The Effects of Varying Degree of MWCNT Carboxylation on Bioactivity in Various In Vivo and In Vitro Exposure Models
Abstract
:1. Introduction
2. Results
2.1. Functionalized Particle Characterizations
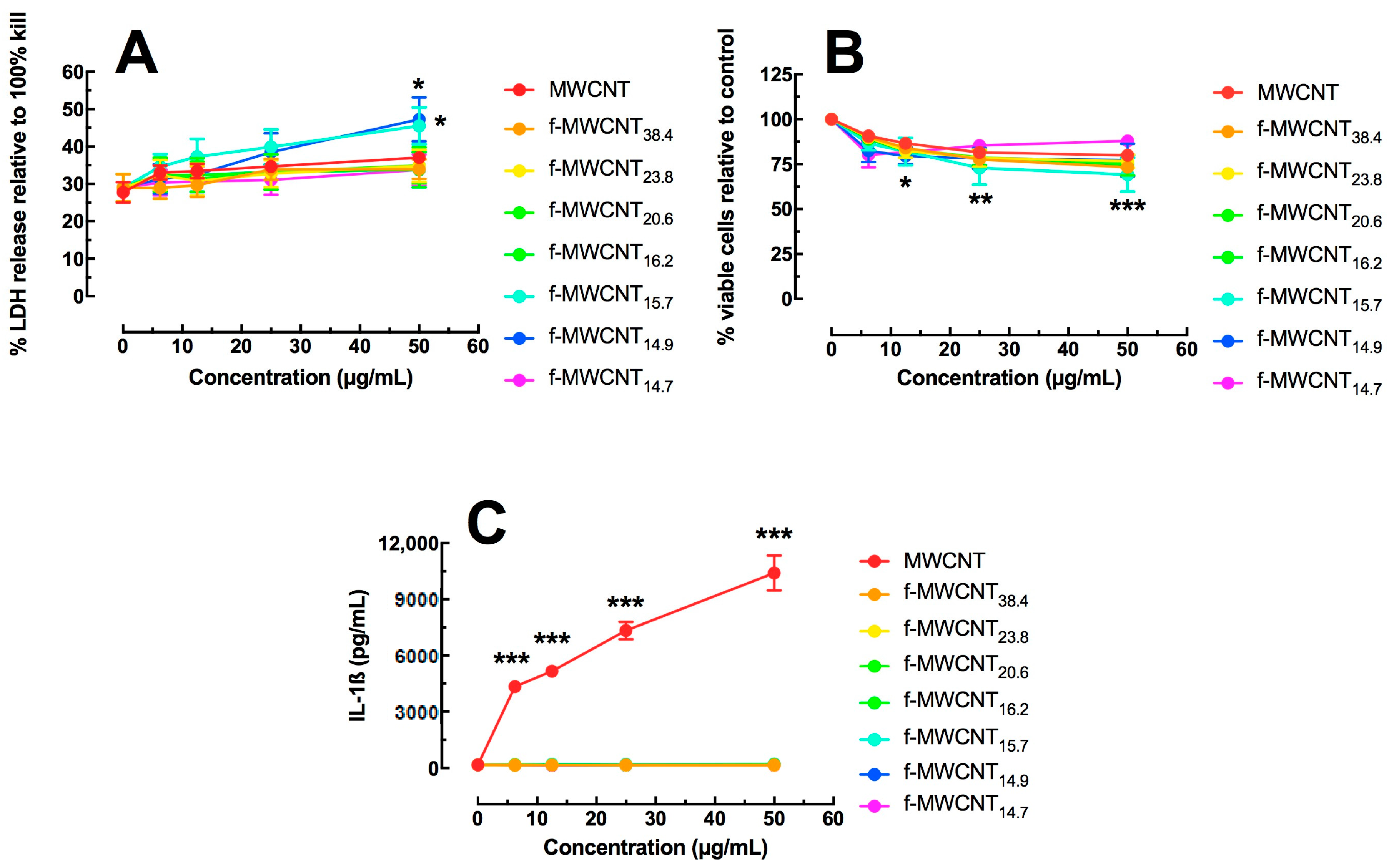
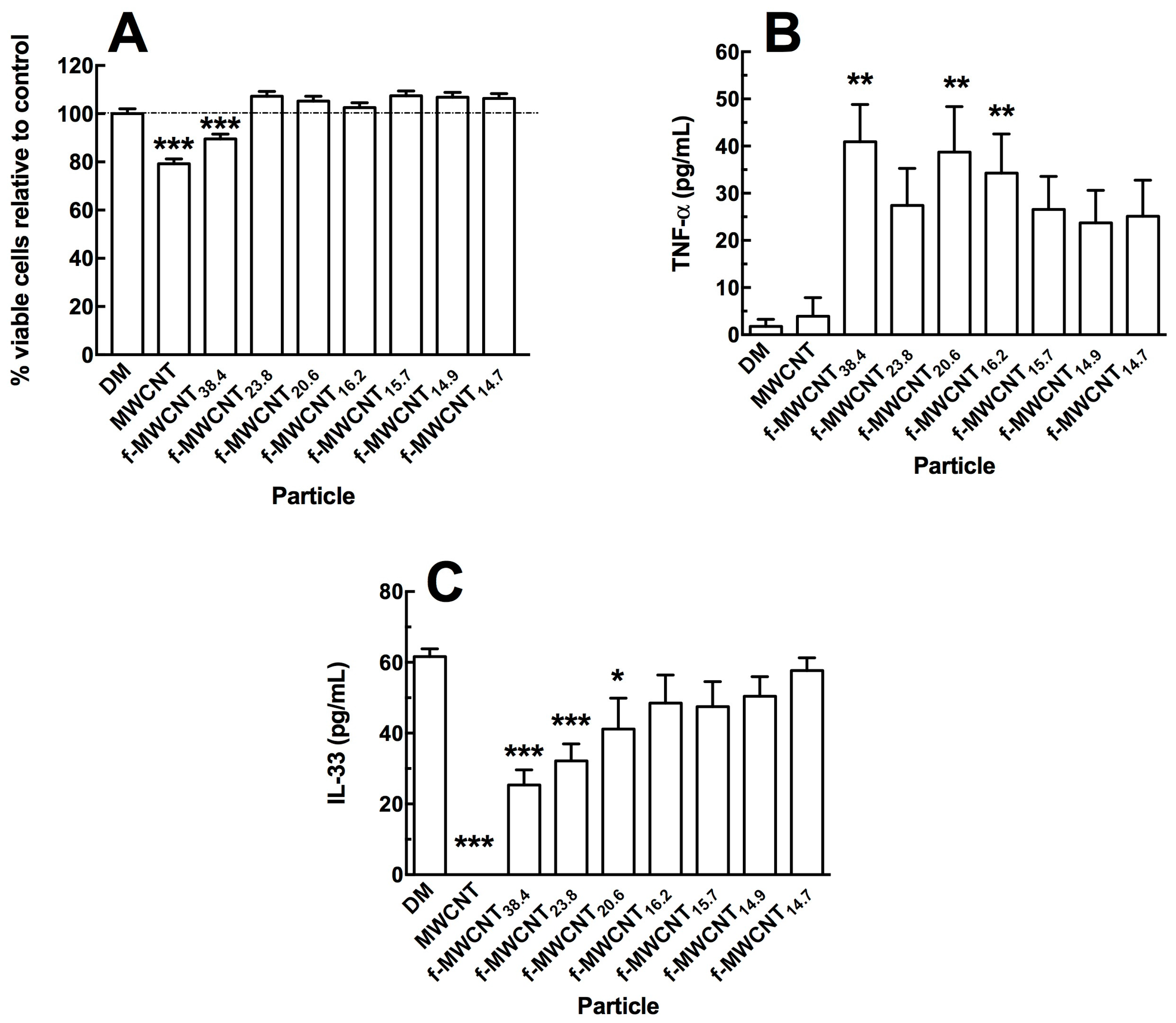
2.2. In Vitro Effects of Varied Carboxylation
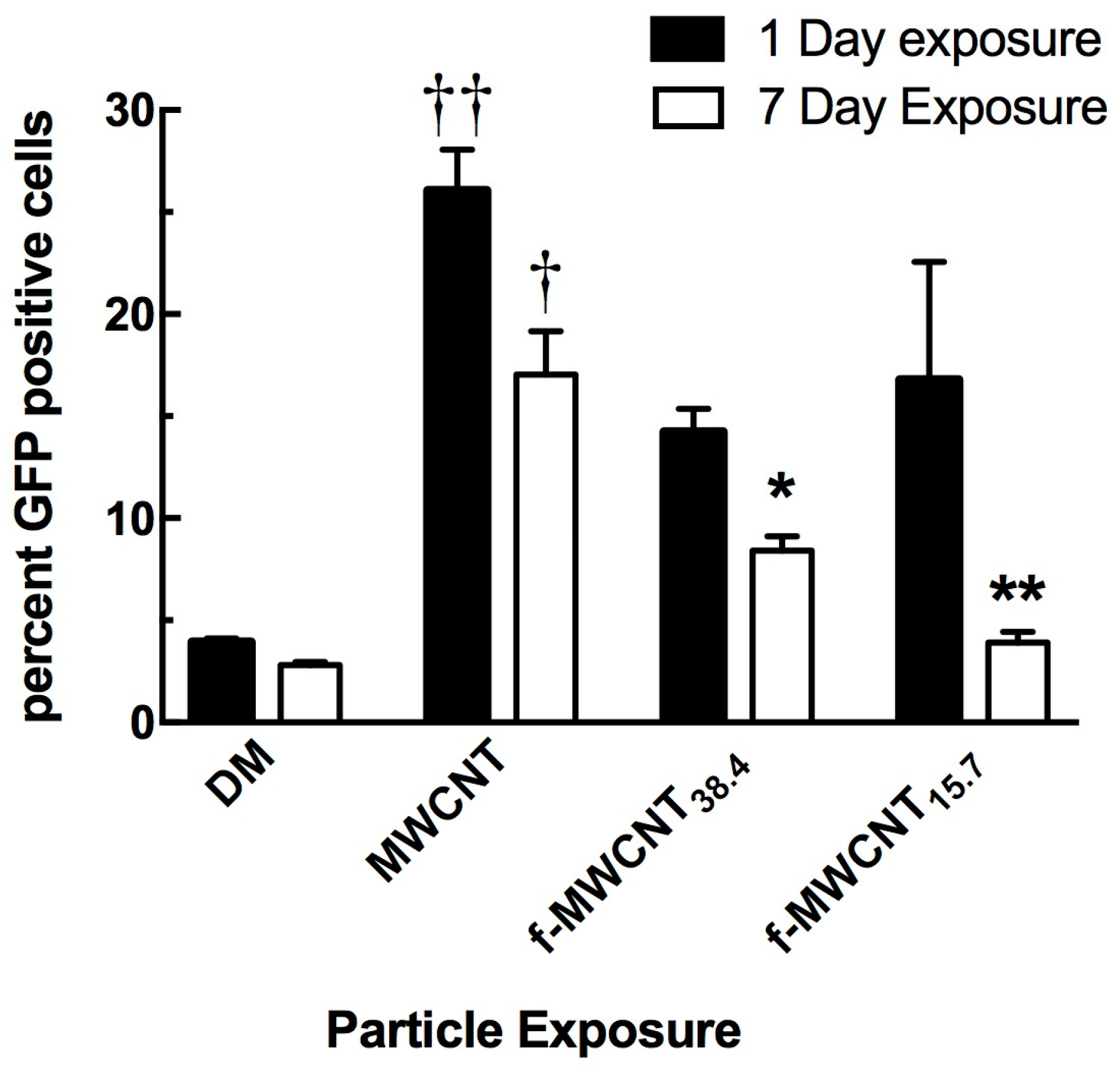
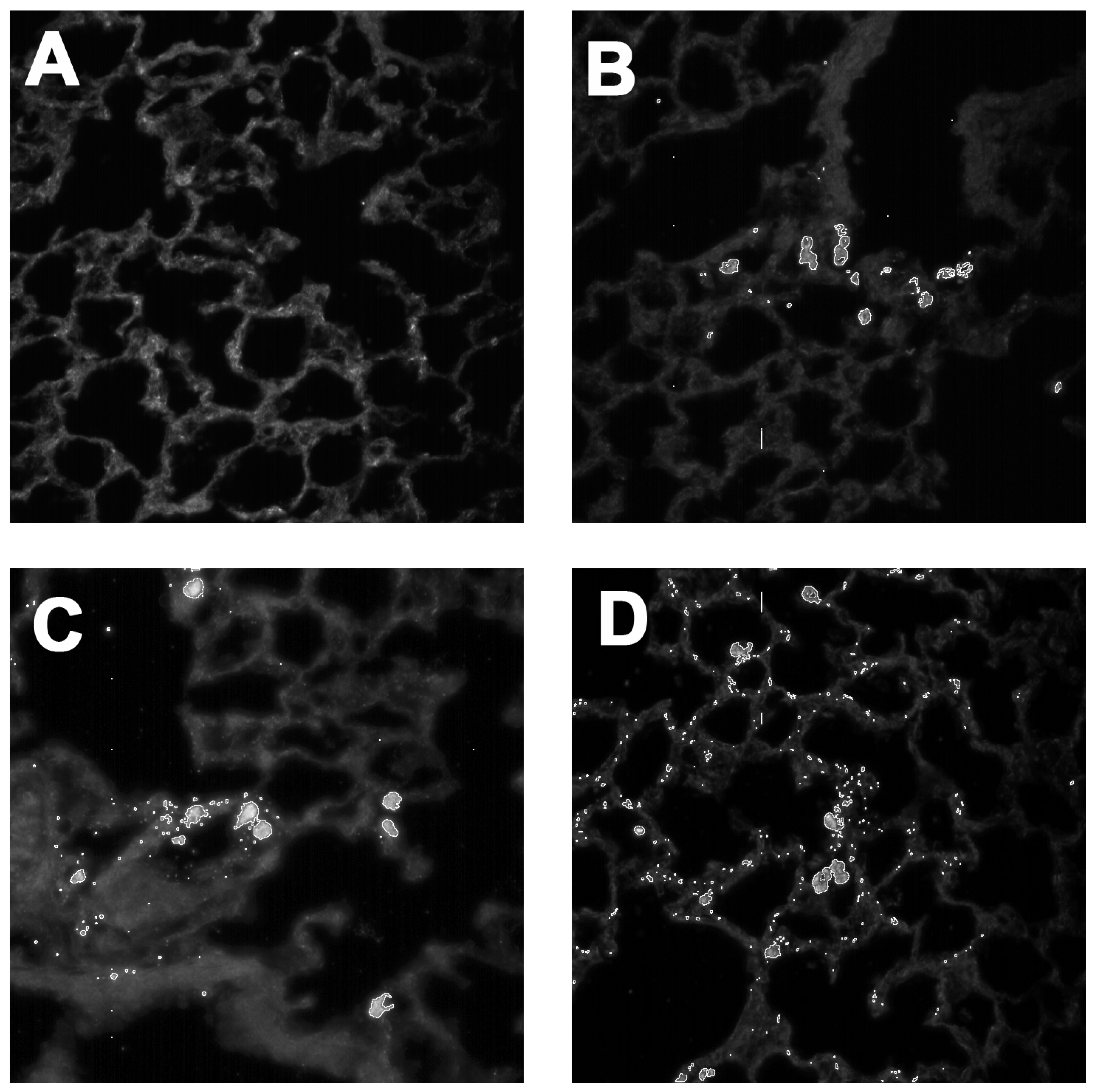
2.3. In Vivo Effects
3. Discussion
4. Materials and Methods
4.1. Preparation of the Functionalized-MWCNTs
4.2. Characterization of the Functionalized-CNTs
4.2.1. Transmission Electron Microscopy of Particles and AM
4.2.2. Particle Size and Zeta Potentials
4.2.3. Surface Area Assessment
4.2.4. CNT Suspensions
4.3. Human THP-1 Cell Line Culturing
4.4. Human A549 Cell Line Culturing
4.5. Toxicity Assays
4.6. Cytokine Assays
4.7. Animals
4.8. Lung Cell Isolation
4.9. Autophagy Determination In Vivo
4.10. Dark-Field Imaging
4.11. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdalla, S.; Al-Marzouki, F.; Al-Ghamdi, A.A.; Abdel-Daiem, A. Different Technical Applications of Carbon Nanotubes. Nanoscale Res. Lett. 2015, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.F., Jr.; Wu, Z.; Mitra, S.; Shaw, P.K.; Holian, A. Effect of MWCNT size, carboxylation, and purification on in vitro and in vivo toxicity, inflammation and lung pathology. Part Fibre Toxicol. 2013, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.F., Jr.; Xiang, C.; Li, M.; Ka, I.; Yang, F.; Ma, D.; Porter, D.W.; Wu, N.; Holian, A. Purification and sidewall functionalization of multiwalled carbon nanotubes and resulting bioactivity in two macrophage models. Inhal. Toxicol. 2013, 25, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Dahm, M.M.; Schubauer-Berigan, M.K.; Evans, D.E.; Birch, M.E.; Fernback, J.E.; Deddens, J.A. Carbon nanotube and nanofiber exposure assessments: An analysis of 14 site visits. Ann. Occup. Hyg. 2015, 59, 705–723. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Yanamala, N.; Kisin, E.R.; Tkach, A.V.; Murray, A.R.; Hubbs, A.; Chirila, M.M.; Keohavong, P.; Sycheva, L.P.; Kagan, V.E.; et al. Long-term effects of carbon containing engineered nanomaterials and asbestos in the lung: One year postexposure comparisons. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L170–L182. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.F., Jr.; Buford, M.; Xiang, C.; Wu, N.; Holian, A. NLRP3 inflammasome activation in murine alveolar macrophages and related lung pathology is associated with MWCNT nickel contamination. Inhal. Toxicol. 2012, 24, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Sager, T.M.; Wolfarth, M.; Leonard, S.S.; Morris, A.M.; Porter, D.W.; Castranova, V.; Holian, A. Role of engineered metal oxide nanoparticle agglomeration in reactive oxygen species generation and cathepsin B release in NLRP3 inflammasome activation and pulmonary toxicity. Inhal. Toxicol. 2016, 28, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Sager, T.; Wolfarth, M.; Keane, M.; Porter, D.; Castranova, V.; Holian, A. Effects of nickel-oxide nanoparticle pre-exposure dispersion status on bioactivity in the mouse lung. Nanotoxicology 2016, 10, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Rahman, L.; Jacobsen, N.R.; Aziz, S.A.; Wu, D.; Williams, A.; Yauk, C.L.; White, P.; Wallin, H.; Vogel, U.; Halappanavar, S. Multi-walled carbon nanotube-induced genotoxic, inflammatory and pro-fibrotic responses in mice: Investigating the mechanisms of pulmonary carcinogenesis. Mutat. Res. 2017, 823, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, X.; Song, L.; Zhang, H.; Liu, L.; Zhu, D.; Song, C.; Leng, X. Carboxylation of multiwalled carbon nanotube enhanced its biocompatibility