Deciphering Auxin-Ethylene Crosstalk at a Systems Level
Abstract
:1. An Overview of Auxin and Ethylene Pathways in Plants
2. Auxin-Ethylene Crosstalk at the Molecular Level
2.1. Auxin Pathways Possess Ethylene Targets
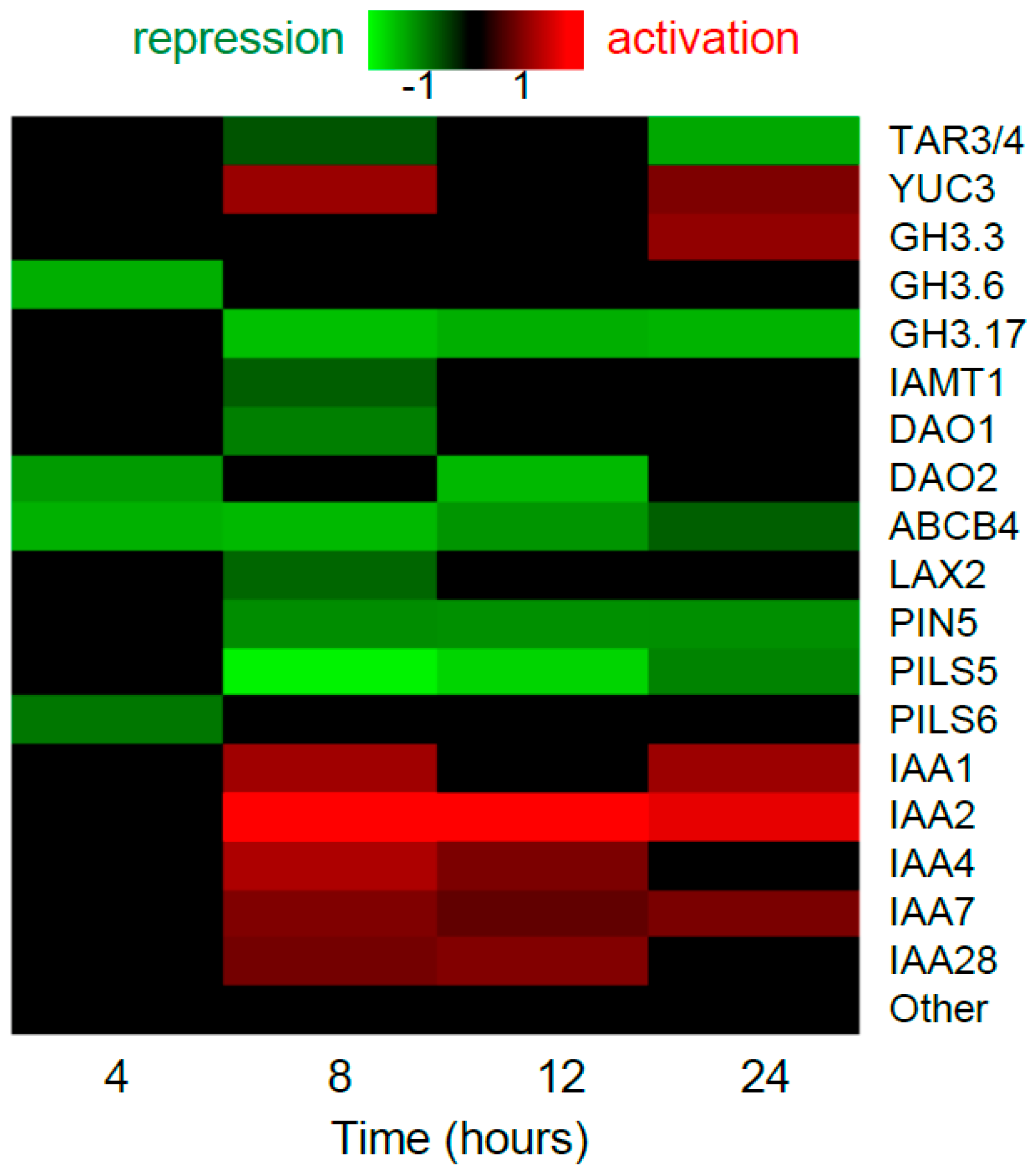
2.1.1. Ethylene Regulates Expression of Auxin Homeostasis Genes
2.1.2. Ethylene Modulates the Abundance of Auxin Transporters
2.1.3. Ethylene Affects Auxin Signaling
2.2. Auxin Reciprocally Regulates Ethylene Pathways
2.2.1. Auxin Drastically Affects Ethylene Biosynthesis
2.2.2. Auxin Might Mediate ACC Transport
2.2.3. Auxin Modulates Ethylene Signaling
3. Auxin-Ethylene Crosstalk at the Systems Level
3.1. Ethylene-Induced Auxin Accumulation: Inhibition of Root Elongation
3.2. Ethylene-Induced Auxin Depletion: Inhibition of Lateral Root Initiation
3.3. Ethylene-Mediated Asymmetry in Auxin Distribution: Apical Hook Formation
3.4. The Convergence of Auxin and Ethylene Pathways in Root Hair Development
4. Mathematical Modeling of Auxin-Ethylene Crosstalk
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mroue, S.; Simeunovic, A.; Robert, H.S. Auxin production as an integrator of environmental cues for developmental growth regulation. J. Exp. Bot. 2018, 69, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu. Rev. Plant Biol. 2018, 69, 417–435. [Google Scholar] [CrossRef]
- Korver, R.A.; Koevoets, I.T.; Testerink, C. Out of shape during stress: A key role for auxin. Trends Plant Sci. 2018, 23, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Radwanski, E.R.; Last, R.L. Tryptophan biosynthesis and metabolism: Biochemical and molecular genetics. Plant Cell 1995, 7, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Marchant, A.; Bhalerao, R.; Casimiro, I.; Eklöf, J.; Casero, P.J.; Bennett, M.; Sandberg, G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 2002, 14, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Péret, B.; Swarup, K.; Ferguson, A.; Seth, M.; Yang, Y.; Dhondt, S.; James, N.; Casimiro, I.; Perry, P.; Syed, A.; et al. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell 2012, 24, 2874–2885. [Google Scholar] [CrossRef] [PubMed]
- Zazímalová, E.; Krecek, P.; Skůpa, P.; Hoyerová, K.; Petrásek, J. Polar transport of the plant hormone auxin—The role of PIN-FORMED (PIN) proteins. Cell. Mol. Life Sci. 2007, 64, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Cho, H.T. The function of ABCB transporters in auxin transport. Plant Signal. Behav. 2013, 8, e22990. [Google Scholar] [CrossRef] [PubMed]
- Barbez, E.; Kubeš, M.; Rolčík, J.; Béziat, C.; Pěnčík, A.; Wang, B.; Rosquete, M.R.; Zhu, J.; Dobrev, P.I.; Lee, Y.; et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 2012, 485, 119–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal Bosco, C.; Dovzhenko, A.; Palme, K. Intracellular auxin transport in pollen: PIN8, PIN5 and PILS5. Plant Signal. Behav. 2012, 7, 1504–1505. [Google Scholar] [CrossRef] [Green Version]
