Differences in the Plasma Proteome of Patients with Hypothyroidism before and after Thyroid Hormone Replacement: A Proteomic Analysis
Abstract
:1. Introduction
2. Results
2.1. Biochemical Parameters of the Study Subjects
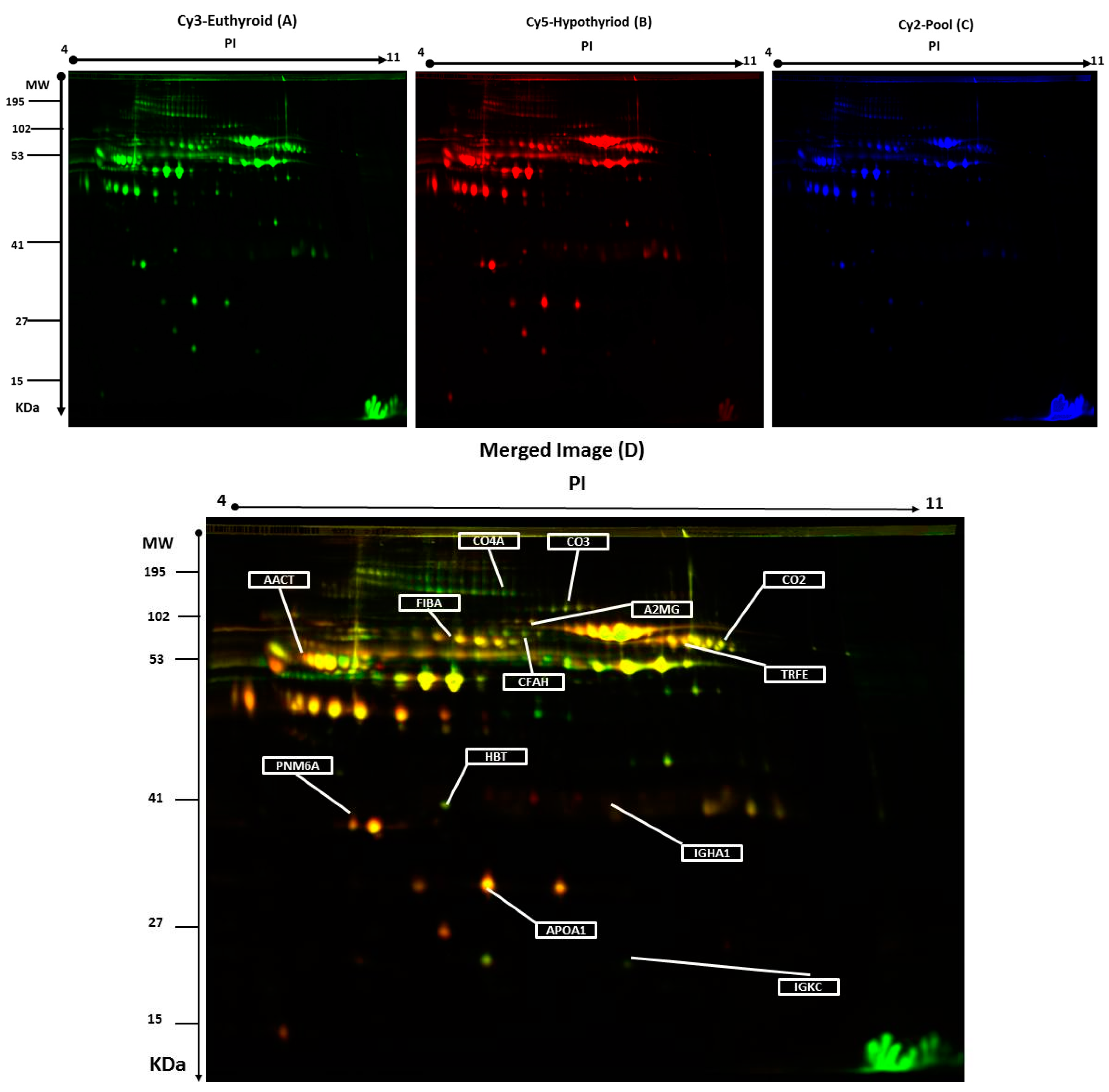
2.2. 2D-DIGE Analysis and Identification of Differentially Abundant Proteins
2.3. Confirmation of Changes in Selected Proteins by Immunoblotting
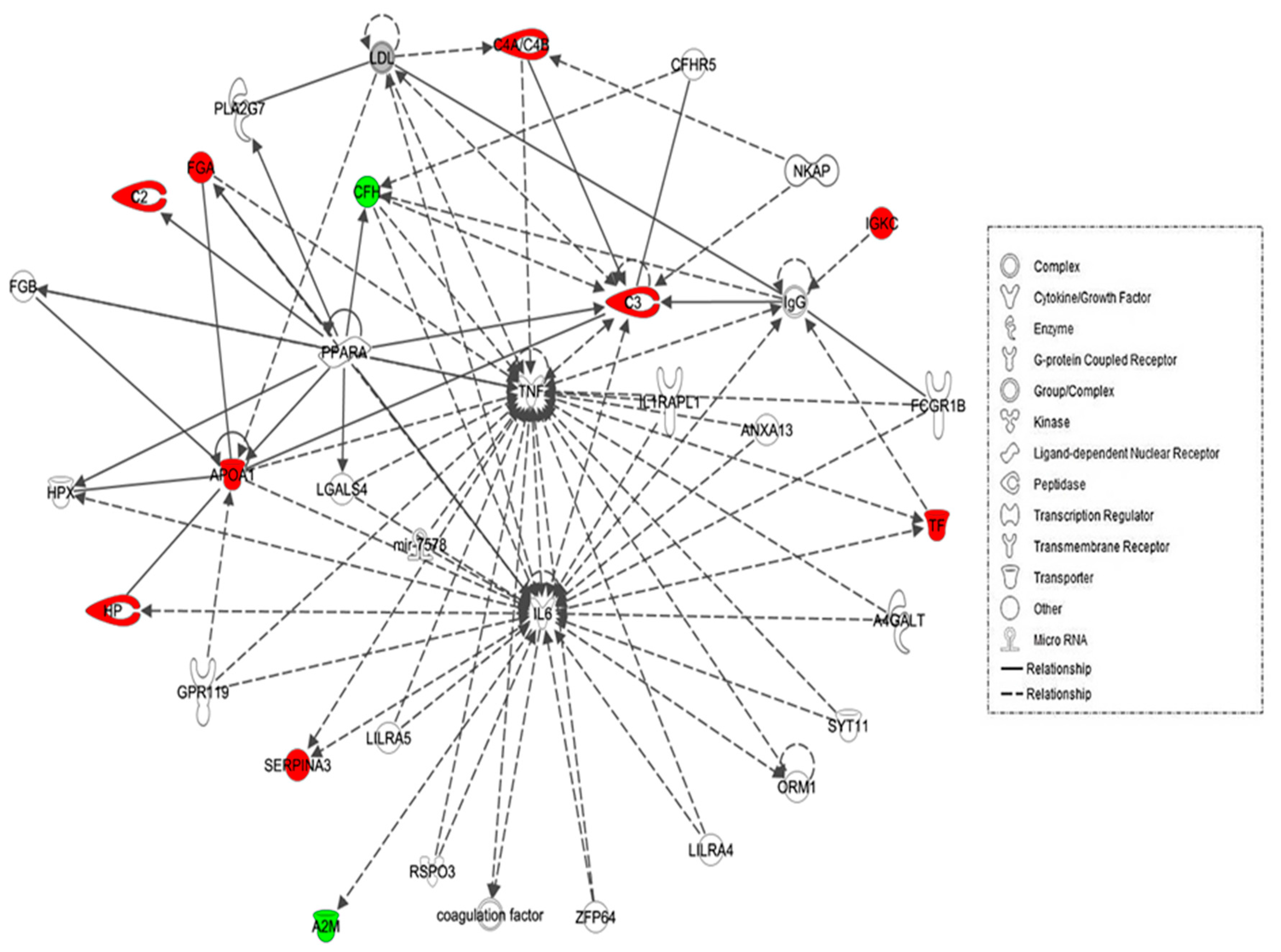
2.4. Protein–Protein Interaction Networks
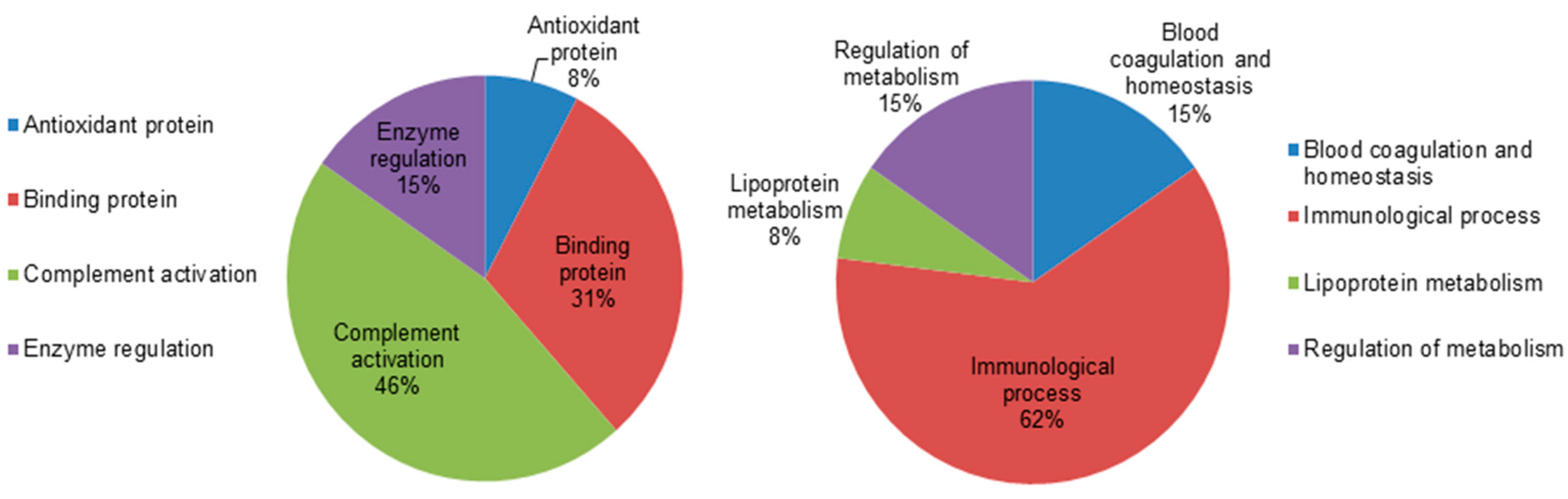
2.5. Classification of Key Proteins Based on Function
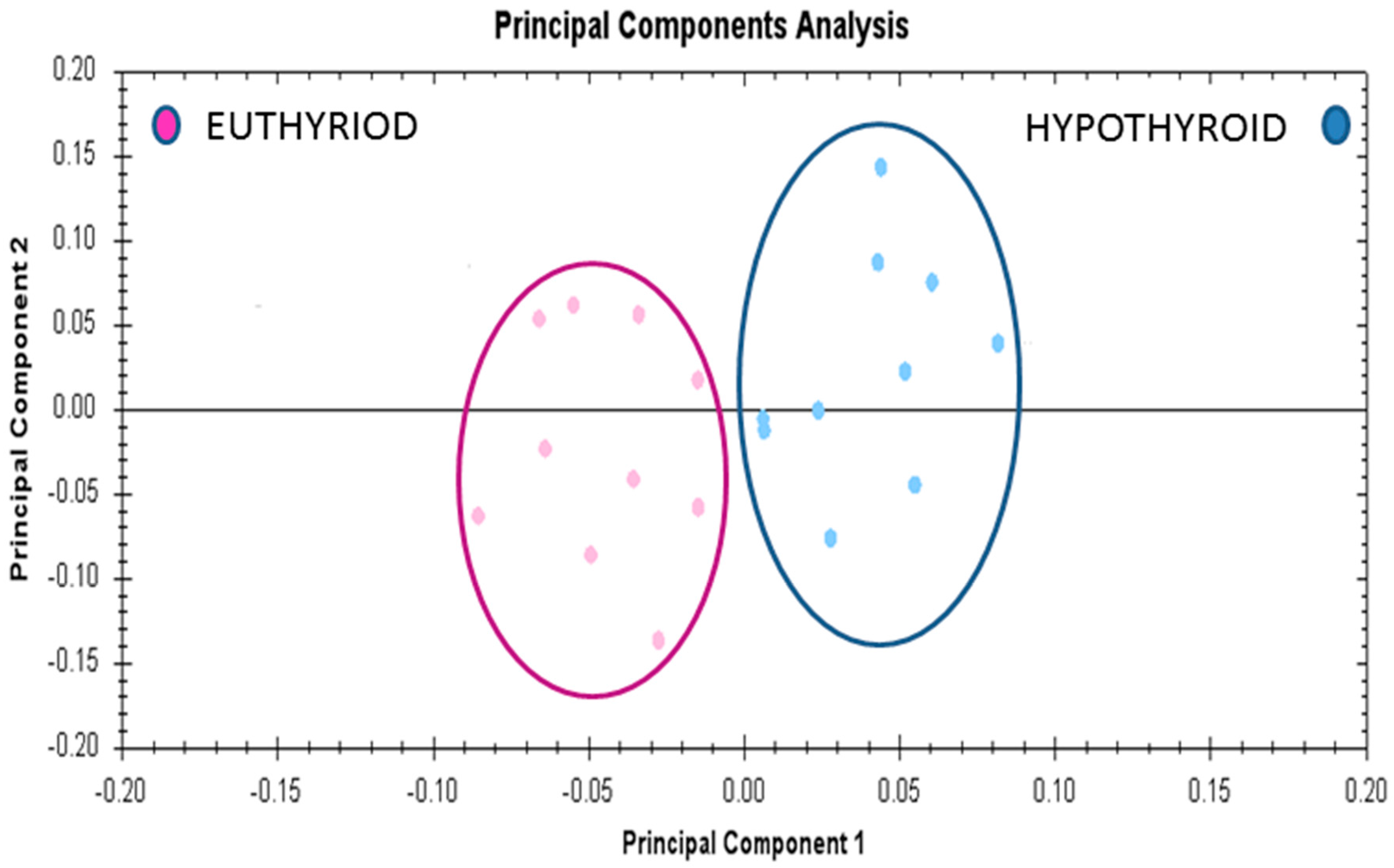
2.6. Principal Component Analyses (PCA) Analyses
3. Discussion
3.1. Proteins with Increased Abundance in the Hypothyroid vs. Euthyroid State
3.2. Proteins Found with Decreased Abundance in Hypothyroid vs. Euthyroid State
4. Materials and Methods
4.1. Ethical Considerations and Informed Consent
4.2. Study Design and Subjects
4.3. Biochemical Analysis
4.4. Sample Processing and Protein Extraction
4.5. CyDye Labeling, 2-D DIGE, and Imaging
4.6. Colloidal Coomassie Blue Staining of the Preparative Gel
4.7. Protein Identification by MALDI-TOF MS
4.8. Pathway Analysis
4.9. Immunoblotting
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N. Update in lipid alterations in subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2012, 97, 326–333. [Google Scholar] [CrossRef] [PubMed]
- De Vito, P.; Incerpi, S.; Pedersen, J.Z.; Luly, P.; Davis, F.B.; Davis, P.J. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid 2011, 21, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Klecha, A.J.; Barreiro Arcos, M.L.; Frick, L.; Genaro, A.M.; Cremaschi, G. Immune-endocrine interactions in autoimmune thyroid diseases. Neuroimmunomodulation 2008, 15, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Klecha, A.J.; Genaro, A.M.; Gorelik, G.; Barreiro Arcos, M.L.; Silberman, D.M.; Schuman, M.; Garcia, S.I.; Pirola, C.; Cremaschi, G.A. Integrative study of hypothalamus-pituitary-thyroid-immune system interaction: Thyroid hormone-mediated modulation of lymphocyte activity through the protein kinase C signaling pathway. J. Endocrinol. 