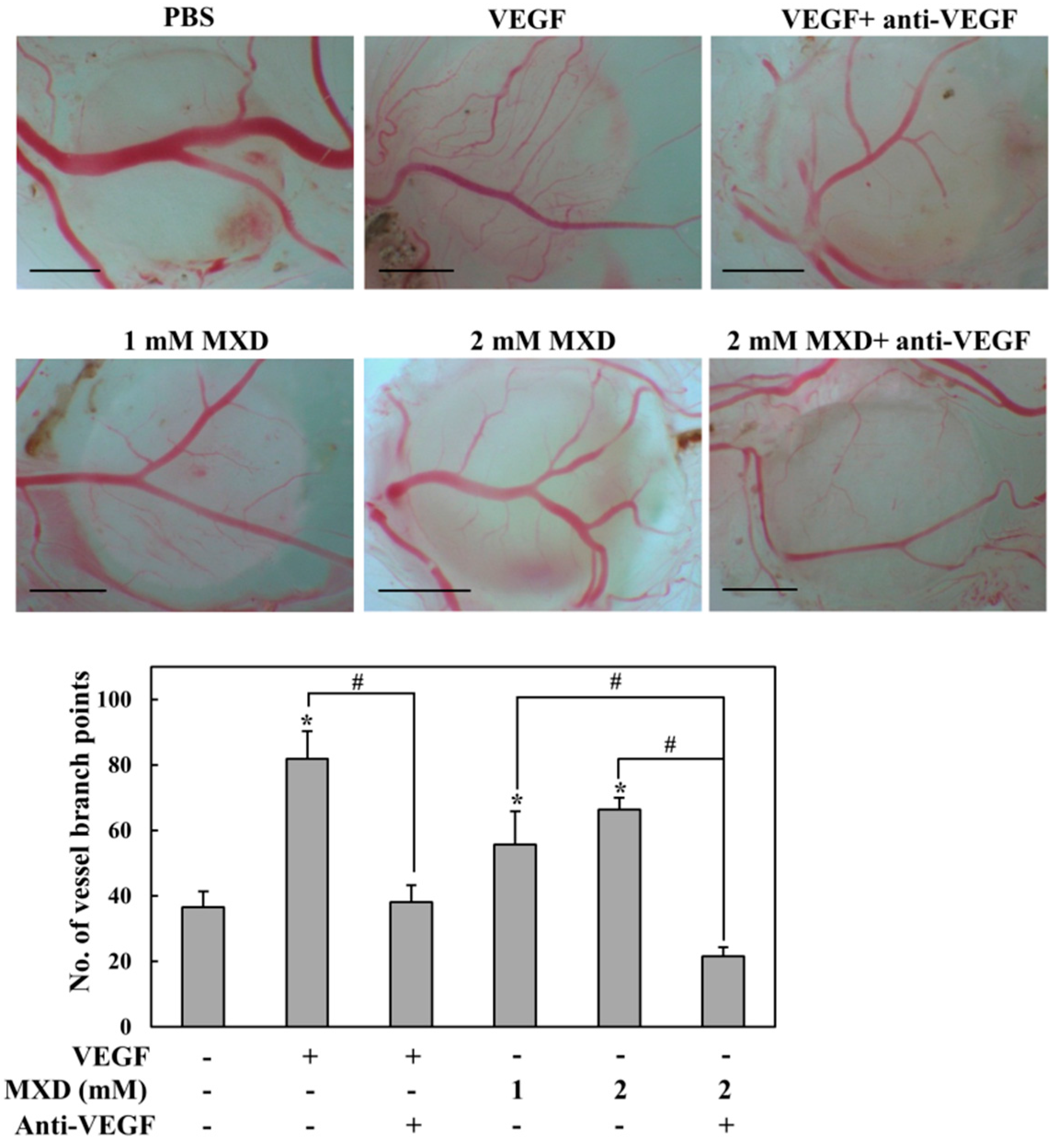
2.5. Millimolar Minoxidil Promotes Angiogenesis in CAM Assay
3. Discussion
4. Materials and Methods
4.1. Chemicals and Animals
4.2. Cell Culture
4.3. Topical Treatment with Minoxidil
4.4. Immunoblot Analysis
4.5. In Vitro VHL Capture Assay
4.6. CAM Assay
4.7. VEGF Analysis
4.8. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fenton, D.A.; Wilkinson, J.D. Topical minoxidil in the treatment of alopecia areata. Br. Med. J. 1983, 287, 1015–1017. [Google Scholar] [CrossRef]
- Olsen, E.A. Topical minoxidil in the treatment of androgenetic alopecia in women. Cutis 1991, 48, 243–248. [Google Scholar] [PubMed]
- Weiss, V.C.; West, D.P. Topical minoxidil therapy and hair regrowth. Arch. Dermatol. 1985, 121, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J.L. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1998, 138, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Headington, J.T. Hair follicle biology and topical minoxidil: Possible mechanisms of action. Dermatologica 1987, 175, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.A.; Moretti, G. Vascular patterns associated with categen hair follicles in the human scalp. Ann. N. Y. Acad. Sci. 1959, 83, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Goldman, C.K.; Tsai, J.C.; Soroceanu, L.; Gillespie, G.Y. Loss of vascular endothelial growth factor in human alopecia hair follicles. J. Investig. Dermatol. 1995, 104, 18S–20S. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, U.; Blume-Peytavi, U.; Kodelja, V.; Sommer, C.; Goerdt, S.; Majewski, S.; Jablonska, S.; Orfanos, C.E. Expression of vascular endothelial growth factor (VEGF) in various compartments of the human hair follicle. Arch. Dermatol. Res. 1998, 290, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Marubayashi, A.; Nakaya, Y.; Fukui, K.; Arase, S. Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: Possible involvement of sulfonylurea receptor 2B as a target of minoxidil. J. Investig. Dermatol. 2001, 117, 1594–1600. [Google Scholar] [PubMed]
- Chen, L.; Endler, A.; Shibasaki, F. Hypoxia and angiogenesis: Regulation of hypoxia-inducible factors via novel binding factors. Exp. Mol. Med. 2009, 41, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Bae, M.K.; Jeong, J.W.; Moon, H.E.; Kim, K.W. Hypoxia-induced angiogenesis during carcinogenesis. J. Biochem. Mol. Biol. 2003, 36, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Song, I.S.; Wang, A.G.; Yoon, S.Y.; Kim, J.M.; Kim, J.H.; Lee, D.S.; Kim, N.S. Regulation of glucose metabolism-related genes and VEGF by HIF-1alpha and HIF-1beta, but not HIF-2alpha, in gastric cancer. Exp. Mol. Med. 2009, 41, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Huh, J.; Kim, W.; Jeong, S.; Min do, S.; Jung, Y. Phospholipase D activates HIF-1-VEGF pathway via phosphatidic acid. Exp. Mol. Med. 2014, 46, e126. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cockman, M.E.; Masson, N.; Mole, D.R.; Jaakkola, P.; Chang, G.W.; Clifford, S.C.; Maher, E.R.; Pugh, C.W.; Ratcliffe, P.J.; Maxwell, P.H. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 2000, 275, 25733–25741. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.E.; Gu, J.; Schau, M.; Bunn, H.F. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 7987–7992. [Google Scholar] [CrossRef] [PubMed]