- Vanderstraeten, L.; van der Straeten, D. Accumulation and transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: Current status, considerations for future research and agronomic applications. Front. Plant Sci. 2017, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Vandenbussche, F.; van der Straeten, D. Regulation of seedling growth by ethylene and the ethylene–auxin crosstalk. Planta 2017, 245, 467–489. [Google Scholar] [CrossRef] [PubMed]
- Zemlyanskaya, E.V.; Omelyanchuk, N.A.; Ermakov, A.A.; Mironova, V.V. Mechanisms regulating ethylene signal transduction in plants. Russ. J. Genet. Appl. Res. 2017, 7, 335–344. [Google Scholar] [CrossRef]
- van de Poel, B.; van der Straeten, D. 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: More than just the precursor of ethylene! Front. Plant Sci. 2014, 5, 640. [Google Scholar] [CrossRef]
- Zhang, F.; Qi, B.; Wang, L.; Zhao, B.; Rode, S.; Riggan, N.D.; Ecker, J.R.; Qiao, H. EIN2-dependent regulation of acetylation of histone H3K14 and non-canonical histone H3K23 in ethylene signalling. Nat. Commun. 2016, 7, 13018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Wang, L.; Qi, B.; Zhao, B.; Ko, E.E.; Riggan, N.D.; Chin, K.; Qiao, H. EIN2 mediates direct regulation of histone acetylation in the ethylene response. Proc. Natl. Acad. Sci. USA 2017, 114, 10274–10279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.N.; Zhong, S.; Weirauch, M.T.; Hon, G.; Pelizzola, M.; Li, H.; Huang, S.S.; Schmitz, R.J.; Urich, M.A.; Kuo, D.; et al. Temporal transcriptional response to ethylene gas drives growth hormone cross-regulation in Arabidopsis. eLife 2013, 2, e00675. [Google Scholar] [CrossRef] [PubMed]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant Sci. 2012, 17, 181–195. [Google Scholar] [CrossRef]
- Lewis, D.R.; Olex, A.L.; Lundy, S.R.; Turkett, W.H.; Fetrow, J.S.; Muday, G.K. A kinetic analysis of the auxin transcriptome reveals cell wall remodeling proteins that modulate lateral root development in Arabidopsis. Plant Cell 2013, 25, 3329–3346. [Google Scholar] [CrossRef]
- Harkey, A.F.; Watkins, J.M.; Olex, A.L.; DiNapoli, K.T.; Lewis, D.R.; Fetrow, J.S.; Binder, B.M.; Muday, G.K. Identification of transcriptional and receptor networks that control root responses to ethylene. Plant Physiol. 2018, 176, 2095–2118. [Google Scholar] [CrossRef]
- Mao, J.L.; Miao, Z.Q.; Wang, Z.; Yu, L.H.; Cai, X.T.; Xiang, C.B. Arabidopsis ERF1 mediates cross-talk between ethylene and auxin biosynthesis during primary root elongation by regulating ASA1 expression. PLoS Genet. 2016, 12, e1005760. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.N.; Robertson-Hoyt, J.; Yun, J.; Benavente, L.M.; Xie, D.Y.; Doležal, K.; Schlereth, A.; Jürgens, G.; Alonso, J.M. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 2008, 133, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nishiyama, R.; Watanabe, Y.; Van Ha, C.; Kojima, M.; An, P.; Tian, L.; Tian, C.; Sakakibara, H.; Tran, L.P. Effects of overproduced ethylene on the contents of other phytohormones and expression of their key biosynthetic genes. Plant Physiol. Biochem. 