2006, 189, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Helmke, S.M.; Hunsucker, S.W.; Netea-Maier, R.T.; Chiang, S.A.; Heinz, D.E.; Shroyer, K.R.; Duncan, M.W.; Haugen, B.R. Quantitative and qualitative differences in protein expression between papillary thyroid carcinoma and normal thyroid tissue. Mol. Carcinog. 2006, 45, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Paricharttanakul, N.M.; Saharat, K.; Chokchaichamnankit, D.; Punyarit, P.; Srisomsap, C.; Svasti, J. Unveiling a novel biomarker panel for diagnosis and classification of well-differentiated thyroid carcinomas. Oncol. Rep. 2016, 35, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.; Karger, S.; Schierhorn, A.; Poncin, S.; Many, M.C.; Fuhrer, D. Proteomic profiling of cold thyroid nodules. Endocrinology 2007, 148, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, E.; Glinni, D.; Cioffi, F.; Moreno, M.; Lombardi, A.; de Lange, P.; Senese, R.; Ceccarelli, M.; Salzano, A.M.; Scaloni, A.; et al. Metabolic effects of the iodothyronine functional analogue TRC150094 on the liver and skeletal muscle of high-fat diet fed overweight rats: An integrated proteomic study. Mol. Biosyst. 2012, 8, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Bischof, J.; Dirk, A.L.; Friedrich, N.; Hammer, E.; Thiele, T.; Fuhrer, D.; Homuth, G.; Brabant, G.; Volker, U. Effect of experimental thyrotoxicosis onto blood coagulation: A proteomics study. Eur. Thyroid J. 2015, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Masood, A.; Al-Naami, M.Y.; Chaurand, P.; Benabdelkamel, H. A proteomics based approach reveals differential regulation of visceral adipose tissue proteins between metabolically healthy and unhealthy obese patients. Mol. Cells 2017, 40, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, E.; Coppola, M.; Cioffi, F.; Goglia, F. Proteomic