Mechanisms of Cardiovascular Protection Associated with Intermittent Hypobaric Hypoxia Exposure in a Rat Model: Role of Oxidative Stress
Abstract
:1. Introduction
2. Results
2.1. Animals Weights
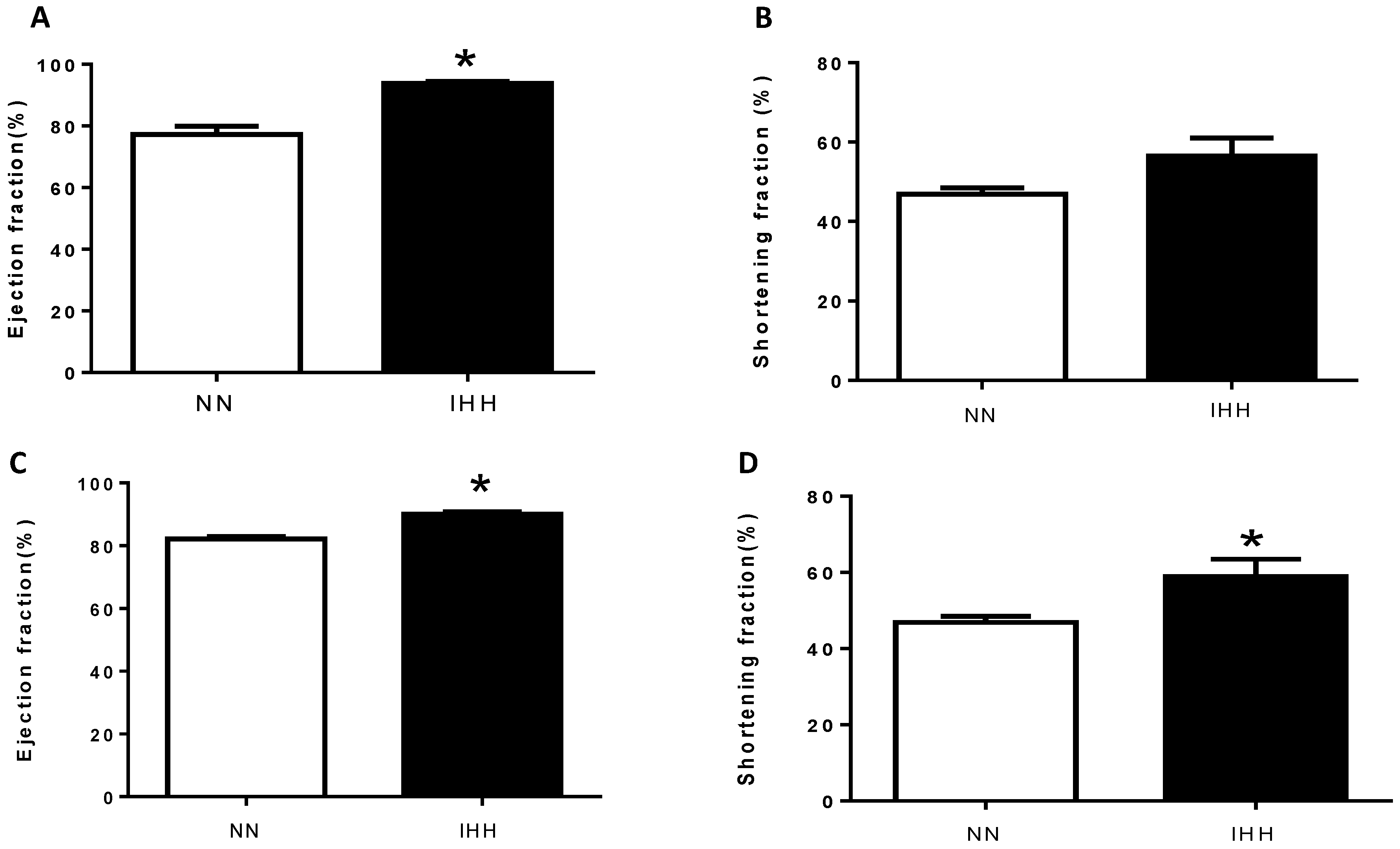
2.2. In Vivo Cardiac Morphometry & Function
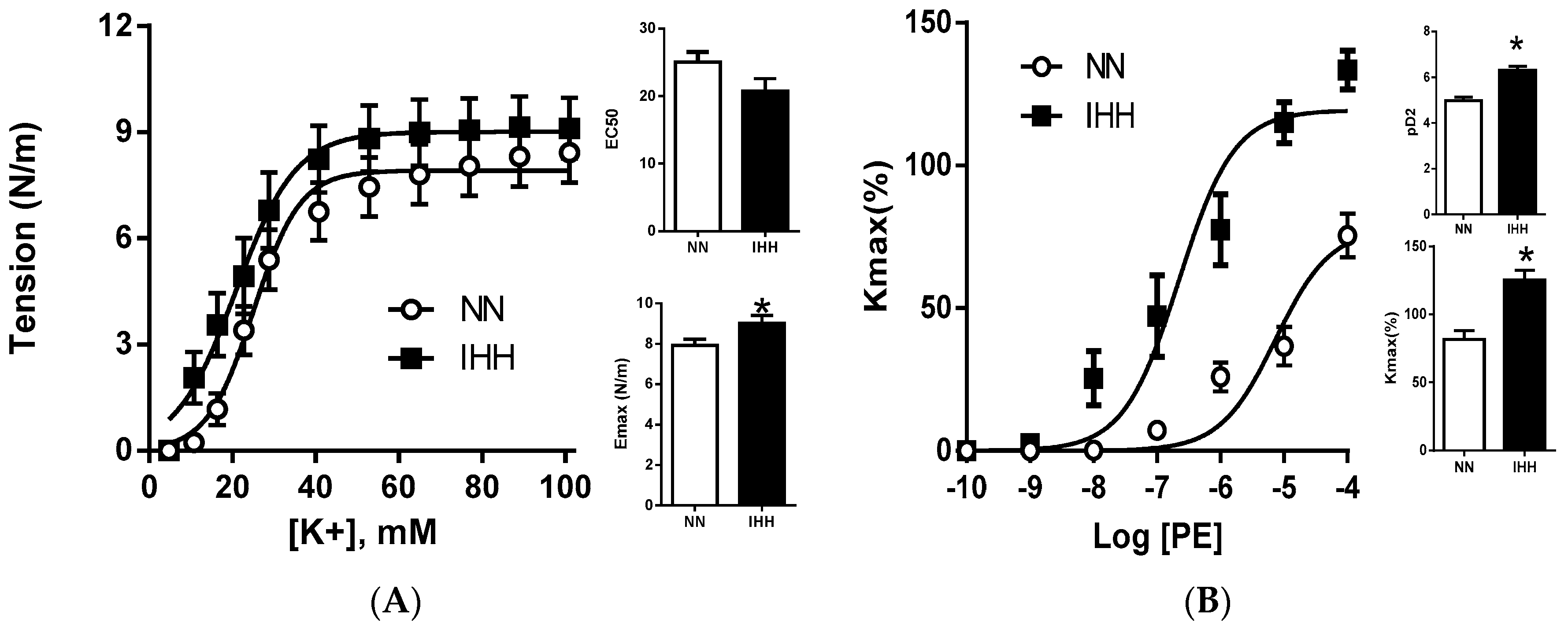
2.3. Ex Vivo Femoral Vascular Function Active Response
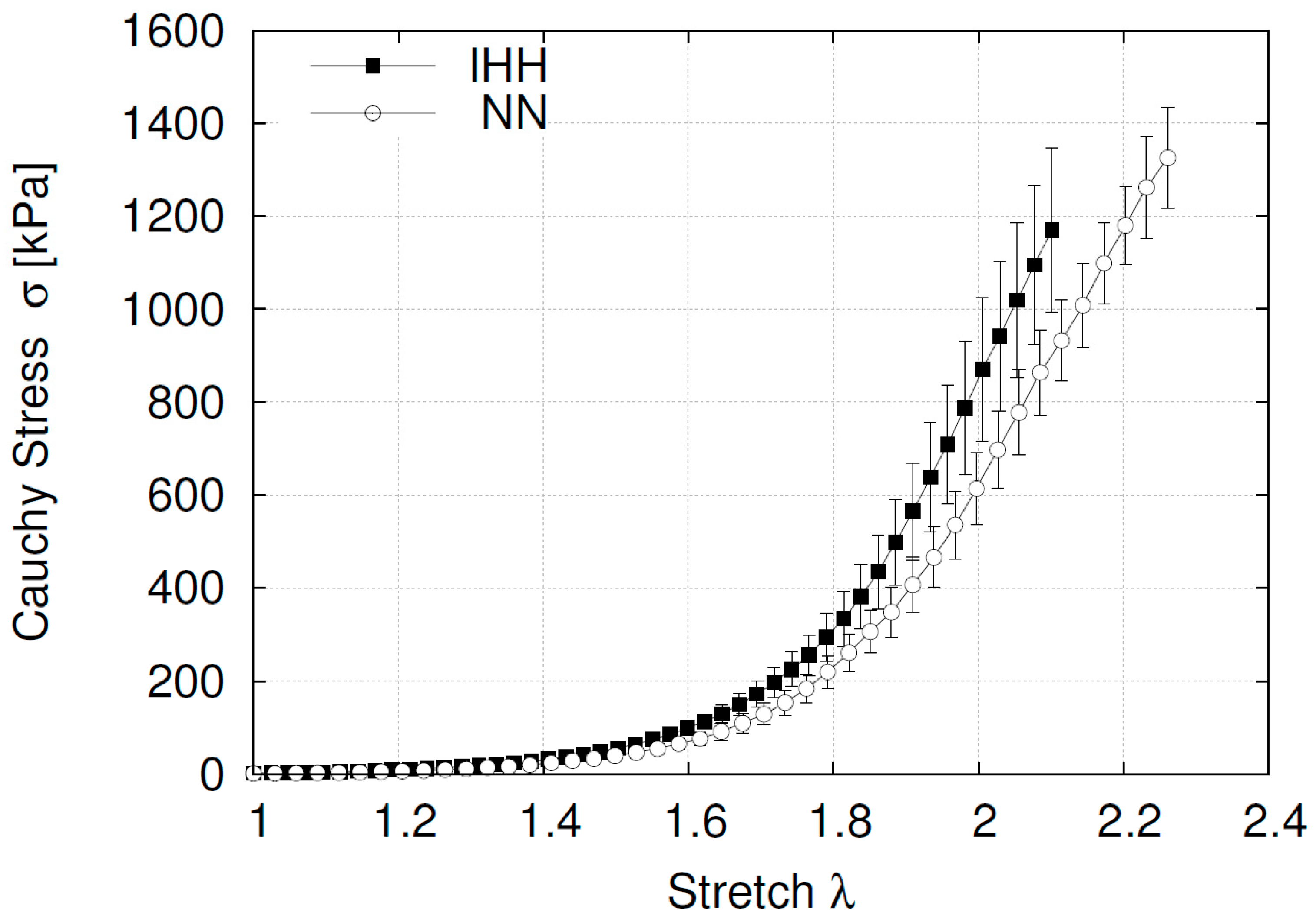
2.4. Ex Vivo Femoral Vascular Function—Passive Response
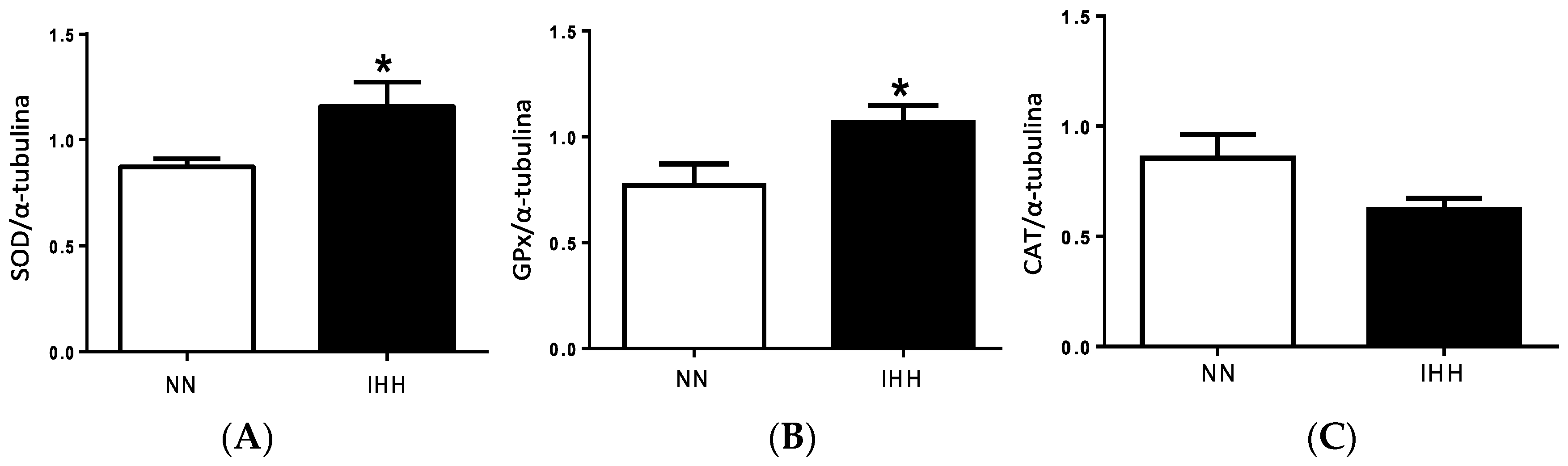
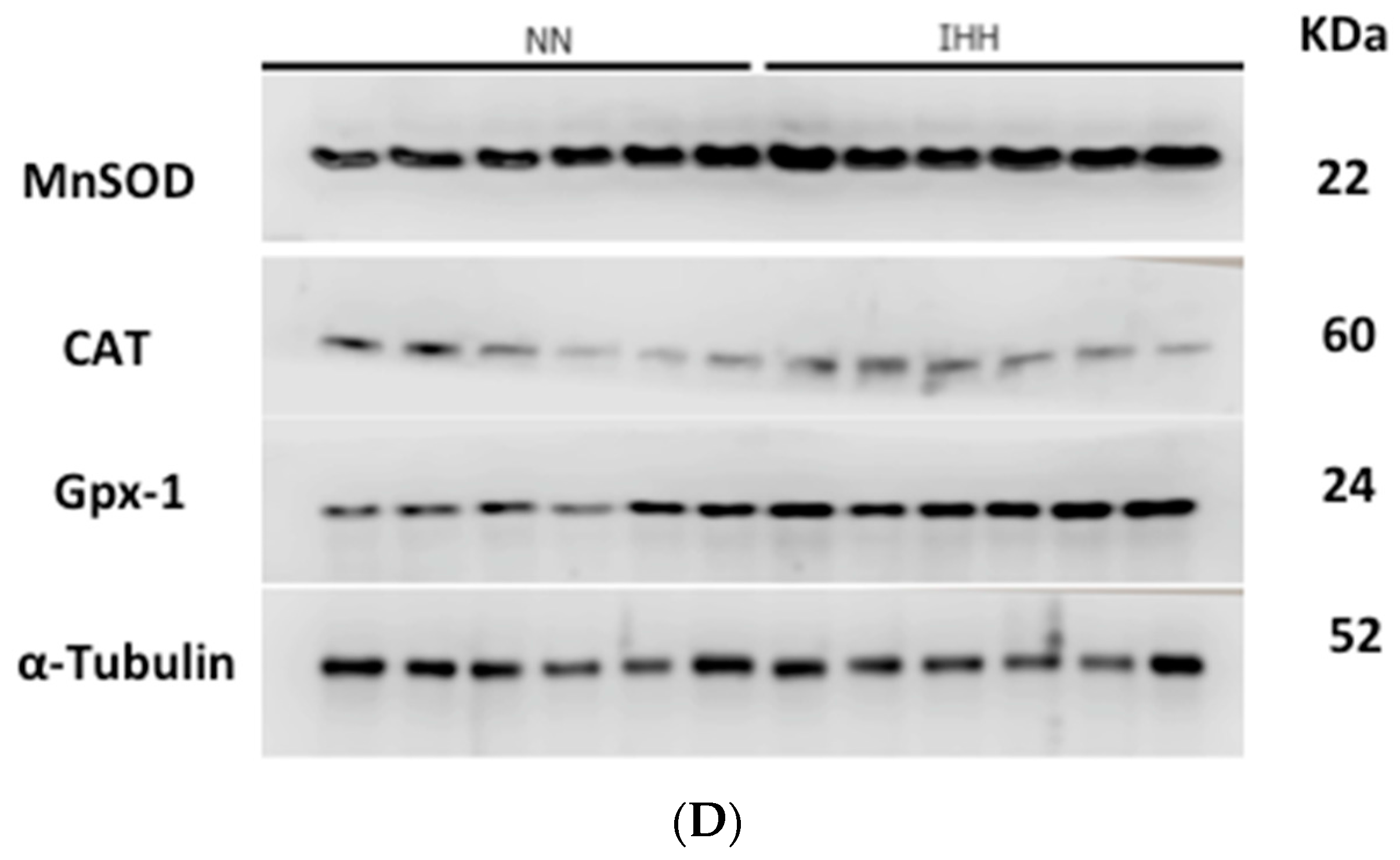
2.5. Cardiac Antioxidant Capacity & Anion Sources
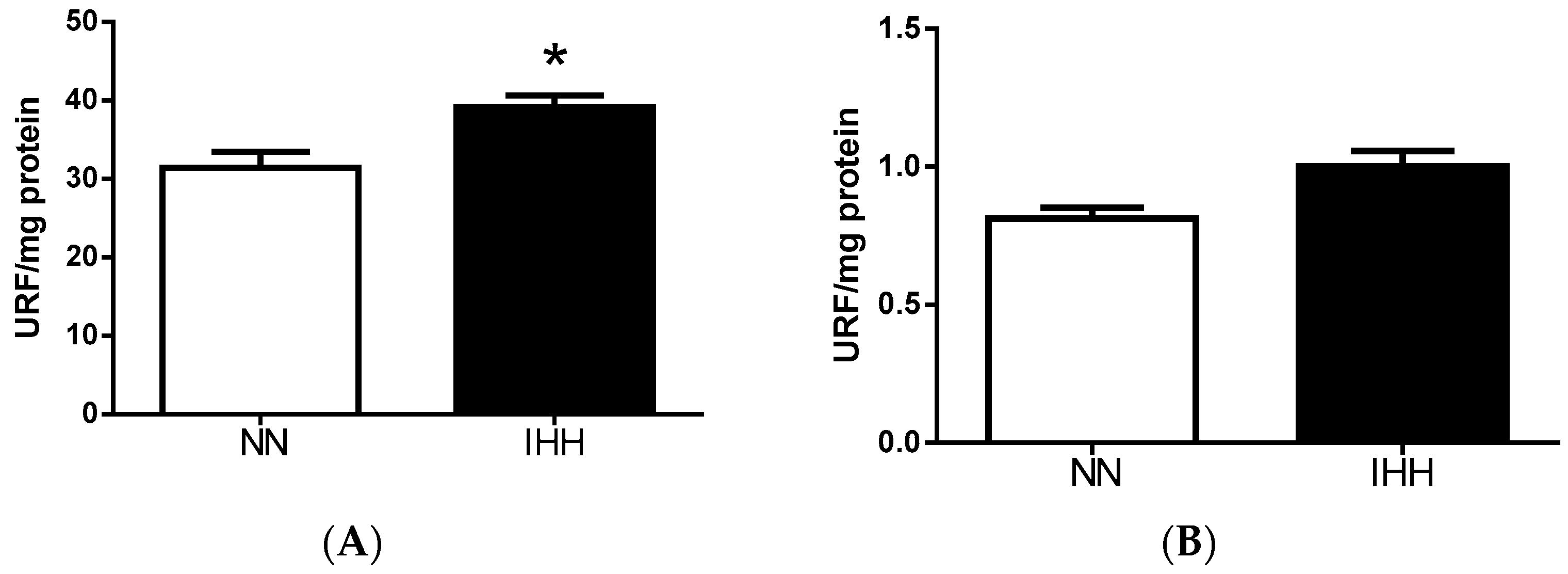
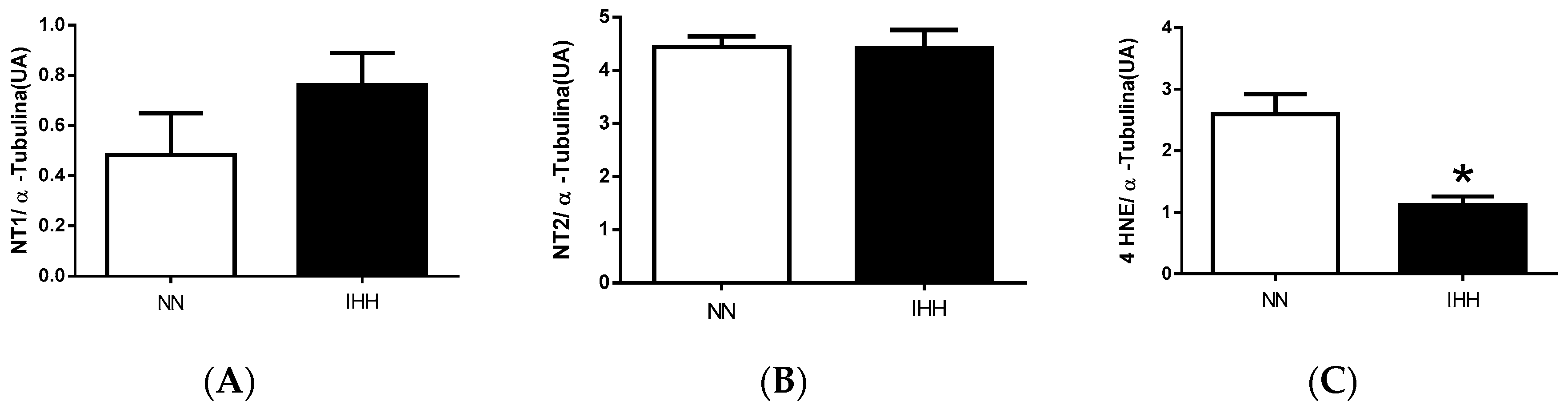
2.6. Cardiac Biomarker of Hypoxia and Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Echocardiography
4.3. Tissue Collection
4.4. Vascular Function Active Response
4.5. Vascular Function Passive Response
4.6. Biomarker of Hypoxia and Oxidative Stress
4.7. Cardiac Antioxidant Enzymes
4.8. Reactive Oxygen Species Sources
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Almendros, I.; Wang, Y.; Gozal, D. The polymorphic and contradictory aspects of intermittent hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.M.; Kumar, R.; Ogren, J.A.; Macey, P.M. Sleep-disordered breathing: Effects on brain structure and function. Respir. Physiol. Neurobiol. 2013, 188, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Matsuo, H.; Ishida, K.; Mori, S.; Miyamura, M. Intermittent hypoxia improves endurance performance and submaximal exercise efficiency. High Alt. Med. Biol. 2003, 4, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Wang, Y.; Gozal, D. Pathological consequences of intermittent hypoxia in the central nervous system. Compr. Physiol. 2012, 2, 1767–1777. [Google Scholar] [PubMed]
- Herrera, E.A.; Farías, J.G.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Castillo, R.L. Pharmacological approaches in either intermittent or permanent hypoxia: A tale of two exposures. Pharmacol. Res. 2015, 101, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.D.; Honigman, B. The effect of altitude-induced hypoxia on heart disease: Do acute, intermittent, and chronic exposures provide cardioprotection? High Alt. Med. Biol. 2011, 12, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Manukhina, E.B.; Belkina, L.M.; Terekhina, O.L.; Abramochkin, D.V.; Smirnova, E.A.; Budanova, O.P.; Mallet, R.T.; Downey, H.F. Normobaric, intermittent hypoxia conditioning is cardio- and vasoprotective in rats. Exp. Biol. Med. (Maywood) 2013, 238, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Beguin, P.C.; Joyeux-Faure, M.; Godin-Ribuot, D.; Levy, P.; Ribuot, C. Acute intermittent hypoxia improves rat myocardium tolerance to ischemia. J. Appl. Physiol. 2005, 99, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Manalo, D.J.; Wei, G.; Rodriguez, E.R.; Fox-Talbot, K.; Lu, H.; Zweier, J.L.; Semenza, G.L. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 2003, 108, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Nagase, H.; Vinod Kumar, S.; Suzuki, Y.J. Acute intermittent hypoxia activates myocardial cell survival signaling. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H751–H757. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, X.Y.; Li, J.; Fu, J.D.; Zhou, Z.N.; Yang, H.T. Intermittent hypoxia protects cardiomyocytes against ischemia-reperfusion injury-induced alterations in Ca2+ homeostasis and contraction via the sarcoplasmic reticulum and Na+/Ca2+ exchange mechanisms. Am. J. Physiol. Cell Physiol. 2006, 290, C1221–C1229. [Google Scholar] [CrossRef] [PubMed]
- Kolar, F.; Neckar, J.; Ostadal, B. MCC-134, a blocker of mitochondrial and opener of sarcolemmal ATP-sensitive K+ channels, abrogates cardioprotective effects of chronic hypoxia. Physiol. Res. 2005, 54, 467–471. [Google Scholar] [PubMed]
- Zhuang, J.; Zhou, Z. Protective effects of intermittent hypoxic adaptation on myocardium and its mechanisms. Biol. Signals Recept. 1999, 8, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.Q.; Yu, Z.; Xie, Y.; Huang, G.Q.; Shu, X.H.; Zhu, Y.; Zhou, Z.N.; Yang, H.T. Therapeutic effect of intermittent hypobaric hypoxia on myocardial infarction in rats. Basic Res. Cardiol. 2011, 106, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; Farías, J.G.; González-Candia, A.; Short, S.E.; Carrasco-Pozo, C.; Castillo, R.L. Ω3 Supplementation and intermittent hypobaric hypoxia induce cardioprotection enhancing antioxidant mechanisms in adult rats. Mar. Drugs 2015, 13, 838–860. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.L.; Zepeda, A.B.; Short, S.E.; Figueroa, E.; Bustos-Obregon, E.; Farías, J.G. Protective effects of polyunsatutared fatty acids supplementation against testicular damage induced by intermittent hypobaric hypoxia in rats. J. Biomed. Sci. 2015, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Martí, S.C.; Pallarés, V.; Capdevila, C.; Hernándiz, A.; Portolés, M.; Cosín, J. Precondicionamiento isquémico. ¿Es siempre un fenómeno beneficioso? Rev. Esp. Cardiol. 1999, 52, 429–436. [Google Scholar] [CrossRef]
- Serebrovskaya, T.V.; Xi, L. Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: Practical analysis on methods and equipment. Exp. Biol. Med. (Maywood) 2016, 241, 1708–1723. [Google Scholar] [CrossRef] [PubMed]
- Boos, C.J.; O’Hara, J.P.; Mellor, A.; Hodkinson, P.D.; Tsakirides, C.; Reeve, N.; Gallagher, L.; Charles, N.D.; Woods, D.R. A Four-Way Comparison of Cardiac Function with Normobaric Normoxia, Normobaric Hypoxia, Hypobaric Hypoxia and Genuine High Altitude. PLoS ONE 2016, 11, e0152868. [Google Scholar] [CrossRef] [PubMed]
- Singal, P.K.; Khaper, N.; Palace, V.; Kumar, D. The role of oxidative stress in the genesis of heart disease. Cardiovasc. Res. 1998, 48, 426–432. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Lokuta, A.J.; Maertz, N.A.; Meethal, S.V.; Potter, K.T.; Kamp, T.J.; Valdivia, H.H.; Haworth, R.A. Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation 2005, 111, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Knyushko, T.V.; Sharov, V.S.; Williams, T.D.; Schöneich, C.; Bigelow, D.J. 3-Nitrotyrosine modification of SERCA2a in the aging heart: A distinct signature of the cellular redox environment. Biochemistry 2005, 44, 13071–13081. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.P. Altitude and the cardiovascular system. La Presse Medicale 2012, 41, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Germack, R.; Leon-Velarde, F.; Barra, R.V.; Farias, J.; Soto, G.; Richalet, J.P. Effect of intermittent hypoxia on cardiovascular function, adrenoceptors and muscarinic receptors in Wistar rats. Exp. Physiol. 2002, 87, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Brodde, O.E. Beta-adrenoceptors in cardiac disease. Pharmacol. Ther. 1993, 60, 405–430. [Google Scholar] [CrossRef]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.M.; Davis, R.T.; Warren, C.M.; Mao, L.; Wolska, B.M.; Solaro, R.J.; Rockman, H.A. β-Arrestin mediates the Frank-Starling mechanism of cardiac contractility. Proc. Natl. Acad. Sci. USA 2016, 113, 14426–14431. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.C.; Zhang, Z.; Zhang, L.N.; Xiong, C.; Feng, C.; Liu, Q.; Liu, X.; Shi, X.L.; Wang, Y.L. Chronic intermittent hypobaric hypoxia protects the heart against ischemia/reperfusion injury through upregulation of antioxidant enzymes in adult guinea pigs. Acta Pharmacol. Sin. 2009, 30, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; Riquelme, R.A.; Ebensperger, G.; Reyes, R.V.; Ulloa, C.E.; Cabello, G.; Krause, B.J.; Parer, J.T.; Giussani, D.A.; Llanos, A.J. Long-term exposure to high-altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension at sea level. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Prabhakar, N.R. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J. Appl. Physiol. 2004, 96, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Craige, S.M.; Kant, S.; Keaney, J.F., Jr. Reactive Oxygen Species in Endothelial Function. Circ. J. 2015, 79, 1145–1155. [Google Scholar]
- Farías, J.G.; Herrera, E.A.; Carrasco-Pozo, C.; Sotomayor-Zárate, R.; Cruz, G.; Morales, P.; Castillo, R.L. Pharmacological models and approaches for pathophysiological conditions associated with hypoxia and oxidative stress. Pharmacol. Ther. 2016, 158, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Joanny, P.; Steinberg, J.; Robach, P.; Richalet, J.P.; Gortan, C.; Gardette, B.; Jammes, Y. Operation Everest III (Comex’97): The effect of simulated severe hypobaric hypoxia on lipid peroxidation and antioxidant defence systems in human blood at rest and after maximal exercise. Resuscitation 2001, 49, 307–314. [Google Scholar] [CrossRef]
- Gazaryan, I.G.; Thomas, B. The status of Nrf2-based therapeutics: Current perspectives and future prospects. Neural Regen. Res. 2016, 11, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, A.B.; Pessoa, A., Jr.; Castillo, R.L.; Figueroa, C.A.; Pulgar, V.M.; Farías, J.G. Cellular and molecular mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell Biochem. Funct. 