2018, 128, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Růzicka, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Perry, P.; Hagenbeek, D.; van der Straeten, D.; Beemster, G.T.; Sandberg, G.; Bhalerao, R.; Ljung, K.; Bennett, M.J. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 2007, 19, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Negi, S.; Sukumar, P.; Muday, G.K. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 2011, 138, 3485–3495. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.Q.; Zhao, P.X.; Mao, J.; Yu, L.; Yuan, Y.; Tang, H.; Liu, Z.B.; Xiang, C. Arabidopsis HB52 mediates the crosstalk between ethylene and auxin by transcriptionally modulating PIN2, WAG1, and WAG2 during primary root elongation. Plant Cell 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nodzynski, T.; Pencík, A.; Rolcík, J.; Friml, J. PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc. Natl. Acad. Sci. USA 2010, 107, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.N.; Yun, J.; Likhacheva, A.V.; Alonso, J.M. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 2007, 19, 2169–2185. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, I.I.; Qudeimat, E.; Potuschak, T.; Du, Y.; Genschik, P.; Vandenbussche, F.; van der Straeten, D. The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc. Natl. Acad. Sci. USA 2018, 115, E4130–E4139. [Google Scholar] [CrossRef] [PubMed]
- Lehman, A.; Black, R.; Ecker, J.R. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 1996, 85, 183–194. [Google Scholar] [CrossRef]
- An, F.; Zhang, X.; Zhu, Z.; Ji, Y.; He, W.; Jiang, Z.; Li, M.; Guo, H. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 2012, 22, 915–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Johnson, P.; Stepanova, A.; Alonso, J.M.; Ecker, J.R. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev. Cell 2004, 7, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, Y.; Chen, X.; Li, L.; Liu, K.; Zhao, H.; Wang, Y.; Han, S. ERF72 interacts with ARF6 and BZR1 to regulate hypocotyl elongation in Arabidopsis. J. Exp. Bot. 2018, 69, 3933–3947. [Google Scholar] [CrossRef] [PubMed]
- Cherenkov, P.; Novikova, D.; Omelyanchuk, N.; Levitsky, V.; Grosse, I.; Weijers, D.; Mironova, V. Diversity of cis-regulatory elements associated with auxin response in Arabidopsis thaliana. J. Exp. Bot. 2018, 69, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Woeste, K.E.; Vogel, J.P.; Kieber, J.J. Factors regulating ethylene biosynthesis in etiolated Arabidopsis thaliana seedlings. Physiol. Plant 1999, 105, 478–484. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chen, Y.C.; Kieber, J.J.; Yoon, G.M. Regulation of the turnover of ACC synthases by phytohormones and heterodimerization in Arabidopsis. Plant J. 2017, 91, 491–504. [Google Scholar] [CrossRef]
- Abel, S.; Nguyen, M.D.; Chow, W.; Theologis, A. ASC4, a primary indoleacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana: Structural characterization, expression in Escherichia coli, and expression characteristics in response to auxin. J. Biol. Chem. 1995, 270, 19093–19099. [Google Scholar] [CrossRef]
