Indoleamine 2,3-Dioxygenase (IDO) Enzyme Links Innate Immunity and Altered T-Cell Differentiation in Non-ST Segment Elevation Acute Coronary Syndrome
Abstract
:1. Introduction
2. Results
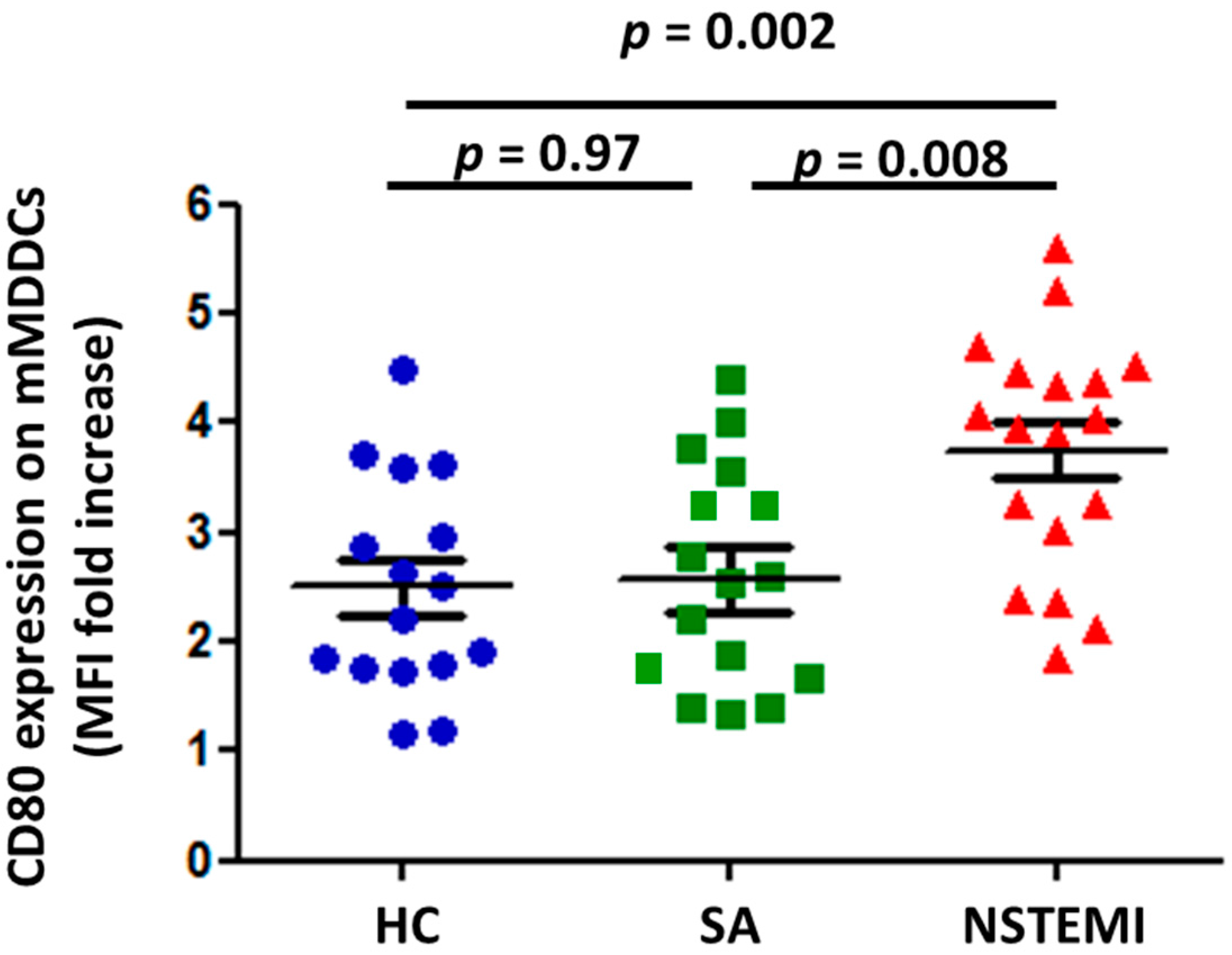
2.1. MDDC Maturation was Altered in NSTEMI Patients
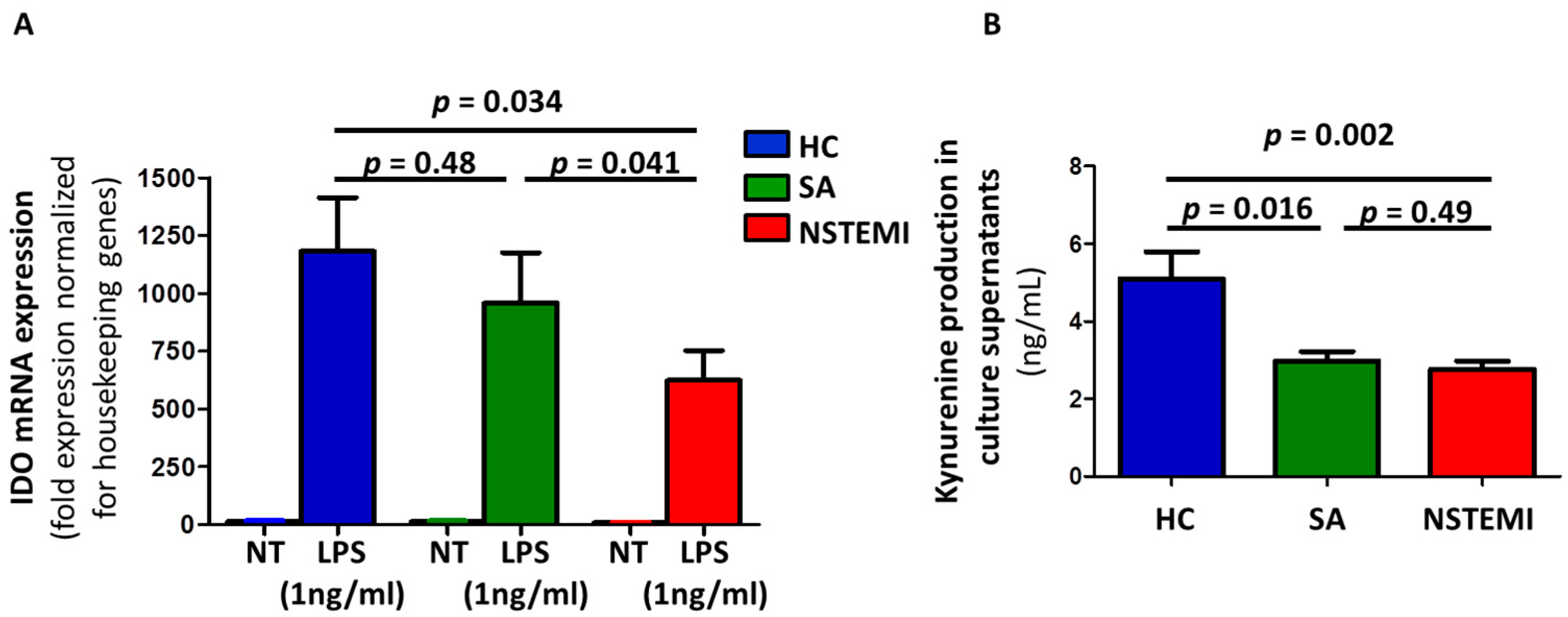
2.2. Altered IDO Induction and Activity in Mmddcs of NSTEMI Patients
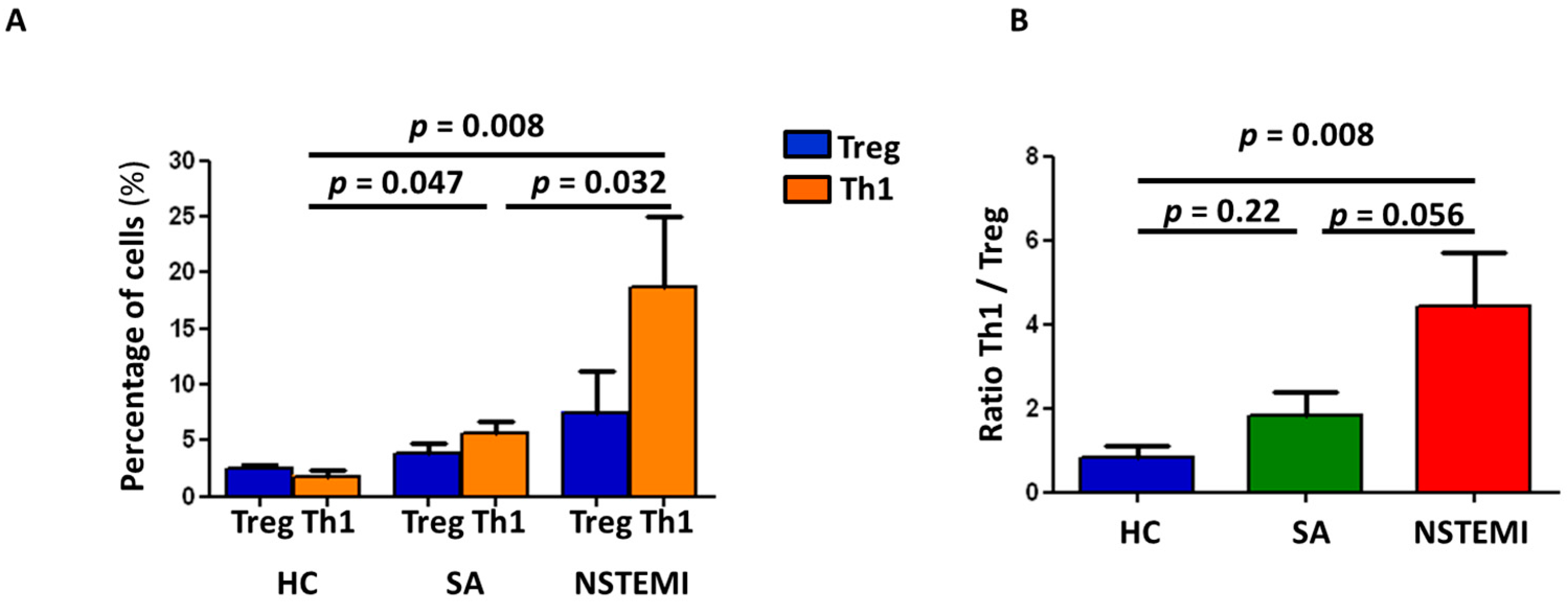
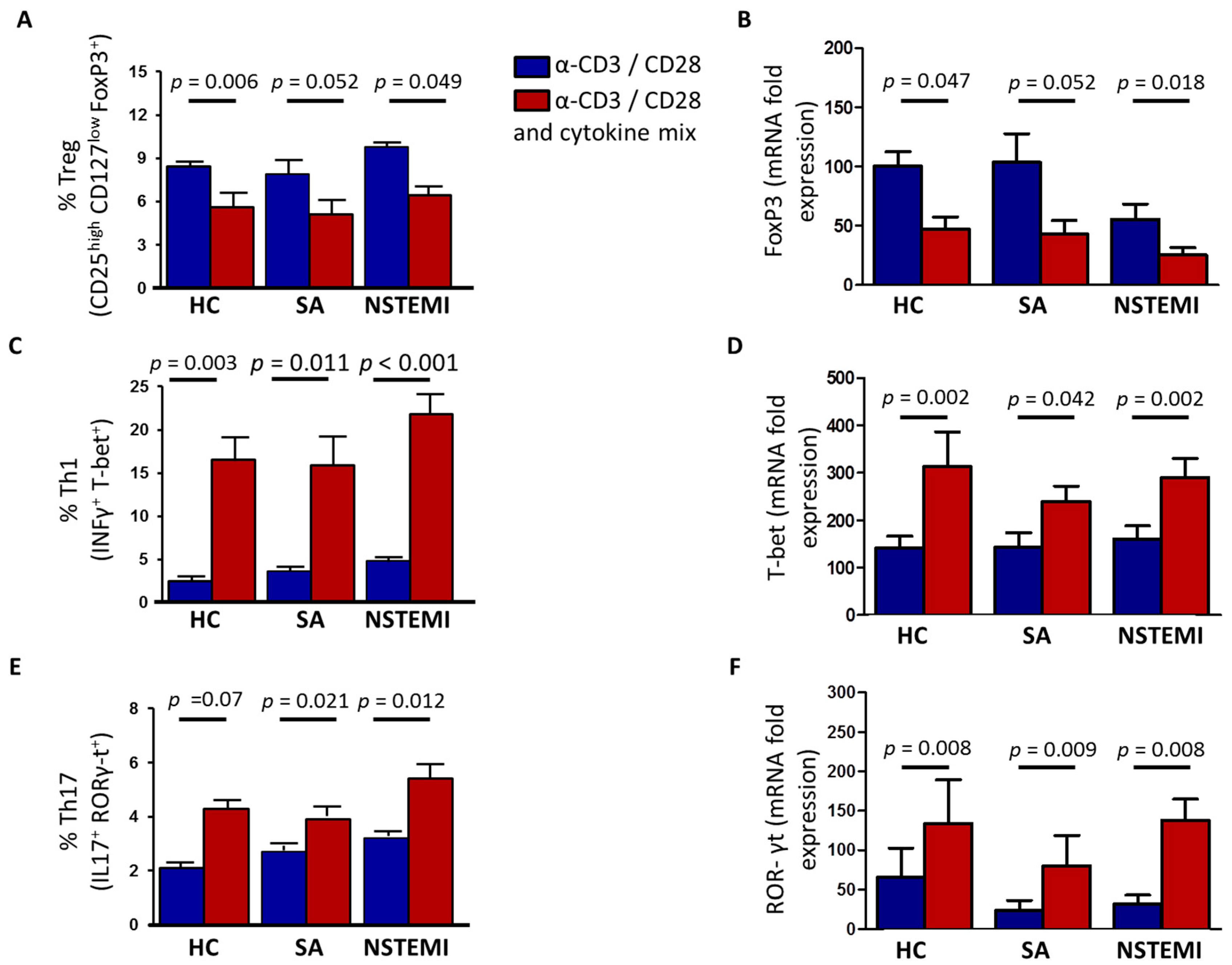
2.3. Altered Th1/Treg Differentiation in NSTEMI and SA Patients
3. Discussion
Study Limitations
4. Materials and Methods
4.1. NSTEMI and SA Patients and Healthy Subjects Enrolled in the Study
4.2. Cell Isolation and Cultures
4.3. Mixed Lymphocyte Reaction
4.4. Flow Cytometric Analysis
4.5. RNA Extraction
4.6. Real Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.7. Kynurenine Production
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Flego, D.; Liuzzo, G.; Weyand, C.M.; Crea, F. Adaptive Immunity Dysregulation in Acute Coronary Syndromes: From Cellular and Molecular Basis to Clinical Implications. J. Am. Coll. Cardiol. 2016, 68, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Goronzy, J.J.; Yang, H.; Kopecky, S.L.; Holmes, D.R.; Frye, R.L.; Weyand, C.M. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 2000, 101, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- De Palma, R.; Del Galdo, F.; Abbate, G.; Chiariello, M.; Calabró, R.; Forte, L.; Cimmino, G.; Papa, M.F.; Russo, M.G.; Ambrosio, G.; et al. Patients with acute coronary syndrome show oligoclonal T-cell recruitment within instable plaque: Evidence for a local, intracoronary immunologic mechanism. Circulation 2006, 113, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Montone, R.A.; Gabriele, M.; Pedicino, D.; Giglio, A.F.; Trotta, F.; Galiffa, V.A.; Previtero, M.; Severino, A.; Biasucci, L.M.; et al. Identification of uniqueadaptive immune systemsignature in acute coronarysyndromes. Int. J. Cardiol. 2013, 168, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Flego, D.; Severino, A.; Trotta, F.; Previtero, M.; Ucci, S.; Zara, C.; Massaro, G.; Pedicino, D.; Biasucci, L.M.; Liuzzo, G.; et al. Increased PTPN22 expression and defective CREB activation impair regulatory T-cell differentiation in non-ST-segment elevation acute coronary syndromes. J. Am. Coll. Cardiol. 2015, 65, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Flego, D.; Severino, A.; Trotta, F.; Previtero, M.; Ucci, S.; Zara, C.; Pedicino, D.; Massaro, G.; Biasucci, L.M.; Liuzzo, G.; et al. Altered CD31 expression and activity in helper T cells of acute coronary syndrome patients. Basic Res. Cardiol. 2014, 109, 448. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Hemmi, H. Dendritic cells: Translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006, 311, 17–58. [Google Scholar] [PubMed]
- Mosmann, T.R.; Livingstone, A.M. Dendritic cells: The immune information management experts. Nat. Immunol. 2004, 5, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Iberg, C.A.; Jones, A.; Hawiger, D. Dendritic Cells as Inducers of Peripheral Tolerance. Trends Immunol. 