Neuroprotective, Neurogenic, and Amyloid Beta Reducing Effect of a Novel Alpha 2-Adrenoblocker, Mesedin, on Astroglia and Neuronal Progenitors upon Hypoxia and Glutamate Exposure
Abstract
:1. Introduction
2. Results
2.1. Mesedin Decreases the Lactate Dehydrogenase (LDH) Release from Astroglial Primary Culture
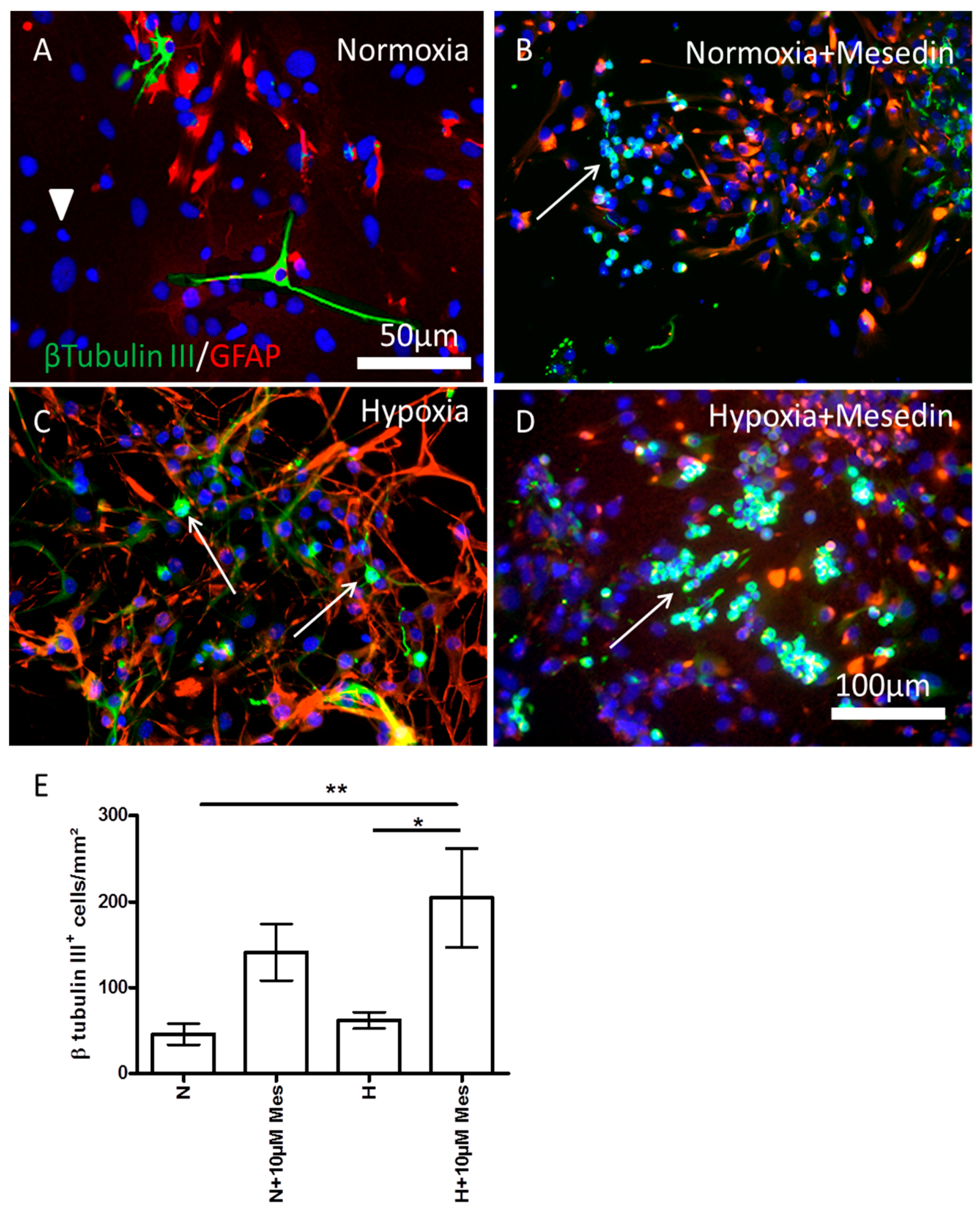
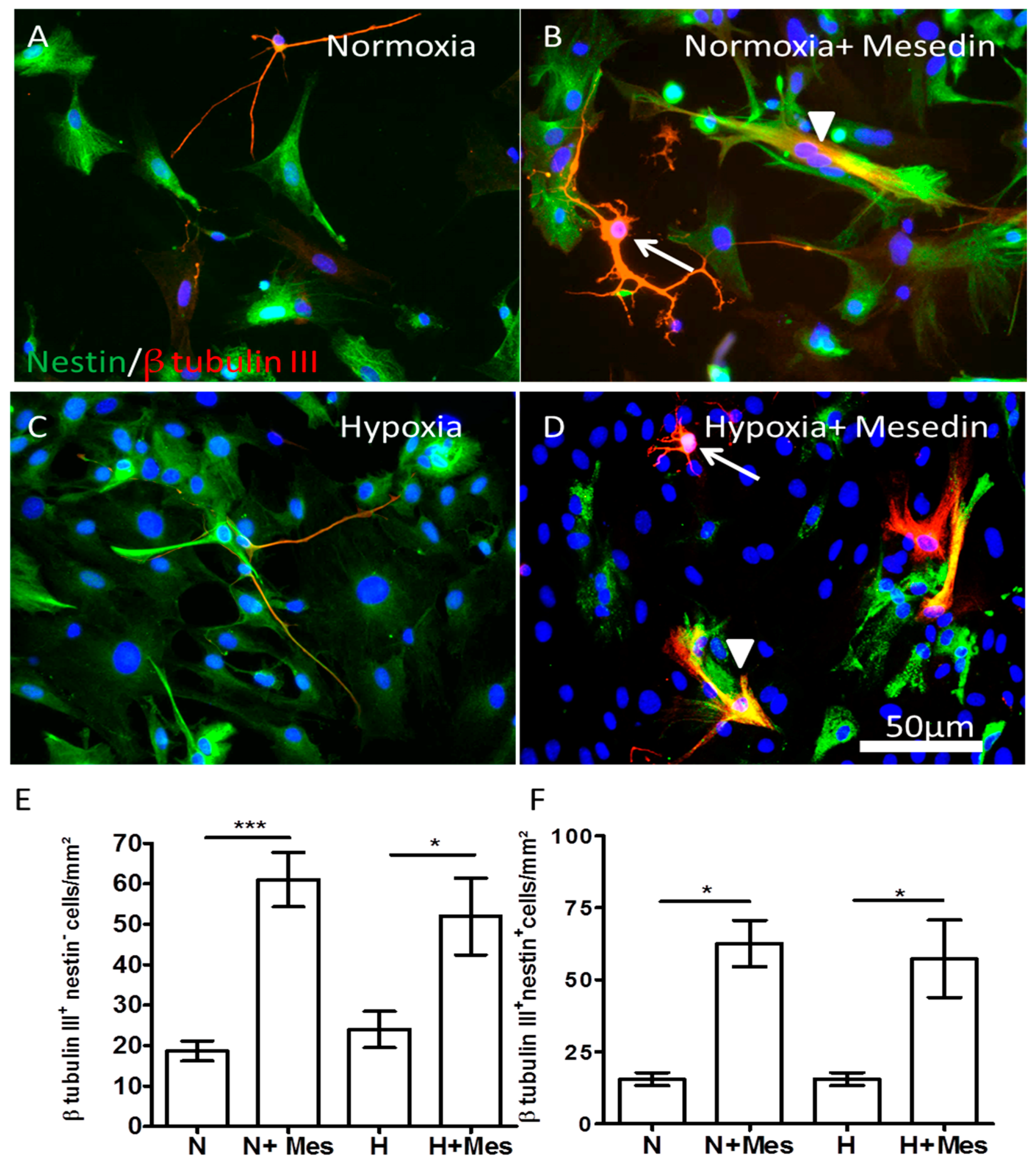
2.2. Mesedin Promotes Neuronal Commitment of Precursor Cells and Their Survival upon Hypoxia
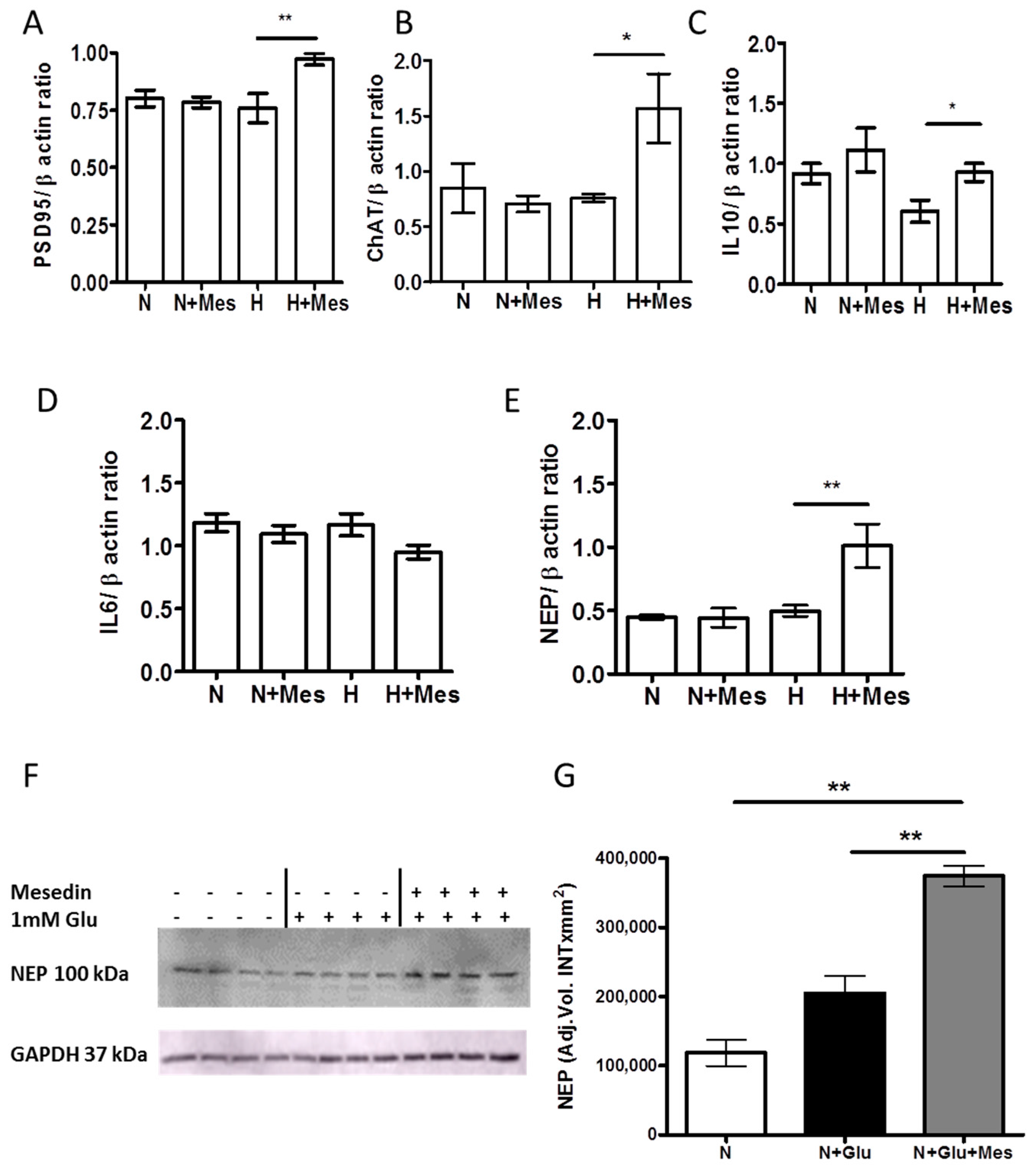
2.3. Mesedin Promotes the Expression of Interleukine-10, Postsynaptic Density Protein 95, and Choline Acetyltransferase in APC upon Hypoxia
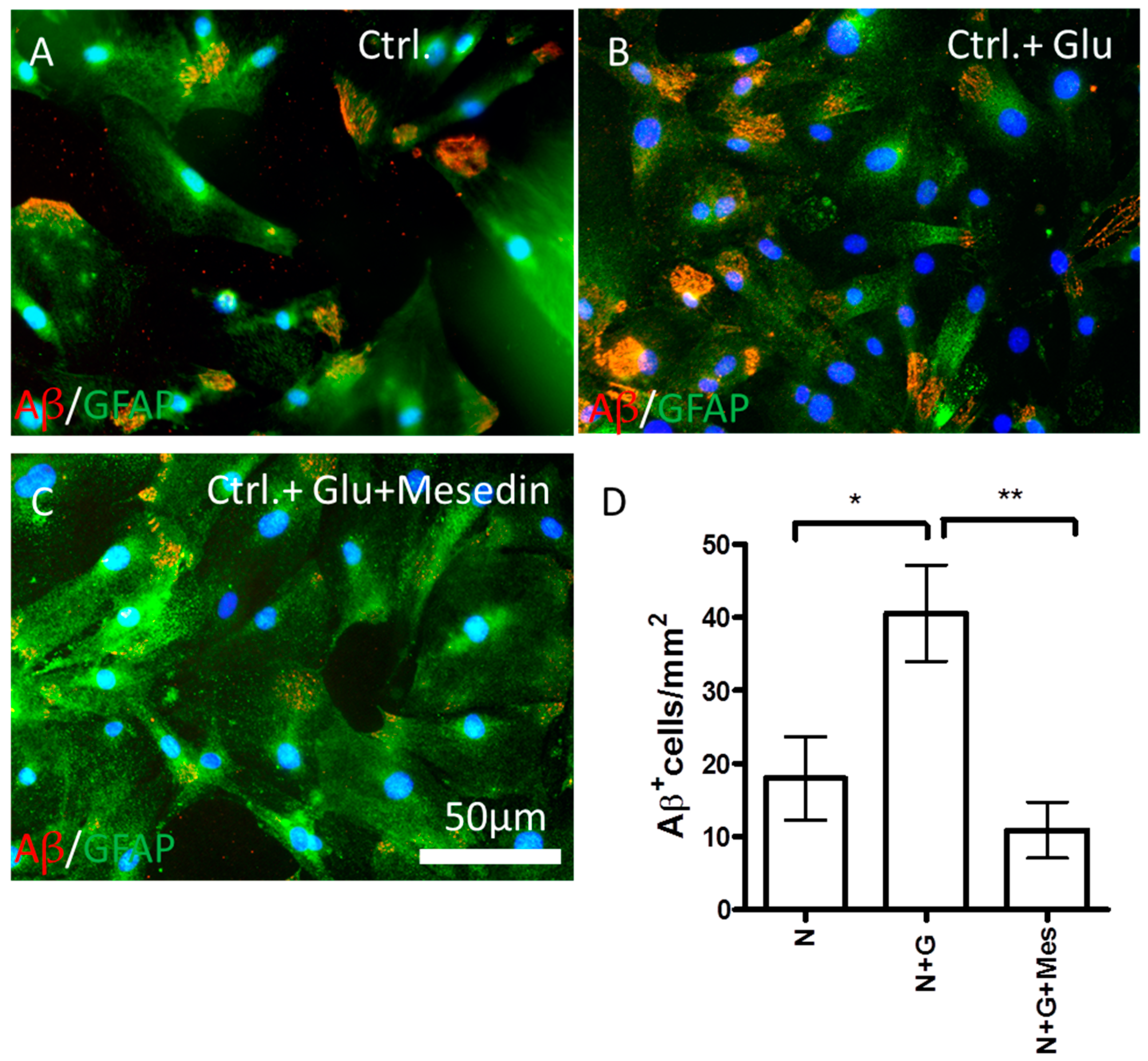
2.4. Mesedin Enhances the Aβ Metabolism by Astroglia from 3×Tg-AD Mice
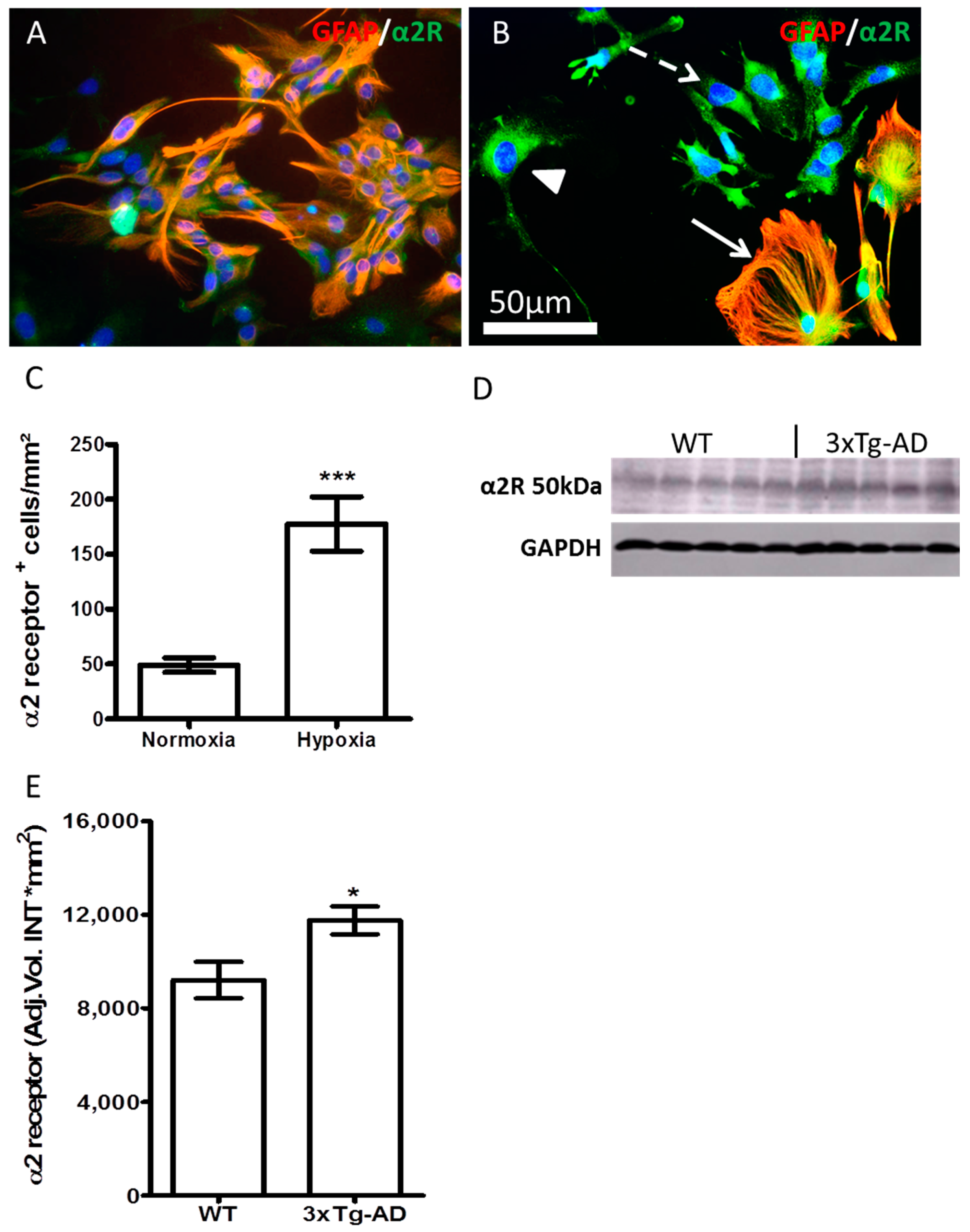
2.5. Hypoxia and AD-Like Condition Increase the Expression of α2 Receptor
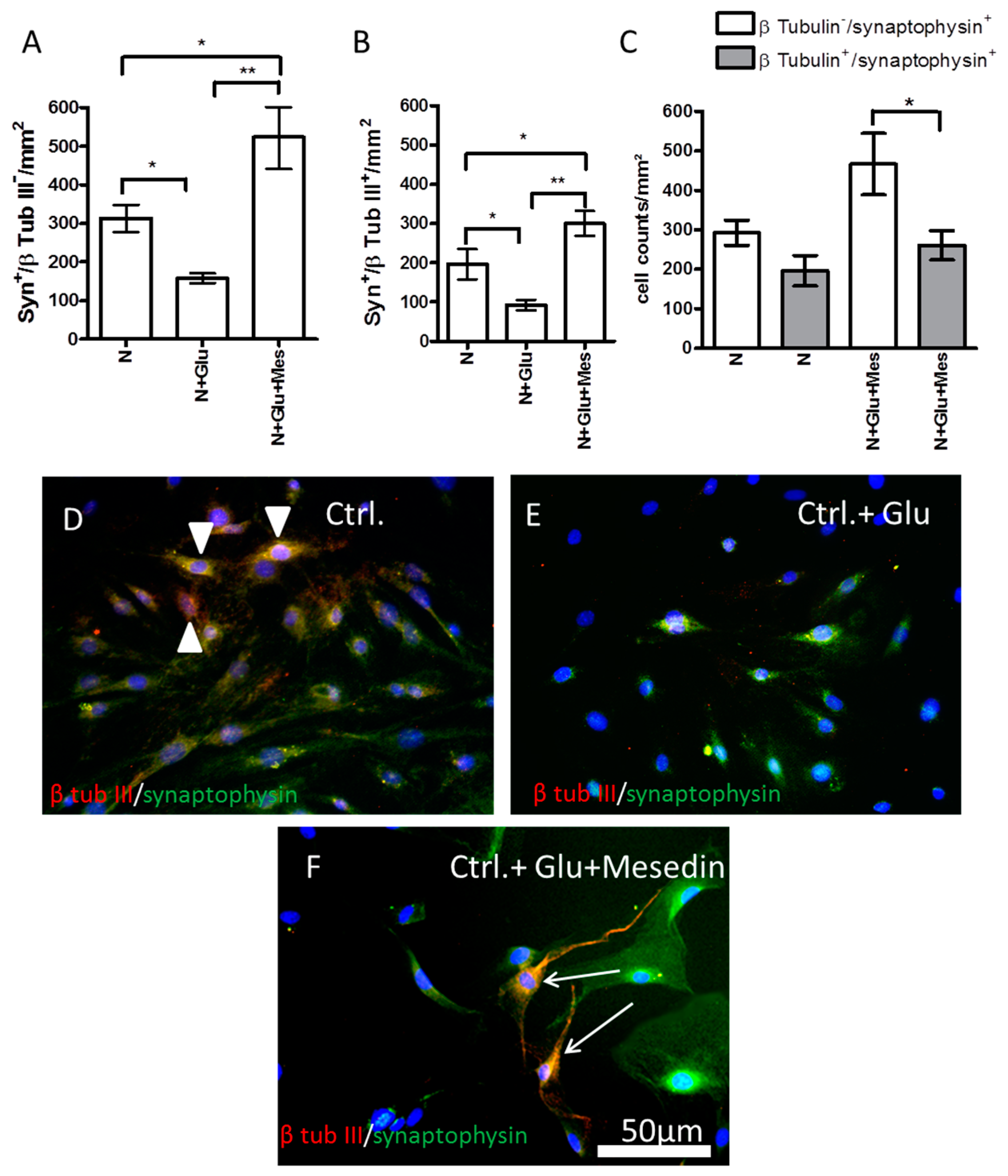
2.6. Increased Expression of Synaptophysin upon Mesedin in Astroglia and Neurons from 3×Tg-AD APC
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Immunocytochemistry
4.3. Western Blot Analysis
4.4. Cytotoxicity Assay
4.5. Quantitative PCR
4.6. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farzanehfar, P. Towards a Better Treatment Option for Parkinson’s Disease: A Review of Adult Neurogenesis. Neurochem. Res. 2016, 41, 3161–3170. [Google Scholar] [CrossRef] [PubMed]
- Hoglinger, G.U.; Rizk, P.; Muriel, M.P.; Duyckaerts, C.; Oertel, W.H.; Caille, I.; Hirsch, E.C. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004, 7, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.P.; Bix, G.J. Neuronal restoration following ischemic stroke: Influences, barriers, and therapeutic potential. Neurorehabil. Neural Repair 2013, 27, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.J.; Verkhratsky, A. Neurogenesis in Alzheimer’s disease. J. Anat. 2011, 219, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Ziabreva, I.; Perry, E.; Perry, R.; Minger, S.L.; Ekonomou, A.; Przyborski, S.; Ballard, C. Altered neurogenesis in Alzheimer’s disease. J. Psychosom. Res. 2006, 61, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, G.J.; Schiller, P.C.; Benoit, J.P.; Montero-Menei, C.N. Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials 2010, 31, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Tan, Y.; Tran, V.; Zhang, N.; Wen, X. Improve the viability of transplanted neural cells with appropriate sized neurospheres coated with mesenchymal stem cells. Med. Hypotheses 2012, 79, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Ronaghi, M.; Erceg, S.; Moreno-Manzano, V.; Stojkovic, M. Challenges of stem cell therapy for spinal cord injury: Human embryonic stem cells, endogenous neural stem cells, or induced pluripotent stem cells? Stem Cells 2010, 28, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, E.; Sanchez-Gomez, M.V.; Cavaliere, F.; Perez-Samartin, A.; Zugaza, J.L.; Trullas, R.; Domercq, M.; Matute, C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 2010, 47, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zhang, X.; Yang, D.; Luo, G.; Chen, S.; Le, W. Hypoxia increases Aβ generation by altering β- and γ-cleavage of APP. Neurobiol. Aging 2009, 30, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Cheng, B.; Davis, D.; Bryant, K.; Lieberburg, I.; Rydel, R.E. β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 1992, 12, 376–389. [Google Scholar] [PubMed]
- Haglund, M.; Sjobeck, M.; Englund, E. Locus ceruleus degeneration is ubiquitous in Alzheimer’s disease: Possible implications for diagnosis and treatment. Neuropathology 2006, 26, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Manaye, K.F.; Mouton, P.R.; Xu, G.; Drew, A.; Lei, D.L.; Sharma, Y.; Rebeck, G.W.; Turner, S. Age-related loss of noradrenergic neurons in the brains of triple transgenic mice. Age 2013, 35, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, S.; Polak, P.E.; Lin, S.X.; Sakharkar, A.J.; Pandey, S.C.; Feinstein, D.L. The noradrenaline precursor l-DOPS reduces pathology in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