with L02 cells through decreased activation of mitochondrial apoptotic pathway. J. Biomed. Mater. Res. A 2014, 102, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Peng, D. Carboxylation of multiwalled carbon nanotube attenuated the cytotoxicity by limiting the oxidative stress initiated cell membrane integrity damage, cell cycle arrestment, and death receptor mediated apoptotic pathway. J. Biomed. Mater. Res. A 2015, 103, 2770–2777. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Thakare, V.S.; Das, M.; Godugu, C.; Jain, A.K.; Mathur, R.; Chuttani, K.; Mishra, A.K. Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chem. Res. Toxicol. 2011, 24, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Bunderson-Schelvan, M.; Hamilton, R.; Trout, K.; Jessop, F.; Gulumian, M.; Holian, A. Approaching a unified theory for particle-induced inflammation. In Biological Effects of Fibrous and Particulate Substances; Otsuki, T., Yoshioka, Y., Holian, A., Eds.; Springer: Tokyo, Japan, 2016; pp. 51–76. [Google Scholar]
- Fanizza, C.; Casciardi, S.; Incoronato, F.; Cavallo, D.; Ursini, C.L.; Ciervo, A.; Maiello, R.; Fresegna, A.M.; Marcelloni, A.M.; Lega, D.; et al. Human epithelial cells exposed to functionalized multiwalled carbon nanotubes: Interactions and cell surface modifications. J. Microsc. 2015, 259, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Ursini, C.L.; Maiello, R.; Ciervo, A.; Fresegna, A.M.; Buresti, G.; Superti, F.; Marchetti, M.; Iavicoli, S.; Cavallo, D. Evaluation of uptake, cytotoxicity and inflammatory effects in respiratory cells exposed to pristine and –OH and –COOH functionalized multi-wall carbon nanotubes. J. Appl. Toxicol. 2016, 36, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Wu, Z.; Mitra, S.; Brown, J.M. Effects of multiwalled carbon nanotube surface modification and purification on bovine serum albumin binding and biological responses. J. Nanomater. 