Characterization and Oral Delivery of Proinsulin-Transferrin Fusion Protein Expressed Using ExpressTec
Abstract
:1. Introduction
2. Results
2.1. Expression, Extraction and Purification of ExpressTec-Proinsulin-Transferrin Fusion Protein (ExpressTec-ProINS-Tf)
2.2. Molecular Characterization of ExpressTec-ProINS-Tf
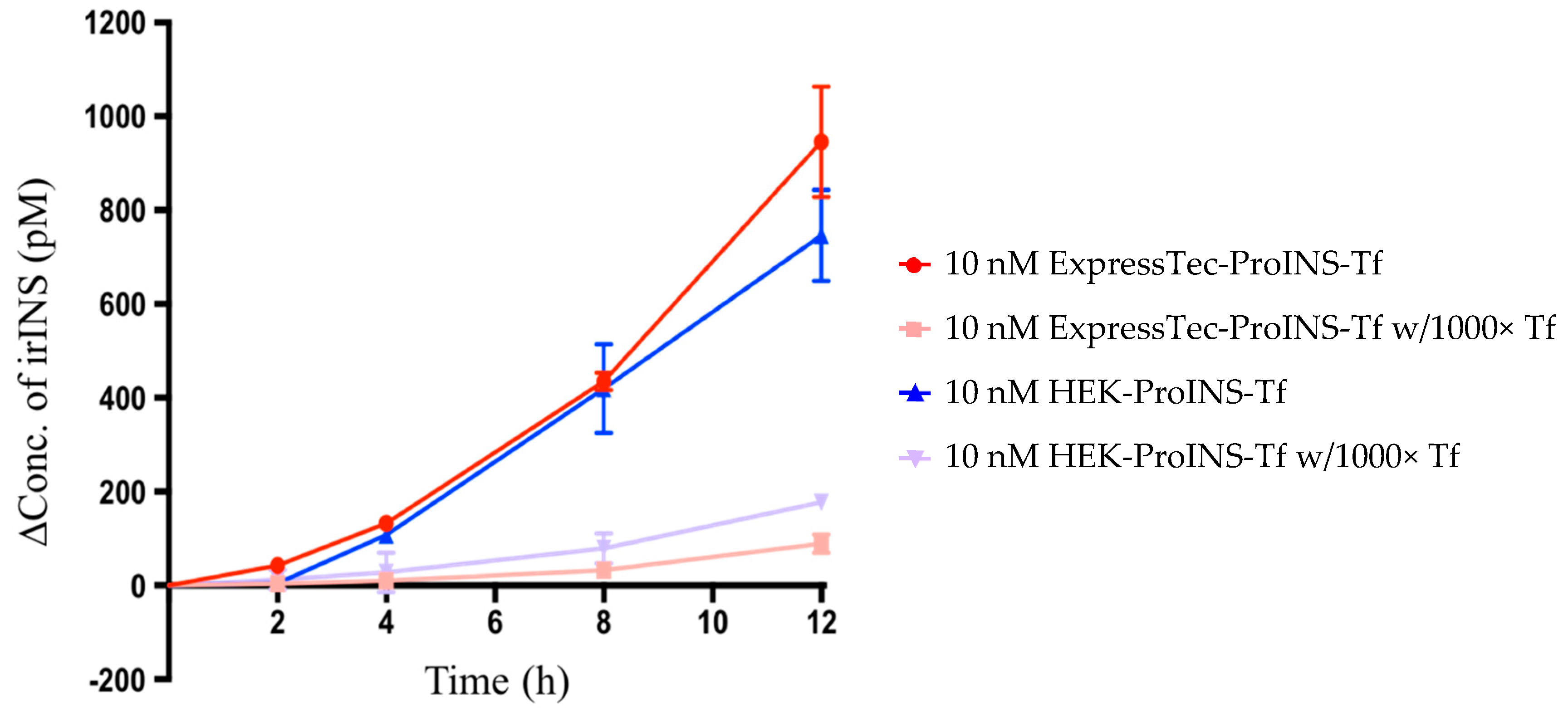
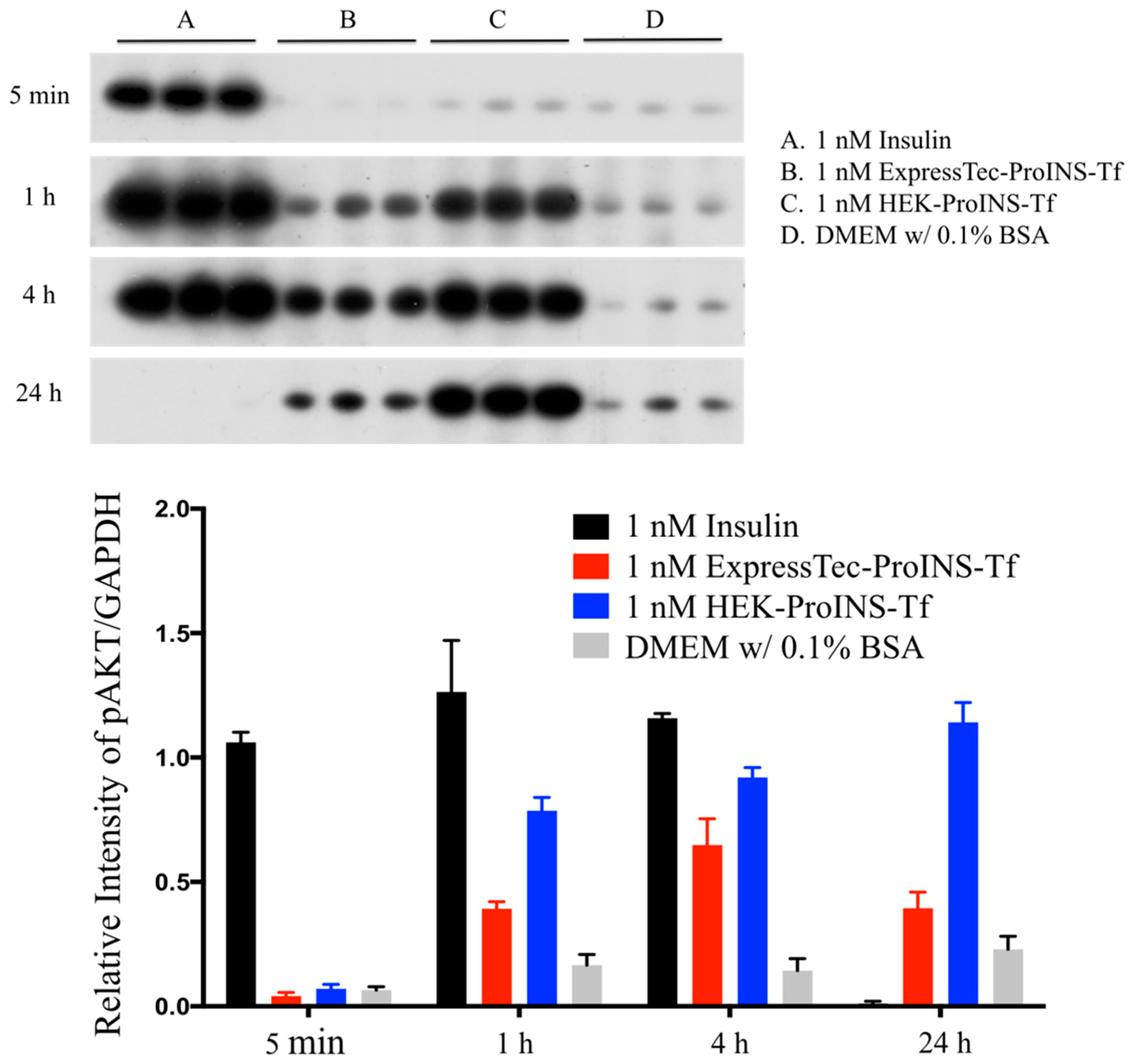
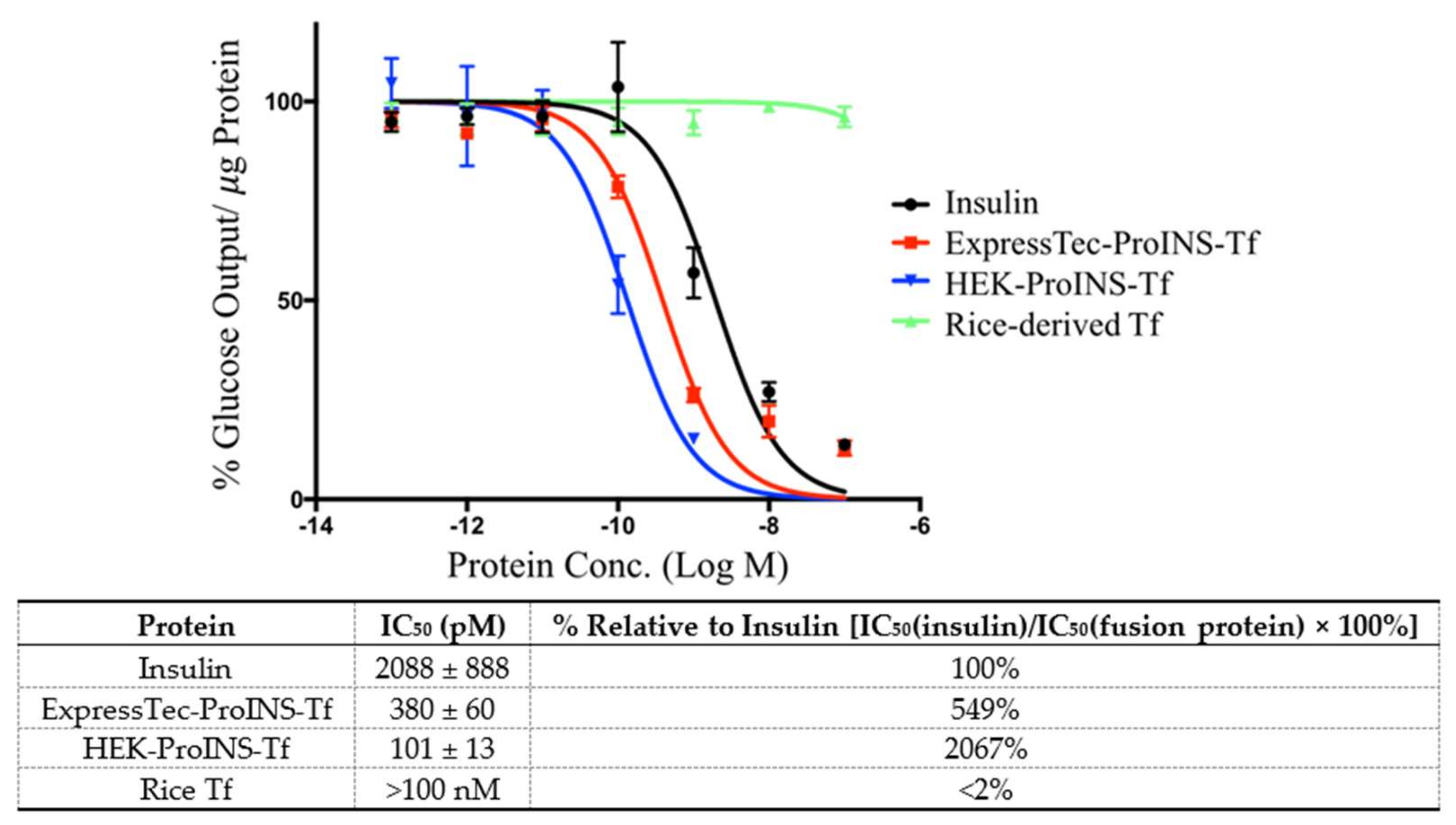
2.3. In Vitro Comparisons between ExpressTec-ProINS-Tf and HEK293-ProINS-Tf
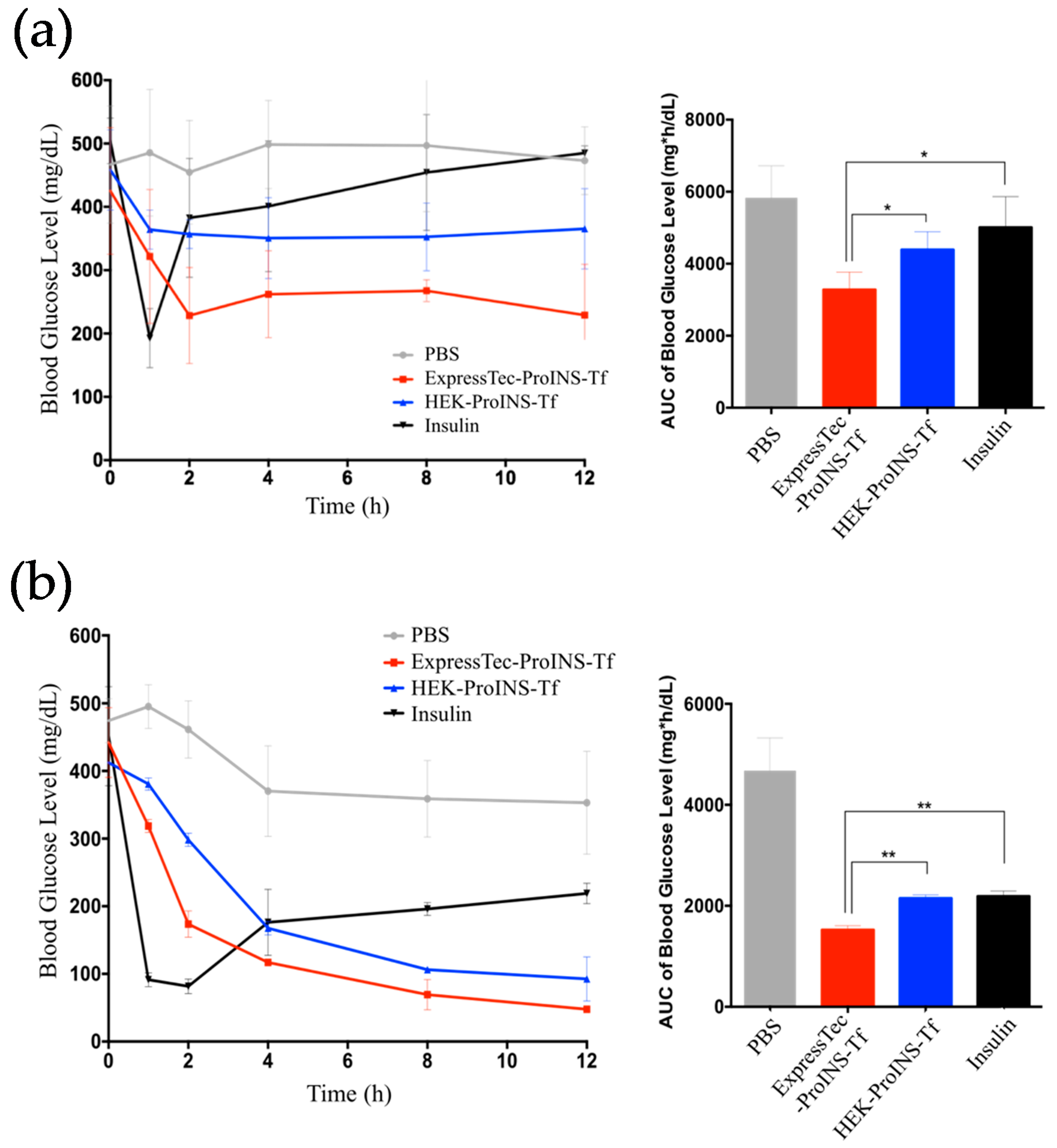
2.4. In Vivo Glucose-Lowering Effects following Subcutaneous Injection in Type 1 Diabetic Mice
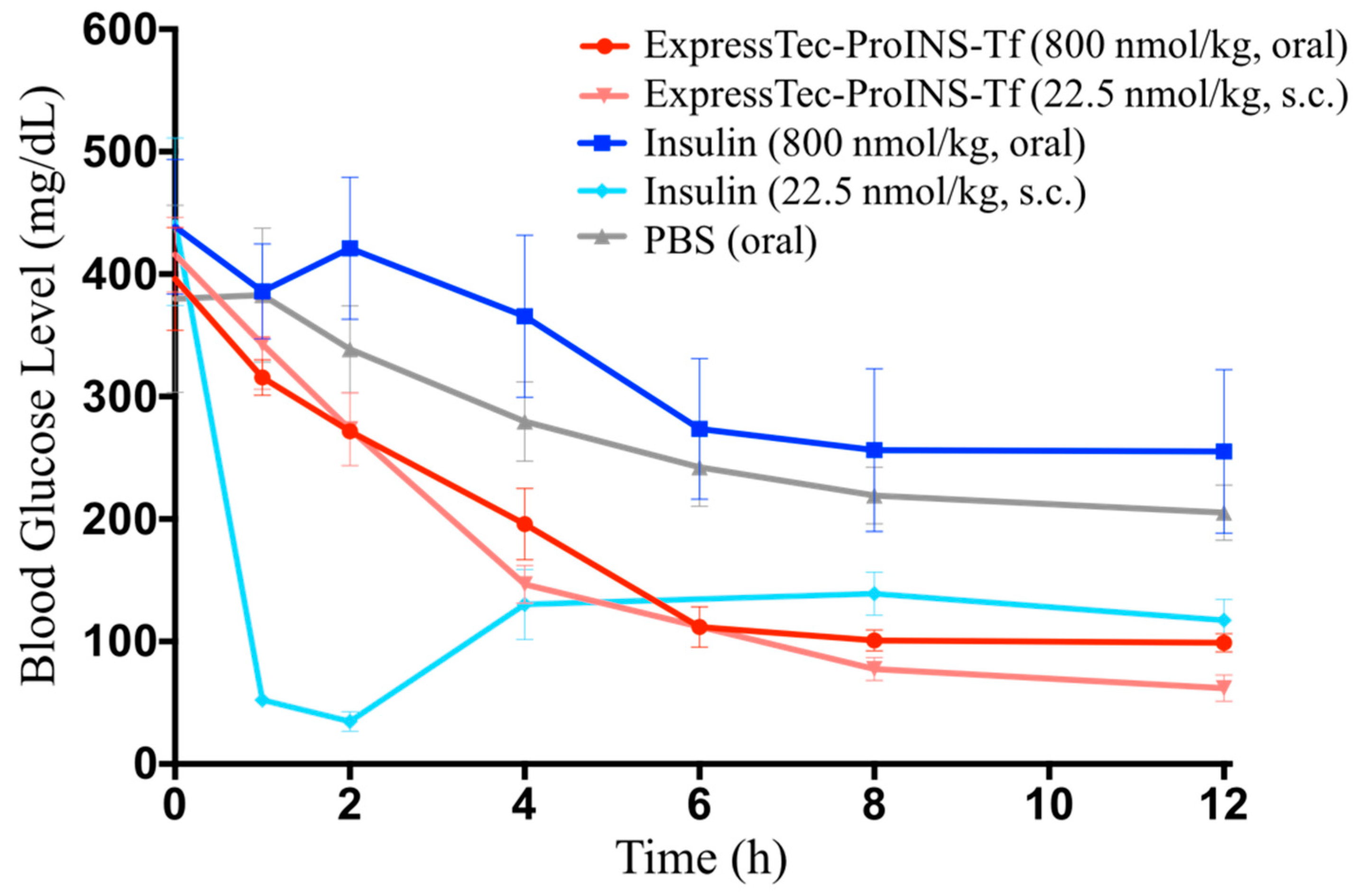
2.5. In Vivo Glucose-Lowering Effects Following Oral Administration in Type 1 Diabetic Mice
3. Discussion
4. Materials and Methods
4.1. Development of Plasmid Construct
4.2. Rice genetic Transformation