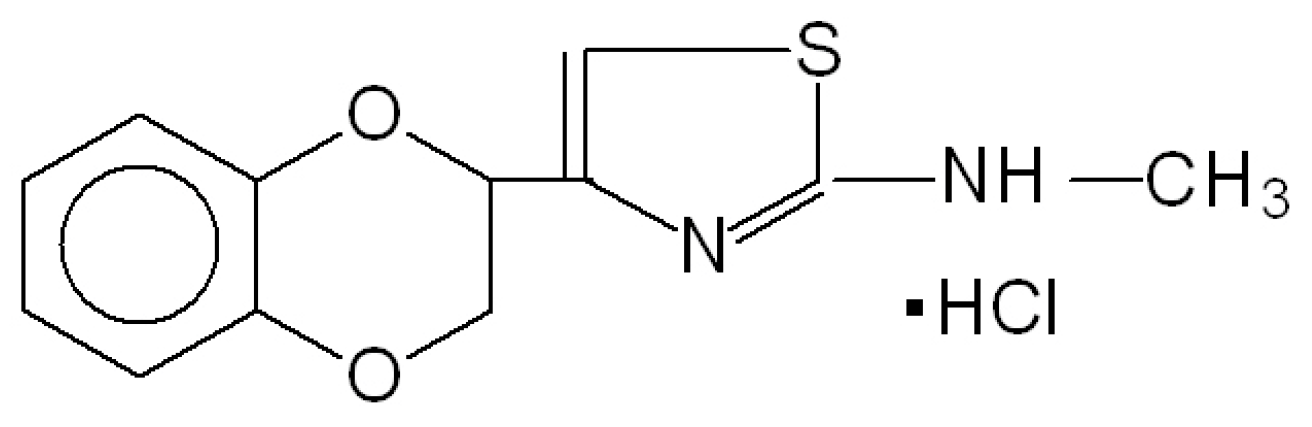
- Vartanyan, S.O.; Avakyan, A.S.; Sargsyan, A.B.; Arutyunyan, S.A.; Noravyan, O.S.; Tsatinyan, A.S. Synthesis and biologic properties of new thiazolylbenzodioxane derivatives. Russ. J. Org. Chem. 2016, 52, 244–248. [Google Scholar] [CrossRef]
- Shirinyan, E.A.; Harutyunyan, S.A. Mesedin—A new anti-hypoxic property possessing peripheral post-synaptic α2-Adrenoblocker. Med. Sci. Educ. 2015, 18, 27–31. [Google Scholar]
- Tananyan, A.; Balasanyan, M. Prevention of focal ischemia induced memory deficit and anxiety by mesedin. Eur. Neuropsychopharmacol. 2014, 24, S610. [Google Scholar] [CrossRef]
- Tananyan, A.G. The effect of mesedin on the behavior and memory of rats with disorders caused by stroke of the brain. Med. Sci. Armen. 2017, 57, 89–98. [Google Scholar]
- Melkonyan, M.M.; Hunanyan, L.S.; Manukyan, A.L.; Grigoryan, A.M.; Harutyunyan, H.A.; Poghosyan, G.A.; Tovmasyan, N.V. The effects of high level noise and α−adrenoblocker on the oxidation intensity in white rats blood. J. Med. Biol. Sci. 2015, 2, 5–10. [Google Scholar]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid β-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Farkas, E.; Luiten, P.G. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog. Neurobiol. 2001, 64, 575–611. [Google Scholar] [CrossRef]
- Salmon, D.P.; Bondi, M.W. Neuropsychological assessment of dementia. Annu. Rev. Psychol. 2009, 60, 257–282. [Google Scholar] [CrossRef] [PubMed]
- McCurry, S.M.; Gibbons, L.E.; Logsdon, R.G.; Teri, L. Anxiety and nighttime behavioral disturbances. Awakenings in patients with Alzheimer’s disease. J. Gerontol. Nurs. 2004, 30, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Tananyan, A.G.; Balasanyan, M.G.; Khostikian, C.G.; Mardanyan, L.S.; Eritsyan, E.L. Influence of mesedin on the morphological shifts in the brain tissue in conditions of local permanent ischemia. Med. Sci. Armen. 2017, 57, 33–42. [Google Scholar]