2016, 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Z.; Yu, F.; Thakkar, M.; Mitra, S. Variation in chemical, colloidal and electrochemical properties of carbon nanotubes with the degree of carboxylation. J. Nanopart. Res. 2017, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Hamilton, R.F.; Bonner, J.C.; Crandall, E.D.; Elder, A.; Fazlollahi, F.; Girtsman, T.A.; Kim, K.; Mitra, S.; Ntim, S.A.; et al. Interlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nanomaterials: The NIEHS Nano GO Consortium. Environ. Health Perspect. 2013, 121, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; Adiseshaiah, P.P.; Crist, R.M. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.N.; Kim, J.E.; Seo, H.W.; Chae, C.; Cho, M.H. Differential toxic responses between pristine and functionalized multiwall nanotubes involve induction of autophagy accumulation in murine lung. J. Toxicol. Environ. Health A 2013, 76, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Jessop, F.; Hamilton, R.F.; Rhoderick, J.F.; Shaw, P.K.; Holian, A. Autophagy deficiency in macrophages enhances NLRP3 inflammasome activity and chronic lung disease following silica exposure. Toxicol. Appl. Pharmacol. 2016, 309, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Allegri, M.; Perivoliotis, D.K.; Bianchi, M.G.; Chiu, M.; Pagliaro, A.; Koklioti, M.A.; Trompeta, A.F.A.; Bergamaschi, E.; Bussolati, O.; Charitidis, C.A. Toxicity determinants of multi-walled carbon nanotubes: The relationship between functionalization and agglomeration. Toxicol. Rep. 2016, 3, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Ji, Z.; Sun, B.; Zhang, H.; Chang, C.H.; Lin, S.; Meng, H.; Liao, Y.P.; Wang, M.; et al. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano 2013, 7, 2352–2368. [Google Scholar] [CrossRef] [PubMed]