2013, 31, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Heyman, S.N.; Leibowitz, D.; Mor-Yosef Levi, I.; Liberman, A.; Eisenkraft, A.; Alcalai, R.; Khamaisi, M.; Rosenberger, C. Adaptive response to hypoxia and remote ischaemia pre-conditioning: A new hypoxia-inducible factors era in clinical medicine. Acta Physiol. (Oxf.) 2016, 216, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R. Physiological adaptation of the cardiovascular system to high altitude. Prog. Cardiovasc. Dis. 2010, 52, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, A.; Léon-Velarde, F.; Rivera-Ch, M.; Palacios, J.A.; Pragnell, T.R.; O’Connor, D.F.; Robbins, P.A. Selected contribution: Acute and sustained ventilatory responses to hypoxia in high-altitude natives living at sea level. J. Appl. Physiol. 2003, 94, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.L.; Farías, J.G.; Herrera, E.A.; Álvarez, P.I.; Short, S.E.; Tapia, L.; Sotomayor-Zárate, R. Effects of chronic intermittent hypoxia and polyunsaturated fatty acids on infarct size and oxidative stress markers in cardiac ischemia reperfusion. Exp. Clin. Cardiol. 2014, 20, 3833–3858. [Google Scholar]
- Hurtado, A. Some clinical aspects of life at high altitudes. Ann. Intern. Med. 1960, 53, 247–258. [Google Scholar] [PubMed]
- Mateika, J.H.; El-Chami, M.; Shaheen, D.; Ivers, B. Intermittent hypoxia: A low-risk research tool with therapeutic value in humans. J. Appl. Physiol. 2015, 118, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Kacimi, R.; Richalet, J.P.; Corsin, A.; Abousahl, I.; Crozatier, B. Hypoxia-induced downregulation of beta-adrenergic receptors in rat heart. J. Appl. Physiol. 1992, 73, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.P.; Vargas, M.; Jimenez, D.; Antezana, A.M.; Hudson, C.; Cortés, G.; Osorio, J.; León, A. Chilean Miners Commuting from Sea Level to 4500 m: A Prospective Study. High Alt. Med. Biol. 2002, 3, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.G.; Jimenez, D.; Osorio, J.; Zepeda, A.B.; Figueroa, C.A.; Pulgar, V.M. Acclimatization to chronic intermittent hypoxia in mine workers: A challenge to mountain medicine in Chile. Biol. Res. 2013, 46, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.; Siqués, P.; León-Velarde, F.; De La Cruz, J.J.; López, V.; Herruzo, R. Chronic intermittent hypoxia at high altitude exposure for over 12 years: Assessment of hematological, cardiovascular, and renal effects. High Alt. Med. Biol. 2007, 8, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Beidleman, B.A.; Muza, S.R.; Fulco, C.S.; Cymerman, A.; Ditzler, D.; Stulz, D.; Staab, J.E.; Skrinar, G.S.; Lewis, S.F.; Sawka, M.N. Intermittent altitude exposures reduce acute mountain sickness at 4300 m. Clin. Sci. (Lond.) 2004, 106, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Ma, H.J.; Liu, Y.; Guan, Y.; Maslov, L.N.; Li, D.P.; Zhang, Y. The anti-arrhythmic effect of chronic intermittent hypobaric hypoxia in rats with metabolic syndrome induced with fructose. Can. J. Physiol. Pharmacol. 2015, 93, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Siques, P.; Brito, J.; Naveas, N.; Pulido, R.; De la Cruz, J.J.; Mamani, M.; León-Velarde, F. Plasma and liver lipid profiles in rats exposed to chronic hypobaric hypoxia: Changes in metabolic pathways. High Alt. Med. Biol. 2014, 15, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yan, G.; Xu, H.; He, W.; Liu, Z.; Ma, G. Hypoxic preconditioning increases survival of cardiac progenitor cells via the pim-1 kinase-mediated anti-apoptotic effect. Circ. J. 2014, 78, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Zhu, W.; Cheng, M.; Chen, L.; Zhang, J.; Sun, T.; Kishore, R.; Phillips, M.I.; Losordo, D.W.; Qin, G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ. Res. 2009, 104, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.G.; Osorio, J.; Soto, G.; Brito, J.; Siques, P.; Reyes, J.G. Sustained acclimatization in Chilean mine workers subjected to chronic intermittent hypoxia. High Alt. Med. Biol. 2006, 7, 302–306. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. A strategy for oxygen conditioning at high altitude: Comparison with air conditioning. J. Appl. Physiol. 2015, 119, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Dosek, A.; Ohno, H.; Acs, Z.; Taylor, A.W.; Radak, Z. High altitude and oxidative stress. Respir. Physiol. Neurobiol. 2007, 158, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Suryakumar, G.; Prasad, R.; Ganju, L. Upregulation of cytoprotective defense mechanisms and hypoxia-responsive proteins imparts tolerance to acute hypobaric hypoxia. High Alt. Med. Biol. 2013, 14, 65–77. [Google Scholar] [CrossRef] [PubMed]