- Chen, Y.; Peng, Y.; Che, P.; Gannon, M.; Liu, Y.; Li, L.; Bu, G.; van Groen, T.; Jiao, K.; Wang, Q. α2A adrenergic receptor promotes amyloidogenesis through disrupting APP-SorLA interaction. Proc. Natl. Acad. Sci. USA 2014, 111, 17296–17301. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, L.; Abu-Rumeileh, S.; Fabris, M.; Pistis, C.; Baldi, A.; Sanvilli, N.; Curcio, F.; Gigli, G.L.; D’Anna, S.; Valente, M. Serum Interleukin-10 Levels Correlate with Cerebrospinal Fluid Amyloid β Deposition in Alzheimer Disease Patients. Neurodegener. Dis. 2017, 17, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.; Strauss, S.; Berger, M.; Volk, B.; Bauer, J. Inflammatory mechanisms in Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 1996, 246, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, S.; Thangavel, R.; Wu, Y.; Khan, M.M.; Kempuraj, D.; Zaheer, A. Enhanced expression of glia maturation factor correlates with glial activation in the brain of triple transgenic Alzheimer’s disease mice. Neurochem. Res. 2013, 38, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kiyota, T.; Walsh, S.M.; Liu, J.; Kipnis, J.; Ikezu, T. Cytokine-mediated inhibition of fibrillar amyloid-β peptide degradation by human mononuclear phagocytes. J. Immunol. 2008, 181, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; Oddo, S.; Sugarman, M.C.; Akbari, Y.; LaFerla, F.M. Age- and region-dependent alterations in Aβ-degrading enzymes: Implications for Aβ-induced disorders. Neurobiol. Aging 2005, 26, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Shirotani, K.; Lu, B.; Gerard, N.P.; Gerard, C.; Hama, E.; Lee, H.J.; Saido, T.C. Metabolic regulation of brain Aβ by neprilysin. Science 2001, 292, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Talantova, M.; Sanz-Blasco, S.; Zhang, X.; Xia, P.; Akhtar, M.W.; Okamoto, S.; Dziewczapolski, G.; Nakamura, T.; Cao, G.; Pratt, A.E.; et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. USA 2013, 110, E2518–E2527. [Google Scholar] [CrossRef] [PubMed]