approaches for the study of tissue specific effects of 3,5,3′-triiodo-l-thyronine and 3,5-diiodo-l-thyronine in conditions of altered energy metabolism. Front. Physiol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; You, S.H.; Zhou, J.; Siddique, M.M.; Bay, B.H.; Zhu, X.; Privalsky, M.L.; Cheng, S.Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012, 122, 2428–2438. [Google Scholar] [CrossRef] [PubMed]
- Pietzner, M.; Engelmann, B.; Kacprowski, T.; Golchert, J.; Dirk, A.L.; Hammer, E.; Iwen, K.A.; Nauck, M.; Wallaschofski, H.; Fuhrer, D.; et al. Plasma proteome and metabolome characterization of an experimental human thyrotoxicosis model. BMC Med. 2017, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Lee, H.Y.; Shih, C.H.; Yen, C.C.; Chen, S.L.; Yang, R.C.; Wang, C.S. Plasma protein regulation by thyroid hormone. J. Endocrinol. 2003, 179, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.R. The immune system as a regulator of thyroid hormone activity. Exp. Biol. Med. 2006, 231, 229–236. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Blanchin, S.; Estienne, V.; Durand-Gorde, J.M.; Carayon, P.; Ruf, J. Complement activation by direct C4 binding to thyroperoxidase in Hashimoto’s thyroiditis. Endocrinology 2003, 144, 5422–5429. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, Y.; Zhang, M.; Lu, H.; Zhang, J.; Wang, H.; Cianflone, K. Thyroid status influence on adiponectin, acylation stimulating protein (ASP) and complement C3 in hyperthyroid and hypothyroid subjects. Nutr. Metab. 2006, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Poorgholami, M.; Izadi, N.; Nemati, M.; Rezayati, M. Immunological and hematological changes in patients with hyperthyroidism or hypothyroidism. Clin. Investig. Med. 2010, 33, E271–E279. [Google Scholar] [CrossRef]