- Ntim, S.A.; Sae-Khow, O.; Witzmann, F.A.; Mitra, S. Effects of polymer wrapping and covalent functionalization on the stability of MWCNT in aqueous dispersions. J. Colloid Interface Sci. 2011, 355, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Thielbeer, F.; Duffin, R.; Johansson, E.M.; Megson, I.L.; MacNee, W.; Bradley, M.; Donaldson, K. Surface functionalization affects the zeta potential, coronal stability and membranolytic activity of polymeric nanoparticles. Nanotoxicology 2014, 8, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, Y.; Yan, B. Functionalized carbon nanotubes for potential medicinal applications. Drug Discov. Today 2010, 15, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Fu, K.; Lin, Y.; Huang, W. Functionalized carbon nanotubes: Properties and applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.F.; Wu, N.; Xiang, C.; Li, M.; Yang, F.; Wolfarth, M.; Porter, D.W.; Holian, A. Synthesis, characterization, and bioactivity of carboxylic acid-functionalized titanium dioxide nanobelts. Part Fibre Toxicol. 2014, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.; Hu, S.; Ruenraroengsak, P.; Chen, S.; Gow, A.; Schwander, S.; Zhang, J.F.; Chung, K.F.; Ryan, M.P.; Porter, A.E.; et al. Carboxylation of multiwalled carbon nanotubes reduces their toxicity in primary human alveolar macrophages. Environ. Sci. Nano 2016, 3, 1340–1350. [Google Scholar] [CrossRef]
- Stern, S.T.; Johnson, D.N. Role for nanomaterial-autophagy interaction in neurodegenerative disease. Autophagy 2008, 4, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Fraczek-Szczypta, A.; Menaszek, E.; Syeda, T.B.; Misra, A.; Alavijeh, M.; Adu, J.; Blazewicz, S. Effect of MWCNT surface and chemical modification on in vitro cellular response. J. Nanopart. Res. 2012, 14, 1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, R.R.; Hubbs, A.F.; Scabilloni, J.F.; Wang, L.; Battelli, L.A.; Friend, S.; Castranova, V.; Porter, D.W. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part Fibre Toxicol. 2011, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Rajavel, K.; Gomathi, R.; Manian, S.; Rajendra Kumar, R.T. In vitro bacterial cytotoxicity of CNTs: Reactive oxygen species mediate cell damage edges over direct physical puncturing. Langmuir 2014, 30, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Ruenraroengsak, P.; Chen, S.; Hu, S.; Melbourne, J.; Sweeney, S.; Thorley, A.J.; Skepper, J.N.; Shaffer, M.S.; Tetley, T.D.; Porter, A.E. Translocation of functionalized multi-walled carbon nanotubes across human pulmonary alveolar epithelium: dominant role of epithelial type 1 cells. ACS Nano 2016, 10, 5070–5085. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, T.; Ntim, S.A.; Ji, Z.; George, S.; Meng, H.; Zhang, H.; Castranova, V.; Mitra, S.; Nel, A.E. Quantitative techniques for assessing and controlling the dispersion and biological effects of multiwalled carbon nanotubes in mammalian tissue culture cells. ACS Nano 2010, 4, 7241–7252. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, T.; Ntim, S.A.; Ji, Z.; Lin, S.; Meng, H.; Chung, C.H.; George, S.; Zhang, H.; Wang, M.; et al. Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung. ACS Nano 2011, 5, 9772–9787. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.S.; Shenderov, K.; Huang, N.N.; Kabat, J.; Abu-Asab, M.; Fitzgerald, K.A.; Sher, A.; Kehrl, J.H. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012, 13, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.; Wandel, M.P.; von Muhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Methods for monitoring autophagy using GFP-LC3 transgenic mice. Methods Enzymol. 2009, 452, 13–23. [Google Scholar] [PubMed]