2017, 38, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Kaliński, P.; Hilkens, C.M.; Wierenga, E.A.; Kapsenberg, M.L. T-cell priming by type-1 and type-2 polarized dendritic cells: The concept of a third signal. Immunol. Today 1999, 20, 561–567. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Orekhov, A.N.; Bobryshev, Y.V. Dendritic cells: A double-edge sword in atherosclerotic inflammation. Curr. Pharm. Des. 2015, 21, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Favre, D.; Mold, J.; Hunt, P.W.; Kanwar, B.; Loke, P.; Seu, L.; Barbour, J.D.; Lowe, M.M.; Jayawardene, A.; Aweeka, F.; et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med. 2010, 2, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Cuffy, M.C.; Silverio, A.M.; Qin, L.; Wang, Y.; Eid, R.; Brandacher, G.; Lakkis, F.G.; Fuchs, D.; Pober, J.S.; Tellides, G. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J. Immunol. 2007, 179, 5246–5254. [Google Scholar] [CrossRef] [PubMed]
- Hwu, P.; Du, M.X.; Lapointe, R.; Do, M.; Taylor, M.W.; Young, H.A. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 2000, 164, 3596–3599. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Ramprasath, T.; Wang, H.; Zou, M.H. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell. Mol. Life Sci. 2017, 74, 2899–2916. [Google Scholar] [CrossRef] [PubMed]
- Niinisalo, P.; Oksala, N.; Levula, M.; Pelto-Huikko, M.; Järvinen, O.; Salenius, J.P.; Kytömäki, L.; Soini, J.T.; Kähönen, M.; Laaksonen, R.; et al. Activation of indoleamine 2,3-dioxygenase-induced tryptophan degradation in advanced atherosclerotic plaques: Tampere vascular study. Ann. Med. 2010, 42, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.R.; Tuseth, N.; Eussen, S.J.; Ueland, P.M.; Strand, E.; Svingen, G.F.; Midttun, Ø.; Meyer, K.; Mellgren, G.; Ulvik, A.; et al. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Liuzzo, G. Pathogenesis of acute coronary syndromes. J. Am. Coll. Cardiol. 2013, 61, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yu, X.; Ding, Y.J.; Fu, Q.Q.; Xie, J.J.; Tang, T.T.; Yao, R.; Chen, Y.; Liao, Y.H. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin. Immunol. 2008, 127, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Biasucci, L.M.; Trotta, G.; Brugaletta, S.; Pinnelli, M.; Digianuario, G.; Rizzello, V.; Rebuzzi, A.G.; Rumi, C.; Maseri, A.; et al. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J. Am. Coll. Cardiol. 2007, 50, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Mälarstig, A.; Eriksson, P.; Hamsten, A.; Lindahl, B.; Wallentin, L.; Siegbahn, A. Raised interleukin-10 is an indicator of poor outcome and enhanced system inflammation in patients with acute coronary syndrome. Heart 2008, 94, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, S.; Dazhu, L.; Qiutang, Z.; Yibo, F.; Yushu, L.; Xiang, W.; Shen, C.L.; Yuan, T. Differentiation of dendritic cells in monocyte cultures isolated from patients with unstable angina. Int. J. Cardiol. 2004, 97, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Yang, K.; Hu, Y.; Zeng, Q. Toll-like receptor-4 and mitogen-activated protein kinase signal system are involved in activation of dendritic cells in patients with acute coronary syndrome. Immunology 2008, 125, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Brochez, L.; Chevolet, I.; Kruse, V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur. J. Cancer 2017, 76, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.E.; Astola, N.; Cribbs, A.P.; Goddard, M.E.; Park, I.; Green, P.; Davies, A.H.; Williams, R.O.; Feldmann, M.; Monaco, C. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc. Natl. Acad. Sci. USA 2015, 112, 13033–13038. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, K.A.; Ovchinnikova, O.; Berg, M.; Baumgartner, R.; Agardh, H.; Pirault, J.; Gisterå, A.; Assinger, A.; Laguna-Fernandez, A.; Bäck, M.; et al. Inhibition of indoleamine 2,3-dioxygenase promotes vascular inflammation and increases atherosclerosis in Apoe−/− mice. Cardiovasc. Res. 2015, 106, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ovchinnikova, O.; Jönsson, A.; Lundberg, A.M.; Berg, M.; Hansson, G.K.; Ketelhuth, D.F. The tryptophan metabolite 3-hydroxyanthranilic acid lowers plasma lipids and decreases atherosclerosis in hypercholesterolaemic mice. Eur. Heart J. 2012, 33, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Daissormont, I.T.; Christ, A.; Temmerman, L.; SampedroMillares, S.; Seijkens, T.; Manca, M.; Rousch, M.; Poggi, M.; Boon, L.; van der Loos, C.; et al. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ. Res. 2011, 109, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Flego, D.; Bianco, M.; Quattrini, A.; Mancini, F.; Carollo, M.; Schiavoni, I.; Ciervo, A.; Ausiello, C.M.; Fedele, G. Chlamydia pneumoniae modulates human monocytes-derived dendritic cells functions driving the induction of Type 1/Type 17 inflammatory response. Microbes Infect. 2013, 15, 105–114. [Google Scholar] [CrossRef] [PubMed]