- Parkes, A.B.; Othman, S.; Hall, R.; John, R.; Lazarus, J.H. Role of complement in the pathogenesis of postpartum thyroiditis: Relationship between complement activation and disease presentation and progression. Eur. J. Endocrinol. 1995, 133, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.F.; Palomaki, G.E.; Neveux, L.M.; Navolotskaia, O.; Ledue, T.B.; Craig, W.Y. Reference distributions for complement proteins C3 and C4: A practical, simple and clinically relevant approach in a large cohort. J. Clin. Lab. Anal. 2004, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ingram, G.; Hakobyan, S.; Robertson, N.P.; Morgan, B.P. Elevated plasma C4a levels in multiple sclerosis correlate with disease activity. J. Neuroimmunol. 2010, 223, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Rizos, C.V.; Elisaf, M.S.; Liberopoulos, E.N. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc. Med. J. 2011, 5, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Robbins, J. Lipoprotein-thyroid hormone interactions. Trends Endocrinol. Metab. 1993, 4, 194–198. [Google Scholar] [CrossRef]
- Kung, A.W.; Pang, R.W.; Janus, E.D. Elevated serum lipoprotein(a) in subclinical hypothyroidism. Clin. Endocrinol. 1995, 43, 445–449. [Google Scholar] [CrossRef]
- Ozata, M.; Yildirimkaya, M.; Yilmaz, K.; Kutluay, T.; Corakci, A.; Beyhan, Z.; Gundogan, M.A. The effects of thyroid status on serum apolipoprotein A-I-containing lipoprotein particles. Horm. Metab. Res. 1998, 30, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hernandez-Ono, A.; Graham, M.J.; Galton, V.A.; Ginsberg, H.N. Type 1 deiodinase regulates ApoA-I gene expression and ApoA-I synthesis independent of thyroid hormone signaling. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.L.; Goemann, I.M.; Meyer, E.L.; Wajner, S.M. Deiodinases: The balance of thyroid hormone: Type 1 iodothyronine deiodinase in human physiology and disease. J. Endocrinol. 2011, 209, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Storch, S.; Kubler, B.; Honing, S.; Ackmann, M.; Zapf, J.; Blum, W.; Braulke, T. Transferrin binds insulin-like growth factors and affects binding properties of insulin-like growth factor binding protein-3. FEBS Lett. 2001, 509, 395–398. [Google Scholar] [CrossRef]