- García-Herrera, C.M.; Atienza, J.M.; Rojo, F.J.; Claes, E.; Guinea, G.V.; Celentano, D.J.; García-Montero, C.; Burgos, R.L. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Med. Biol. Eng. Comput. 2012, 50, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippi, N.R.; Bird, C.E.; Marcus, N.J.; Olson, E.B.; Chesler, N.C.; Morgan, B.J. Time course of intermittent hypoxia-induced impairments in resistance artery structure and function. Respir. Physiol. Neurobiol. 2010, 28, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ocaranza, M.P.; Moya, J.; Barrientos, V.; Alzamora, R.; Hevia, D.; Morales, C.; Pinto, M.; Escudero, N.; García, L.; Novoa, U.; et al. Angiotensin-(1–9) reverses experimental hypertension and cardiovascular damage by inhibition of the angiotensin converting enzyme/Ang II axis. J. Hypertens. 2014, 32, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; Verkerk, M.M.; Derks, J.B.; Giussani, D.A. Antioxidant treatment alters peripheral vascular dysfunction induced by postnatal glucocorticoid therapy in rats. PLoS ONE 2010, 5, e9250. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.; González-Candia, A.; Montt, C.; Ebensperger, G.; Chubretovic, M.; Serón-Ferré, M.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Melatonin reduces oxidative stress and improves vascular function in hypertensive newborn sheep. J. Pineal Res. 2015, 58, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Cañas, D.; Herrera, E.A.; García-Herrera, C.; Celentano, D.; Krause, B.J. Fetal Growth Restriction Induces Heterogeneous Effects on Vascular Biomechanical and Functional Properties in Guinea Pigs (Cavia porcellus). Front. Physiol. 2017, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Castillo, R.L.; Beltrán, C.; Miranda, A.; Fuentes, J.; Gotteland, M. Molecular mechanisms of gastrointestinal protection by quercetin against indomethacin-induced damage: Role of NF-κB and Nrf2. J. Nutr. Biochem. 2016, 27, 289–298. [Google Scholar] [CrossRef] [PubMed]

| Organs Weight | NN | % NN | IHH | % IHH |
|---|---|---|---|---|
| Body weight (g) | 445 ± 15 | - | 422 ± 13 | - |
| Heart (g) | 1.582 ± 0.071 | 0.355 ± 0.016 | 1.521 ± 0.049 | 0.359 ± 0.011 |
| Lung (g) | 2.631 ± 0.215 | 0.590 ± 0.048 | 2.021 ± 0.294 | 0.477 ± 0.069 |
| Spleen (g) | 1.115 ± 0.062 | 0.250 ± 0.013 | 1.347 ± 0.082 * | 0.318 ± 0.019 |
| Liver (g) | 12.483 ± 0.886 | 2.803 ± 0.199 | 10.968 ± 0.883 | 2.593 ± 0.209 |