| HC | SA | NSTEMI | p-Value | |
|---|---|---|---|---|
| Number | 22 | 27 | 37 | |
| Sex (M/F) | 14/8 | 22/5 | 33/4 | 0.06 |
| Age (mean ± SD) | 64 ± 27 | 47 ± 32 | 66 ± 11 | 0.74 |
| RISK FACTORS | ||||
| Hypercholesterolemia, n (%) | 10 (45) | 13 (35) | 15 (41) | 0.82 |
| Hypertension, n (%) | 12 (54) | 19 (70) | 31 (83) | 0.051 |
| Smoking habit, n (%) | 3 (14) | 17 (63) | 21 (57) | <0.001 |
| Family History of IHD, n (%) | 5 (23) | 2 (7) | 14 (38) | 0.020 |
| Diabetes, n (%) | 5 (23) | 10 (37) | 7 (19) | 0.24 |
| Previous History | ||||
| NSTEMI, n (%) | NA | NA | 9 (24) | - |
| Previous PCI/CABG, n (%) | NA | NA | 9 (24) | - |
| Medications (at the time of blood sampling) | ||||
| Aspirin, n (%) | 2 (10) | 8 (30) | 17 (46) | 0.013 |
| Ticlopidin/Clopidogrel, n (%) | 1 (5) | 3 (11) | 7 (19) | 0.27 |
| β-blockers, n (%) | 3 (14) | 6 (22) | 12 (32) | 0.26 |
| ACE-inhibitors/ARBs, n (%) | 4 (18) | 8 (30) | 15 (41) | 0.20 |
| Statins, n (%) | 6 (27) | 8 (30) | 16 (43) | 0.37 |
| Insulin, n (%) | 2 (10) | 3 (11) | 3 (8) | 0.73 |
| Oral antidiabetic drugs, n (%) | 3 (14) | 7 (26) | 4 (11) | 0.71 |
| In-hospital Management | ||||
| cTnI > 0.01 ng/mL, n (%) | 0 | 0 | 37 (100) | - |
| Multi-vessel disease, n (%) | 0 | 13 (48) | 17 (46) | 0.86 |
| PCI/CABG for the index event, n (%) | 0 | 13 (48) | 28 (76) | <0.001 |
| Laboratory Assay (mean ± SD) | ||||
| Total Cholesterol (mg/dL) | 201 ± 40.9 | 193.8 ± 40.5 | 188 ± 36.6 | 0.58 |
| LDL (mg/dL) | 104 ± 37.1 | 107.4 ± 41.3 | 115 ± 35.28 | 0.42 |