- Marziali, L.N.; Garcia, C.I.; Pasquini, J.M. Transferrin and thyroid hormone converge in the control of myelinogenesis. Exp. Neurol. 2015, 265, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Erem, C.; Kavgaci, H.; Ersoz, H.O.; Hacihasanoglu, A.; Ukinc, K.; Karti, S.S.; Deger, O.; Telatari, M. Blood coagulation and fibrinolytic activity in hypothyroidism. Int. J. Clin. Pract. 2003, 57, 78–81. [Google Scholar] [PubMed]
- Chadarevian, R.; Jublanc, C.; Bruckert, E.; Giral, P.; Ankri, A.; Leenhardt, L.; Chapman, J.; Turpin, G. Effect of levothyroxine replacement therapy on coagulation and fibrinolysis in severe hypothyroidism. J. Endocrinol. Investig. 2005, 28, 398–404. [Google Scholar] [CrossRef]
- Van Vlierberghe, H.; Langlois, M.; Delanghe, J. Haptoglobin polymorphisms and iron homeostasis in health and in disease. Clin. Chim. Acta 2004, 345, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.A.; O’Donnell, J.J. Approaches to the treatment of cytomegalovirus retinitis: Ganciclovir and foscarnet. J. Acquir. Immune Defic. Syndr. 1991, 4, S11–S15. [Google Scholar] [PubMed]
- Braghin, E.; Galimberti, D.; Scarpini, E.; Bresolin, N.; Baron, P. α1-antichymotrypsin induces TNF-α production and NF-κB activation in the murine N9 microglial cell line. Neurosci. Lett. 2009, 467, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Belbin, O.; Kalsheker, N.; Morgan, K. SERPINA3 (aka α-1-antichymotrypsin). Front. Biosci. 2007, 12, 2821–2835. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.L.; Rizzo, N.; Liguori, C.; Zucca, G.; Faa, G. α-1-antichymotrypsin immunoreactivity in papillary carcinoma of the thyroid gland. Histopathology 1998, 33, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Hamada, N.; Saito, H.; Yoshimoto, M.; Iwasaki, H.; Ozaki, O.; Okamoto, Y.; Ito, K.; Morii, H. Inhibition by immunoglobulin G of synthesis of thyroid hormone in thyroid cultures from hypothyroid patients with goitrous Hashimoto’s thyroiditis. Acta Endocrinol. 