- Salceda, S.; Caro, J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 1997, 272, 22642–22647. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, K.; Makino, Y.; Pereira, T.; Poellinger, L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000, 19, 4298–4309. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Cappel, M.J.; Flynn, G.L.; Weiner, N.D.; Kreuter, J.; Ferry, J.J. Drug and vehicle deposition from topical applications: Use of in vitro mass balance technique with minoxidil solutions. J. Pharm. Sci. 1992, 81, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Weiner, N.; Flynn, G.L.; Ferry, J.J. Drug and vehicle deposition from topical applications: Localization of minoxidil within skin strata of the hairless mouse. Skin Pharmacol. 1994, 7, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Shanmugam, S.; Lee, W.S.; Lee, W.M.; Kim, J.O.; Oh, D.H.; Kim, D.D.; Kim, J.S.; Yoo, B.K.; Choi, H.G.; et al. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int. J. Pharm. 2009, 377, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Debar, S.; Meier-Davis, S.; Martin, R.; Damaj, B. The Novel Excipient, Dodecyl-2-N, N-dimethylaminopropionate Hydrochloride (DDAIP-HCl) Improves the Flux of Minoxidil in Human Skin. Hair Ther. Transplant. 2012, 2, 105. [Google Scholar] [CrossRef]
- Hewitson, K.S.; Schofield, C.J. The HIF pathway as a therapeutic target. Drug Discov. Today 2004, 9, 704–711. [Google Scholar] [CrossRef]
- Kong, D.; Park, E.J.; Stephen, A.G.; Calvani, M.; Cardellina, J.H.; Monks, A.; Fisher, R.J.; Shoemaker, R.H.; Melillo, G. Echinomycin, a Small-Molecule Inhibitor of Hypoxia-Inducible Factor-1 DNA-Binding Activity. Cancer Res. 2005, 65, 9047–9055. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Isaacs, J.S.; Lee, S.; Trepel, J.; Neckers, L. Microtubule disruption utilizes an NFkappa B-dependent pathway to stabilize HIF-1alpha protein. J. Biol. Chem. 2003, 278, 7445–7452. [Google Scholar] [CrossRef] [PubMed]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.S.; Jung, Y.J.; Mimnaugh, E.G.; Martinez, A.; Cuttitta, F.; Neckers, L.M. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J. Biol. Chem. 2002, 277, 29936–29944. [Google Scholar] [CrossRef] [PubMed]
- Bruick, R.K.; McKnight, S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 2001, 294, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.S.; Jung, Y.J.; Mole, D.R.; Lee, S.; Torres-Cabala, C.; Chung, Y.L.; Merino, M.; Trepel, J.; Zbar, B.; Toro, J.; et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: Novel role of fumarate in regulation of HIF stability. Cancer Cell 2005, 8, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, H.; Choi, D.; Kim, D.; Park, S.Y.; Kim, Y.J.; Kim, Y.M.; Jung, Y. Quercetin activates an angiogenic pathway, hypoxia inducible factor (HIF)-1-vascular endothelial growth factor, by inhibiting HIF-prolyl hydroxylase: A structural analysis of quercetin for inhibiting HIF-prolyl hydroxylase. Mol. Pharmacol. 2007, 71, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Lee, D.Y.; Choi, Y.H.; Yea, S.S.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; Kim, S.K.; et al. Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced asthma. Life Sci. 2008, 82, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Yum, S.; Doh, H.J.; Hong, S.; Jeong, S.; Kim, D.D.; Park, M.; Jung, Y. Piceatannol, a hydroxystilbene natural product, stabilizes HIF-1alpha protein by inhibiting HIF prolyl hydroxylase. Eur. J. Pharmacol. 2013, 699, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Buhl, A.E.; Waldon, D.J.; Kawabe, T.T.; Holland, J.M. Minoxidil stimulates mouse vibrissae follicles in organ culture. J. Investig. Dermatol. 1989, 92, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Buhl, A.E.; Waldon, D.J.; Baker, C.A.; Johnson, G.A. Minoxidil sulfate is the active metabolite that stimulates hair follicles. J. Investig. Dermatol. 1990, 95, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Kivirikko, K.I.; Myllyla, R.; Pihlajaniemi, T. Protein hydroxylation: Prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989, 3, 1609–1617. [Google Scholar] [PubMed]
- Choi, D.; Han, J.; Lee, Y.; Choi, J.; Han, S.; Hong, S.; Jeon, H.; Kim, Y.M.; Jung, Y. Caffeic acid phenethyl ester is a potent inhibitor of HIF prolyl hydroxylase: structural analysis and pharmacological implication. J. Nutr. Biochem. 2010, 21, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.L.; Schutt, W.H.; Caldwell, I.W. Hypertrichosis due to diazoxide. Br. J. Dermatol. 1975, 93, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.G. The culture of dermal papilla cells from human hair follicles. Br. J. Dermatol. 1984, 110, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Kim, S.C.; Hyun, J.H.; Kang, J.H.; Park, D.B.; Lee, Y.J.; Yoo, E.S.; Kang, H.K. Promotion effect of Schisandra nigra on the growth of hair. Eur. J. Dermatol. 2009, 19, 119–125. [Google Scholar] [PubMed]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates beta-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C.; Faller, D.V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991, 19, 2499. [Google Scholar] [CrossRef] [PubMed]
- Park, B.C.; Thapa, D.; Lee, J.S.; Park, S.Y.; Kim, J.A. Troglitazone inhibits vascular endothelial growth factor-induced angiogenic signaling via suppression of reactive oxygen species production and extracellular signal-regulated kinase phosphorylation in endothelial cells. J. Pharmacol. Sci. 2009, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yum, S.; Jeong, S.; Kim, D.; Lee, S.; Kim, W.; Yoo, J.-W.; Kim, J.-A.; Kwon, O.S.; Kim, D.-D.; Min, D.S.; et al. Minoxidil Induction of VEGF Is Mediated by Inhibition of HIF-Prolyl Hydroxylase. Int. J. Mol. Sci. 2018, 19, 53. https://doi.org/10.3390/ijms19010053
Yum S, Jeong S, Kim D, Lee S, Kim W, Yoo J-W, Kim J-A, Kwon OS, Kim D-D, Min DS, et al. Minoxidil Induction of VEGF Is Mediated by Inhibition of HIF-Prolyl Hydroxylase. International Journal of Molecular Sciences. 2018; 19(1):53. https://doi.org/10.3390/ijms19010053
Chicago/Turabian StyleYum, Soohwan, Seongkeun Jeong, Dohoon Kim, Sunyoung Lee, Wooseong Kim, Jin-Wook Yoo, Jung-Ae Kim, Oh Sang Kwon, Dae-Duk Kim, Do Sik Min, and et al. 2018. "Minoxidil Induction of VEGF Is Mediated by Inhibition of HIF-Prolyl Hydroxylase" International Journal of Molecular Sciences 19, no. 1: 53. https://doi.org/10.3390/ijms19010053