| Particle | Hydrodynamic Size (nm) | Zeta Potential (mV) | Endotoxin (ng/5 μg MWCNT) |
|---|---|---|---|
| MWCNT-source | 396 ± 22.0 | −10.3 ± 0.39 | 0.02 |
| f-MWCNT38.4 | 169.1 ± 50.9 | −12.7 ± 0.34 | 2.4 |
| f-MWCNT23.8 | 57.6 ± 2.3 | −10.4 ± 0.96 | 2.95 |
| f-MWCNT20.6 | 67.0 ± 9.4 | −11.1 ± 0.42 | 2.9 |
| f-MWCNT16.2 | 44.2 ± 32.5 | −11.5 ± 1.01 | 2.7 |
| f-MWCNT15.7 | 272.8 ± 1.1 | −11.1 ± 1.3 | 3.25 |
| f-MWCNT14.9 | 234.6 ± 7.2 | −10.2 ± 1.0 | 3.3 |
| f-MWCNT14.7 | 41.8 ± 1.2 | −11.5 ± 1.2 | 3.2 |
| Particle | Treatment Time (min) | % by Weight | C:O | SSA (m2/g) | ||
|---|---|---|---|---|---|---|
| C | O | Ni | ||||
| MWCNTsource | 0 | 93.9 | 4.90 | 1.20 | N/A | 174 |
| f-MWCNT38 | 5 | 90.1 | 9.06 | 0.84 | 13.3 | 220 |
| f-MWCNT24 | 10 | 88.9 | 10.7 | 0.40 | 11.1 | 247 |
| f-MWCNT21 | 20 | 88.2 | 11.8 | - | 10.0 | 246 |
| f-MWCNT16 | 40 | 86.0 | 14.0 | - | 8.2 | 266 |
| f-MWCNT15 | 60 | 85.2 | 14.8 | - | 7.7 | 263 |
| f-MWCNT15 | 90 | 84.9 | 15.1 | - | 7.5 | 251 |
| f-MWCNT14 | 120 | 84.4 | 15.6 | - | 7.2 | 271 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamilton, R.F., Jr.; Wu, Z.; Mitra, S.; Holian, A. The Effects of Varying Degree of MWCNT Carboxylation on Bioactivity in Various In Vivo and In Vitro Exposure Models. Int. J. Mol. Sci. 2018, 19, 354. https://doi.org/10.3390/ijms19020354
Hamilton RF Jr., Wu Z, Mitra S, Holian A. The Effects of Varying Degree of MWCNT Carboxylation on Bioactivity in Various In Vivo and In Vitro Exposure Models. International Journal of Molecular Sciences. 2018; 19(2):354. https://doi.org/10.3390/ijms19020354
Chicago/Turabian StyleHamilton, Raymond F., Jr., Zheqiong Wu, Somenath Mitra, and Andrij Holian. 2018. "The Effects of Varying Degree of MWCNT Carboxylation on Bioactivity in Various In Vivo and In Vitro Exposure Models" International Journal of Molecular Sciences 19, no. 2: 354. https://doi.org/10.3390/ijms19020354