1990, 123, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Yamada, T.; Sato, A.; Inazawa, K.; Aizawa, T. Elevation of serum immunoglobulin G in Hashimoto’s thyroiditis and decrease after treatment with l-thyroxine in hypothyroid patients. Intern. Med. 2010, 49, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Hye, A.; Lynham, S.; Thambisetty, M.; Causevic, M.; Campbell, J.; Byers, H.L.; Hooper, C.; Rijsdijk, F.; Tabrizi, S.J.; Banner, S.; et al. Proteome-based plasma biomarkers for Alzheimer’s disease. Brain 2006, 129, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Holers, V.M. The spectrum of complement alternative pathway-mediated diseases. Immunol. Rev. 2008, 223, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Okroj, M.; Corrales, L.; Stokowska, A.; Pio, R.; Blom, A.M. Hypoxia increases susceptibility of non-small cell lung cancer cells to complement attack. Cancer Immunol. Immunother. 2009, 58, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Schuller, M.; Jenne, D.; Voltz, R. The human PNMA family: Novel neuronal proteins implicated in paraneoplastic neurological disease. J. Neuroimmunol. 2005, 169, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Campillos, M.; Doerks, T.; Shah, P.K.; Bork, P. Computational characterization of multiple Gag-like human proteins. Trends Genet. 2006, 22, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Fujieda, M.; Otsuka, G.; Yang, J.P.; Okamoto, T.; Seo, H. A potential role of activated NF-κB in the pathogenesis of euthyroid sick syndrome. J. Clin. Investig. 2000, 106, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, R.A.; Watson, P.F.; Weetman, A.P. Cytokines and thyroid function. Adv. Neuroimmunol. 1996, 6, 359–386. [Google Scholar] [CrossRef]