| Left kidney (g) | 1.317 ± 0.051 | 0.295 ± 0.011 | 1.402 ± 0.152 | 0.314 ± 0.036 |
| Right kidney (g) | 1.369 ± 0.047 | 0.307 ± 0.010 | 1.329 ± 0.074 | 0.331 ± 0.017 |
| Cardiac Parameters | NN1 | IHH1 | NN4 | IHH4 |
|---|---|---|---|---|
| LVDD (mm) | 7.139 ± 0.426 | 6.498 ± 0.374 * | 7.525 ± 0.166 | 5.957 ± 0.398 * |
| LVSD (mm) | 4.131 ± 0.334 | 2.965 ± 0.305 * | 3.987 ± 0.110 | 2.642 ± 0.396 * |
| IVSD (mm) | 1.571 ± 0.106 | 1.600 ± 0.108 | 1.762 ± 0.147 | 2.000 ± 0.121 |
| LVWD (mm) | 2.501 ± 0.291 | 3.151 ± 0.245 | 2.887 ± 0.161 | 3.014 ± 0.192 |
| LADD (mm) | 3.922 ± 0.135 | 3.527 ± 0.310 | 4.482 ± 0.110 | 3.857 ± 0.03 |
| ADD (mm) | 3.382 ± 0.247 | 3.015 ± 0.154 | 2.907 ± 0.165 | 2.437 ± 0.247 |
| Vmax (cm/s) | 83.41 ± 4.21 | 128.40 ± 4.71 * | 69.58 ± 2.66 | 139.05 ± 4.67 * |
| Vmed (cm/s) | 47.05 ± 3.46 | 72.84 ± 4.84 * | 50.97 ± 2.14 | 77.07 ± 4.54 * |
| GPmax (mmHg) | 2.832 ± 0.273 | 6.615 ± 0.511 * | 1.966 ± 0.144 | 6.941 ± 1.059 * |
| GPmed (mmHg) | 0.918 ± 0.131 | 2.227 ± 0.303 * | 1.467 ± 0.236 | 2.437 ± 0.247 * |
| E-Wave (cm/s) | 80.72 ± 7.76 | 56.93 ± 5.42 | 83.47 ± 6.62 | 86.47 ± 3.77 |
| HR (bpm) | 242 ± 19 | 243 ± 8 | 220 ± 11 | 261 ± 15 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, M.; González-Candia, A.; Rodríguez, J.; Carrasco-Pozo, C.; Cañas, D.; García-Herrera, C.; Herrera, E.A.; Castillo, R.L. Mechanisms of Cardiovascular Protection Associated with Intermittent Hypobaric Hypoxia Exposure in a Rat Model: Role of Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 366. https://doi.org/10.3390/ijms19020366
Aguilar M, González-Candia A, Rodríguez J, Carrasco-Pozo C, Cañas D, García-Herrera C, Herrera EA, Castillo RL. Mechanisms of Cardiovascular Protection Associated with Intermittent Hypobaric Hypoxia Exposure in a Rat Model: Role of Oxidative Stress. International Journal of Molecular Sciences. 2018; 19(2):366. https://doi.org/10.3390/ijms19020366
Chicago/Turabian StyleAguilar, Miguel, Alejandro González-Candia, Jorge Rodríguez, Catalina Carrasco-Pozo, Daniel Cañas, Claudio García-Herrera, Emilio A. Herrera, and Rodrigo L. Castillo. 2018. "Mechanisms of Cardiovascular Protection Associated with Intermittent Hypobaric Hypoxia Exposure in a Rat Model: Role of Oxidative Stress" International Journal of Molecular Sciences 19, no. 2: 366. https://doi.org/10.3390/ijms19020366