- Yamagami, T.; Tsuchisaka, A.; Yamada, K.; Haddon, W.F.; Harden, L.A.; Theologis, A. Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J. Biol. Chem. 2003, 278, 49102–49112. [Google Scholar] [CrossRef]
- Tsuchisaka, A.; Theologis, A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 2004, 136, 2982–3000. [Google Scholar] [CrossRef]
- Zheng, Z.; Guo, Y.; Novák, O.; Dai, X.; Zhao, Y.; Ljung, K.; Noel, J.P.; Chory, J. Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nat. Chem. Biol. 2013, 9, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Lee, S.; Song, W.Y.; Lee, R.A.; Lee, I.; Ha, K.; Koo, J.C.; Park, S.K.; Nam, H.G.; Lee, Y.; et al. Genetic identification of ACC-RESISTANT2 reveals involvement of LYSINE HISTIDINE TRANSPORTER1 in the uptake of 1-aminocyclopropane-1-carboxylic acid in Arabidopsis thaliana. Plant Cell Physiol. 2015, 56, 572–582. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Brumos, J.; Li, H.; Ji, Y.; Ke, M.; Gong, X.; Zeng, Q.; Li, W.; Zhang, X.; An, F.; et al. A small-molecule screen identifies l-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 2011, 23, 3944–3960. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.N.; Hoyt, J.M.; Hamilton, A.A.; Alonso, J.M. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 2005, 17, 2230–2242. [Google Scholar] [CrossRef] [PubMed]
- Staal, M.; de Cnodder, T.; Simon, D.; Vandenbussche, F.; van der Straeten, D.; Verbelen, J.P.; Elzenga, T.; Vissenberg, K. Apoplastic alkalinization is instrumental for the inhibition of cell elongation in the Arabidopsis root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid. Plant Physiol. 2011, 155, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 2018, 47, 306–318.e5. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Ivanchenko, M.G.; Muday, G.K. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008, 55, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.; Grieneisen, V.A.; Hofhuis, H.; Hove, C.A.; Hogeweg, P.; Marée, A.F.; Scheres, B. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008, 6, e307. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; Muday, G.K.; Dubrovsky, J.G. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J. 2008, 55, 335–347. [Google Scholar] [CrossRef]
- Aloni, R. Role of hormones in controlling vascular differentiation and the mechanism of lateral root initiation. Planta 2013, 238, 819–830. [Google Scholar] [CrossRef]
- Žádníková, P.; Smet, D.; Zhu, Q.; van der Straeten, D.; Benková, E. Strategies of seedlings to overcome their sessile nature: Auxin in mobility control. Front. Plant Sci. 2015, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Alabadí, D.; Blázquez, M.A. Differential growth at the apical hook: All roads lead to auxin. Front. Plant Sci. 2013, 4, 441. [Google Scholar] [CrossRef] [PubMed]