- Nizari, S.; Guo, L.; Davis, B.M.; Normando, E.M.; Galvao, J.; Turner, L.A.; Bizrah, M.; Dehabadi, M.; Tian, K.; Cordeiro, M.F. Non-amyloidogenic effects of α2 adrenergic agonists: Implications for brimonidine-mediated neuroprotection. Cell Death Dis. 2016, 7, e2514. [Google Scholar] [CrossRef] [PubMed]
- Lourhmati, A.; Buniatian, G.H.; Paul, C.; Verleysdonk, S.; Buecheler, R.; Buadze, M.; Proksch, B.; Schwab, M.; Gleiter, C.H.; Danielyan, L. Age-dependent astroglial vulnerability to hypoxia and glutamate: The role for erythropoietin. PLoS ONE 2013, 8, e77182. [Google Scholar] [CrossRef] [PubMed]
- Hertz, L.; Lovatt, D.; Goldman, S.A.; Nedergaard, M. Adrenoceptors in brain: Cellular gene expression and effects on astrocytic metabolism and [Ca2+]i. Neurochem. Int. 2010, 57, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Stamer, W.D.; Anthony, T.L.; Kumar, D.V.; St John, P.A.; Regan, J.W. Expression of α2-adrenergic receptor subtypes in prenatal rat spinal cord. Brain Res. Dev. Brain Res. 2002, 133, 93–104. [Google Scholar] [CrossRef]
- Volgin, D.V.; Swan, J.; Kubin, L. Single-cell RT-PCR gene expression profiling of acutely dissociated and immunocytochemically identified central neurons. J. Neurosci. Methods 2004, 136, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Moyse, E.; Jourdan, F.; Colpaert, F.; Martel, J.C.; Marien, M. Effects of the α2-adrenoreceptor antagonist dexefaroxan on neurogenesis in the olfactory bulb of the adult rat in vivo: Selective protection against neuronal death. Neuroscience 2003, 117, 281–291. [Google Scholar] [CrossRef]
- Rizk, P.; Salazar, J.; Raisman-Vozari, R.; Marien, M.; Ruberg, M.; Colpaert, F.; Debeir, T. The α2-adrenoceptor antagonist dexefaroxan enhances hippocampal neurogenesis by increasing the survival and differentiation of new granule cells. Neuropsychopharmacology 2006, 31, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.E.; Bowser, D.N. Astrocytic adrenoceptors and learning: α1-adrenoceptors. Neurochem. Int. 2010, 57, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Gazarini, L.; Stern, C.A.; Carobrez, A.P.; Bertoglio, L.J. Enhanced noradrenergic activity potentiates fear memory consolidation and reconsolidation by differentially recruiting α1- and β-adrenergic receptors. Learn. Mem. 2013, 20, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Torkaman-Boutorabi, A.; Danyali, F.; Oryan, S.; Ebrahimi-Ghiri, M.; Zarrindast, M.R. Hippocampal α-adrenoceptors involve in the effect of histamine on spatial learning. Physiol. Behav. 2014, 129, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Doze, V.A.; Papay, R.S.; Goldenstein, B.L.; Gupta, M.K.; Collette, K.M.; Nelson, B.W.; Lyons, M.J.; Davis, B.A.; Luger, E.J.; Wood, S.G.; et al. Long-term α1A-adrenergic receptor stimulation improves synaptic plasticity, cognitive function, mood, and longevity. Mol. Pharmacol. 2011, 80, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, D.J.; Nanavaty, I.; Prosper, B.W.; Marathe, S.; Husain, B.F.; Kernie, S.G.; Bartlett, P.F.; Vaidya, V.A. Opposing effects of α2- and β-adrenergic receptor stimulation on quiescent neural precursor cell activity and adult hippocampal neurogenesis. PLoS ONE 2014, 9, e98736. [Google Scholar] [CrossRef] [PubMed]
- Traver, S.; Salthun-Lassalle, B.; Marien, M.; Hirsch, E.C.; Colpaert, F.; Michel, P.P. The neurotransmitter noradrenaline rescues septal cholinergic neurons in culture from degeneration caused by low-level oxidative stress. Mol. Pharmacol. 2005, 67, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Troadec, J.D.; Marien, M.; Darios, F.; Hartmann, A.; Ruberg, M.; Colpaert, F.; Michel, P.P. Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. J. Neurochem. 2001, 79, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Nolan, Y.M. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: Can exercise compensate? Neurosci. Biobehav. Rev. 2016, 61, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, T.; Ingraham, K.L.; Swan, R.J.; Jacobsen, M.T.; Andrews, S.J.; Ikezu, T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012, 19, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Yang, J.J.; Liao, J.F.; Wang, C.H.; Chiu, T.H.; Hsu, F.C. Chronic hypobaric hypoxia induces tolerance to acute hypoxia and up-regulation in α-2 adrenoceptor in rat locus coeruleus. Brain Res. 2006, 1106, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Hypoxia/ischemia activate processing of Amyloid Precursor Protein: Impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2017, 140, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Wierenga, C.E.; Hays, C.C.; Zlatar, Z.Z. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 411–419. [Google Scholar]
- Debeir, T.; Marien, M.; Ferrario, J.; Rizk, P.; Prigent, A.; Colpaert, F.; Raisman-Vozari, R. In vivo upregulation of endogenous NGF in the rat brain by the α2-adrenoreceptor antagonist dexefaroxan: Potential role in the protection of the basalocortical cholinergic system during neurodegeneration. Exp. Neurol. 2004, 190, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, D.M.; Hu, B.; Zheng, Y.L.; Zhang, Z.F.; Wang, Y.J. NGF-Dependent activation of TrkA pathway: A mechanism for the neuroprotective effect of troxerutin in D-galactose-treated mice. Brain Pathol. 2010, 20, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Zhou, Z.Y.; Hemmings, H.C., Jr. α2-Adrenergic Receptor and Isoflurane Modulation of Presynaptic Ca2+ Influx and Exocytosis in Hippocampal Neurons. Anesthesiology 2016, 125, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Buosi, A.S.; Matias, I.; Araujo, A.P.; Batista, C.; Gomes, F.C. Heterogeneity in Synaptogenic Profile of Astrocytes from Different Brain Regions. Mol. Neurobiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Hamprecht, B.; Loffler, F. Primary glial cultures as a model for studying hormone action. Methods Enzymol. 1985, 109, 341–345. [Google Scholar] [PubMed]