| HDL (mg/dL) | 54.14 ± 13.3 | 50.8 ± 12.4 | 45.2 ± 11.2 | 0.063 |
| Triglycerides (mg/dL) | 117.9 ± 59.5 | 109.1 ± 40.6 | 149.6 ± 58.3 | 0.065 |
| WBC (109/L) | 8.27 ± 2.5 | 7.59 ± 2.56 | 8.74 ± 3.5 | 0.63 |
| Lymphocytes (109/L) | 1.82 ± 0.6 | 1.84 ± 0.4 | 1.44 ± 0.6 | 0.49 |
| Neutrophil (109/L) | 6.43 ± 3.12 | 4.69 ± 1.36 | 5.83 ± 2.86 | 0.14 |
| Monocytes (109/L) | 0.54 ± 0.26 | 0.44 ± 0.14 | 0.46 ± 0.20 | 0.20 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zara, C.; Severino, A.; Flego, D.; Ruggio, A.; Pedicino, D.; Giglio, A.F.; Trotta, F.; Lucci, C.; D’Amario, D.; Vinci, R.; et al. Indoleamine 2,3-Dioxygenase (IDO) Enzyme Links Innate Immunity and Altered T-Cell Differentiation in Non-ST Segment Elevation Acute Coronary Syndrome. Int. J. Mol. Sci. 2018, 19, 63. https://doi.org/10.3390/ijms19010063
Zara C, Severino A, Flego D, Ruggio A, Pedicino D, Giglio AF, Trotta F, Lucci C, D’Amario D, Vinci R, et al. Indoleamine 2,3-Dioxygenase (IDO) Enzyme Links Innate Immunity and Altered T-Cell Differentiation in Non-ST Segment Elevation Acute Coronary Syndrome. International Journal of Molecular Sciences. 2018; 19(1):63. https://doi.org/10.3390/ijms19010063
Chicago/Turabian StyleZara, Chiara, Anna Severino, Davide Flego, Aureliano Ruggio, Daniela Pedicino, Ada Francesca Giglio, Francesco Trotta, Claudia Lucci, Domenico D’Amario, Ramona Vinci, and et al. 2018. "Indoleamine 2,3-Dioxygenase (IDO) Enzyme Links Innate Immunity and Altered T-Cell Differentiation in Non-ST Segment Elevation Acute Coronary Syndrome" International Journal of Molecular Sciences 19, no. 1: 63. https://doi.org/10.3390/ijms19010063