- Žádníková, P.; Wabnik, K.; Abuzeineh, A.; Gallemi, M.; van der Straeten, D.; Smith, R.S.; Inzé, D.; Friml, J.; Prusinkiewicz, P.; Benková, E. A model of differential growth-guided apical hook formation in plants. Plant Cell 2016, 28, 2464–2477. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, K.; Boutté, Y.; Singh, R.K.; Gendre, D.; Bhalerao, R.P. Ethylene regulates differential growth via BIG ARF-GEF-dependent post-Golgi secretory trafficking in Arabidopsis. Plant Cell 2017, 29, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Petrasek, J.; Zadnikova, P.; Hoyerova, K.; Pesek, B.; Raz, V.; Swarup, R.; Bennett, M.; Zazímalová, E.; Benková, E.; et al. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 2010, 137, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Zádníková, P.; Petrásek, J.; Marhavy, P.; Raz, V.; Vandenbussche, F.; Ding, Z.; Schwarzerová, K.; Morita, M.T.; Tasaka, M.; Hejátko, J.; et al. Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 2010, 137, 607–617. [Google Scholar] [CrossRef]
- Béziat, C.; Kleine-Vehn, J. The road to auxin-dependent growth repression and promotion in apical hooks. Curr. Biol. 2018, 28, R519–R525. [Google Scholar] [CrossRef] [PubMed]
- Boutté, Y.; Jonsson, K.; McFarlane, H.E.; Johnson, E.; Gendre, D.; Swarup, R.; Friml, J.; Samuels, L.; Robert, S.; Bhalerao, R.P. ECHIDNA-mediated post-Golgi trafficking of auxin carriers for differential cell elongation. Proc. Natl. Acad. Sci. USA 2013, 110, 16259–16264. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Huang, L.; Yan, A.; Liu, Y.; Liu, B.; Yu, C.; Zhang, A.; Schiefelbein, J.; Gan, Y. Multiple phytohormones promote root hair elongation by regulating a similar set of genes in the root epidermis in Arabidopsis. J. Exp. Bot. 2016, 67, 6363–6372. [Google Scholar] [CrossRef] [Green Version]
- Grierson, C.; Nielsen, E.; Ketelaarc, T.; Schiefelbein, J. Root hairs. Arabidopsis Book 2014, 12, e0172. [Google Scholar] [CrossRef]
- Balcerowicz, D.; Schoenaers, S.; Vissenberg, K. Cell fate determination and the switch from diffuse growth to planar polarity in Arabidopsis root epidermal cells. Front. Plant Sci. 2015, 6, 1163. [Google Scholar] [CrossRef] [PubMed]
- Mangano, S.; Denita-Juarez, S.P.; Choi, H.S.; Marzol, E.; Hwang, Y.; Ranocha, P.; Velasquez, S.M.; Borassi, C.; Barberini, M.L.; Aptekmann, A.A.; et al. Molecular link between auxin and ROS-mediated polar growth. Proc. Natl. Acad. Sci. USA 2017, 114, 5289–5294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenaers, S.; Balcerowicz, D.; Breen, G.; Hill, K.; Zdanio, M.; Mouille, G.; Holman, T.J.; Oh, J.; Wilson, M.H.; Nikonorova, N.; et al. The auxin-regulated CrRLK1L kinase ERULUS controls cell wall composition during root hair tip growth. Curr. Biol. 2018, 28, 722–732.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, H.; Fang, X.; Zhang, Y.; Jin, C. Auxin acts downstream of ethylene and nitric oxide to regulate magnesium deficiency-induced root hair development in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, P.; Li, B.; Li, P.; Wen, X.; An, F.; Gong, Y.; Xin, Y.; Zhu, Z.; Wang, Y.; et al. Ethylene promotes root hair growth through coordinated EIN3/EIL1 and RHD6/RSL1 activity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 13834–13839. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Men, S.; Fischer, U.; Stepanova, A.N.; Alonso, J.M.; Ljung, K.; Grebe, M. Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat. Cell Biol. 2009, 11, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Resnick, N.; Mayzlish-Gati, E.; Kaplan, Y.; Wininger, S.; Hershenhorn, J.; Koltai, H. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J. Exp. Bot. 2011, 62, 2915–2924. [Google Scholar] [CrossRef] [Green Version]