- Marchiori, R.C.; Pereira, L.A.; Naujorks, A.A.; Rovaris, D.L.; Meinerz, D.F.; Duarte, M.M.; Rocha, J.B. Improvement of blood inflammatory marker levels in patients with hypothyroidism under levothyroxine treatment. BMC Endocr. Disord. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Lin, S.Y.; Yeh, Y.Y.; Hsiao, H.H.; Wu, C.Y.; Chen, S.T.; Wang, A.H. A modified protein precipitation procedure for efficient removal of albumin from serum. Electrophoresis 2005, 26, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Benabdelkamel, H.; Masood, A.; Moustafa, A.; Sallam, R.; Bassas, A.; Duncan, M. Proteomic analysis of mature adipocytes from obese patients in relation to aging. Exp. Gerontol. 2013, 48, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Hypothyroid | Euthyroid | p Value |
|---|---|---|---|
| Number of patients | 10 | 10 | |
| Fasting glucose (mmol/L) | 5.3 ± 0.4 | 5.0 ± 0.5 | 0.078 |
| Urea (mmol/L) | 4.7 ± 0.7 | 4.6 ± 0.9 | 0.392 |
| Creatinine (mmol/L) | 72.5 ± 13.1 | 76.1 ± 23.3 | 0.404 |
| Aspartate transaminase (IU/L) | 33.4 ± 6.6 | 37.5 ± 9.0 | 0.407 |
| Alanine transaminase (IU/L) | 18.0 ± 5.8 | 17.4 ± 3.9 | 0.169 |
| Alkaline phosphatase (IU/L) | 94.9 ± 25.9 | 96.8 ± 31.2 | 0.133 |
| Haemoglobin (g/L) | 13.1 ± 1.7 | 13.5 ± 2 | 0.50 |
| FT4 (pmol/L) | 8.3 ± 5.5 | 18.8 ± 3.7 | 0.000 |
| TSH (mIU/l) | 33.9 ± 22.1 | 1.6 ± 0.9 | 0.000 |
| Total Cholesterol (mmol/L) | 4.6 ± 0.6 | 4.8 ± 0.7 | 0.193 |
| Triglycerides (mmol/L) | 1.2 ± 0.3 | 1.4 ± 0.3 | 0.184 |
| High Density Lipoprotein cholesterol (mmol/L) | 2.9 ± 0.8 | 3.0 ± 0.6 | 0.373 |
| Low density Lipoprotein cholesterol (mmol/L) | 1.2 ± 0.4 | 1.0 ± 0.3 | 0.087 |
| TNF-α | 4.7 ± 1.4 | 3.2 ± 2 | 0.095 |
| IL-6 | 1.0 ± 0.65 | 0.68 ± 0.35 | 0.087 |
| Accession No a | MASCOT ID | Protein Name | Pi b | MW c | Coverage % | Score d | Ratio of Hypothyroid/Euthyroid | Fold Change e | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| P06681 | CO2_HUMAN | Complement C2 | 7.23 | 83,268 | 22 | 64 | up | 3.3 | 0.002 |
| P02787 | TRFE_HUMAN | Serotransferrin | 6.81 | 77,000 | 45 | 67 | up | 2.2 | 0.002 |