- Hansson, E. Cellular composition of a cerebral hemisphere primary culture. Neurochem. Res. 1984, 9, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Oyarzabal, E.A.; Sung, Y.F.; Chu, C.H.; Wang, Q.; Chen, S.L.; Lu, R.B.; Hong, J.S. Microglial Regulation of Immunological and Neuroprotective Functions of Astroglia. Glia 2015, 63, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.; Mueller, L.; Buecheler, R.; Proksch, B.; Schwab, M.; Gleiter, C.H.; Danielyan, L. Interplay between endothelin and erythropoietin in astroglia: The role in protection against hypoxia. Int. J. Mol. Sci. 2014, 15, 2858–2875. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, L.; Gembizki, O.; Proksch, B.; Weinmann, M.; Morgalla, M.; Wiesinger, H.; Buniatian, G.H.; Gleiter, C.H. The blockade of endothelin A receptor protects astrocytes against hypoxic injury: Common effects of BQ-123 anderythropoietin on the rejuvenation of the astrocyte population. Eur. J. Cell Biol. 2005, 84, 567–579. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melkonyan, M.M.; Hunanyan, L.; Lourhmati, A.; Layer, N.; Beer-Hammer, S.; Yenkoyan, K.; Schwab, M.; Danielyan, L. Neuroprotective, Neurogenic, and Amyloid Beta Reducing Effect of a Novel Alpha 2-Adrenoblocker, Mesedin, on Astroglia and Neuronal Progenitors upon Hypoxia and Glutamate Exposure. Int. J. Mol. Sci. 2018, 19, 9. https://doi.org/10.3390/ijms19010009
Melkonyan MM, Hunanyan L, Lourhmati A, Layer N, Beer-Hammer S, Yenkoyan K, Schwab M, Danielyan L. Neuroprotective, Neurogenic, and Amyloid Beta Reducing Effect of a Novel Alpha 2-Adrenoblocker, Mesedin, on Astroglia and Neuronal Progenitors upon Hypoxia and Glutamate Exposure. International Journal of Molecular Sciences. 2018; 19(1):9. https://doi.org/10.3390/ijms19010009
Chicago/Turabian StyleMelkonyan, Magda M., Lilit Hunanyan, Ali Lourhmati, Nikolas Layer, Sandra Beer-Hammer, Konstantin Yenkoyan, Matthias Schwab, and Lusine Danielyan. 2018. "Neuroprotective, Neurogenic, and Amyloid Beta Reducing Effect of a Novel Alpha 2-Adrenoblocker, Mesedin, on Astroglia and Neuronal Progenitors upon Hypoxia and Glutamate Exposure" International Journal of Molecular Sciences 19, no. 1: 9. https://doi.org/10.3390/ijms19010009