- Yi, K.; Menand, B.; Bell, E.; Dolan, L. A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat. Genet. 2010, 42, 264–267. [Google Scholar] [CrossRef]
- Bruex, A.; Kainkaryam, R.M.; Wieckowski, Y.; Kang, Y.H.; Bernhardt, C.; Xia, Y.; Zheng, X.; Wang, J.Y.; Lee, M.M.; Benfey, P.; et al. A gene regulatory network for root epidermis cell differentiation in Arabidopsis. PLoS Genet. 2012, 8, e1002446. [Google Scholar] [CrossRef]
- Liu, J.; Mehdi, S.; Topping, J.; Tarkowski, P.; Lindsey, K. Modelling and experimental analysis of hormonal crosstalk in Arabidopsis. Mol. Syst. Biol. 2010, 6, 373. [Google Scholar] [CrossRef]
- Casson, S.A.; Chilley, P.M.; Topping, J.F.; Evans, I.M.; Souter, M.A.; Lindsey, K. The POLARIS gene of Arabidopsis encodes a predicted peptide required for correct root growth and leaf vascular patterning. Plant Cell 2002, 14, 1705–1721. [Google Scholar] [CrossRef] [PubMed]
- Chilley, P.M.; Casson, S.A.; Tarkowski, P.; Hawkins, N.; Wang, K.L.; Hussey, P.J.; Beale, M.; Ecker, J.R.; Sandberg, G.K.; Lindsey, K. The POLARIS peptide of Arabidopsis regulates auxin transport and root growth via effects on ethylene signaling. Plant Cell 2006, 18, 3058–3072. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Zhang, X.; Mudge, A.; Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Spatiotemporal modelling of hormonal crosstalk explains the level and patterning of hormones and gene expression in Arabidopsis thaliana wild-type and mutant roots. New Phytol. 2015, 207, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk complexities between auxin, cytokinin, and ethylene in Arabidopsis root development: From experiments to systems modeling, and back again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef] [PubMed]
- Raz, V.; Koornneef, M. Cell division activity during apical hook development. Plant Physiol. 2001, 125, 219–226. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemlyanskaya, E.V.; Omelyanchuk, N.A.; Ubogoeva, E.V.; Mironova, V.V. Deciphering Auxin-Ethylene Crosstalk at a Systems Level. Int. J. Mol. Sci. 2018, 19, 4060. https://doi.org/10.3390/ijms19124060
Zemlyanskaya EV, Omelyanchuk NA, Ubogoeva EV, Mironova VV. Deciphering Auxin-Ethylene Crosstalk at a Systems Level. International Journal of Molecular Sciences. 2018; 19(12):4060. https://doi.org/10.3390/ijms19124060
Chicago/Turabian StyleZemlyanskaya, Elena V., Nadya A. Omelyanchuk, Elena V. Ubogoeva, and Victoria V. Mironova. 2018. "Deciphering Auxin-Ethylene Crosstalk at a Systems Level" International Journal of Molecular Sciences 19, no. 12: 4060. https://doi.org/10.3390/ijms19124060