| P01024 | CO3_HUMAN | Complement C3 | 6.39 | 187,030 | 14 | 60 | up | 1.7 | 0.006 |
| P01834 | IGKC_HUMAN | Ig κ chain C region | 6.11 | 11,602 | 85 | 57 | up | 2.2 | 0.009 |
| P01011 | AACT_HUMAN | α-1-antichymotrypsin | 5.33 | 47,621 | 27 | 72 | up | 2.3 | 0.009 |
| P0C0L4 | CO4A_HUMAN | Complement C4-A | 6.66 | 1,926,500 | 28 | 63 | up | 3.4 | 0.01 |
| P00738 | HPT_HUMAN | Haptoglobin | 6.3 | 45,177 | 68 | 60 | up | 1.9 | 0.015 |
| P02671 | FIBA_HUMAN | Fibrinogen α chain | 5.10 | 94,914 | 23 | 75 | up | 1.7 | 0.010 |
| P02647 | APOA1_HUMAN | Apolipoprotein A-I | 5.56 | 30,759 | 34 | 69 | up | 2.2 | 0.013 |
| P01876 | IgGA1_HUMAN | Ig α-1 chain C region | 6.08 | 37,631 | 45 | 166 | up | 1.5 | 0.042 |
| P08603 | CFAH_HUMAN | Complement factor H | 6.23 | 139,068 | 27 | 72 | down | 2.5 | 0.033 |
| P0CW24 | PNM6A_HUMAN | Paraneoplastic antigen-like protein 6A | 5.24 | 43,875 | 35 | 133 | down | 2.1 | 0.034 |
| P01023 | A2MG_HUMAN | α-2-macroglobulin | 6.00 | 163,189 | 27 | 69 | down | 1.9 | 0.035 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfadda, A.A.; Benabdelkamel, H.; Masood, A.; Jammah, A.A.; Ekhzaimy, A.A. Differences in the Plasma Proteome of Patients with Hypothyroidism before and after Thyroid Hormone Replacement: A Proteomic Analysis. Int. J. Mol. Sci. 2018, 19, 88. https://doi.org/10.3390/ijms19010088
Alfadda AA, Benabdelkamel H, Masood A, Jammah AA, Ekhzaimy AA. Differences in the Plasma Proteome of Patients with Hypothyroidism before and after Thyroid Hormone Replacement: A Proteomic Analysis. International Journal of Molecular Sciences. 2018; 19(1):88. https://doi.org/10.3390/ijms19010088
Chicago/Turabian StyleAlfadda, Assim A., Hicham Benabdelkamel, Afshan Masood, Anwar A. Jammah, and Aishah A. Ekhzaimy. 2018. "Differences in the Plasma Proteome of Patients with Hypothyroidism before and after Thyroid Hormone Replacement: A Proteomic Analysis" International Journal of Molecular Sciences 19, no. 1: 88. https://doi.org/10.3390/ijms19010088