4.2.1. Particle Bombardment Transformation
4.2.2. Polymerase Chain Reaction (PCR) Analysis of Transgenic Plants with ProINS-Tf Gene
4.2.3. Plant Culture in Greenhouse
4.3. Expression Analysis of Recombinant ProINS-Tf Protein from Transgenic Rice
4.4. Expression and Purification of ProINS-Tf from HEK293 Cells
4.5. TfR-Mediated Conversion Study in H4IIE Cells
4.6. Phosphorylation of Akt
4.7. Inhibition of Glucose Production in H4IIE Cells
4.8. Type 1 Diabetes Mouse Model
4.9. In Vivo Glucose-Lowering Efficacies via Subcutaneous Injection
4.10. In Vivo Glucose-Lowering Efficacies via Oral Administration
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Chen, Y.S.; Zaro, J.L.; Shen, W.C. Receptor-mediated activation of a proinsulin-transferrin fusion protein in hepatoma cells. J. Controll. Release 2011, 155, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, J.; Zaro, J.L.; Shen, W.C. Proinsulin-transferrin fusion protein as a novel long-acting insulin analog for the inhibition of hepatic glucose production. Diabetes 2014, 63, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zaro, J.L.; Shen, W.C. Proinsulin-transferrin fusion protein exhibits a prolonged and selective effect on the control of hepatic glucose production in an experimental model of type 1 diabetes. Mol. Pharm. 2016, 13, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, R.S. Role of the liver in glucose homeostasis. Diabetes Care 1980, 3, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Geho, W.B. The importance of the liver in insulin replacement therapy in insulin-deficient diabetes. Diabetes 2014, 63, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zaro, J.L.; Shen, W.C. Tissue barriers and novel approaches to achieve hepatoselectivity of subcutaneously-injected insulin therapeutics. Tissue Barriers 2016, 4, e1156804. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Schillberg, S.; Buyel, J.F.; Twyman, R.M. Commercial aspects of pharmaceutical protein production in plants. Curr. Pharm. Des. 2013, 19, 5471–5477. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.; Drake, P.M.; Christou, P. The production of recombinant pharmaceutical proteins in plants. Nat. Rev. Genet. 2003, 4, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Diao, H.; Feng, Z.C.; Lau, A.; Wang, R.; Jevnikar, A.M.; Ma, S. A fusion protein derived from plants holds promising potential as a new oral therapy for type 2 diabetes. Plant. Biotechnol. J. 2014, 12, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Nandi, S.; Bryan, P.; Pettit, S.; Nguyen, D.; Santos, M.A.; Huang, N. Expression, purification, and characterization of recombinant human transferrin from rice (Oryza sativa L.). Protein Expr. Purif. 2010, 74, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, H.F.; Pettit, S.C.; Zaro, J.L.; Huang, N.; Shen, W.C. Characterization of transferrin receptor-mediated endocytosis and cellular iron delivery of recombinant human serum transferrin from rice (Oryza sativa L.). BMC Biotechnol. 2012, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Amet, N.; Wang, W.; Shen, W.C. Human growth hormone-transferrin fusion protein for oral delivery in hypophysectomized rats. J. Controll. Release 2010, 141, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ann, D.K.; Shen, W.C. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proc. Natl. Acad. Sci. USA 2005, 102, 7292–7296. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lee, H.F.; Zaro, J.L.; Shen, W.C. Effects of receptor binding on plasma half-life of bifunctional transferrin fusion proteins. Mol. Pharm. 2011, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Li, H.; Sun, H.; Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 2002, 54, 561–587. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, S.; Ortegren, U.; Karlsson, M.; Ruishalme, I.; Stralfors, P. Rapid insulin-dependent endocytosis of the insulin receptor by caveolae in primary adipocytes. PLoS ONE 2009, 4, e5985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherrington, A.D. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 1999, 48, 1198–1214. [Google Scholar] [CrossRef] [PubMed]
- Kavimandan, N.J.; Losi, E.; Wilson, J.J.; Brodbelt, J.S.; Peppas, N.A. Synthesis and characterization of insulin-transferrin conjugates. Bioconjug. Chem. 2006, 17, 1376–1384. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-S.; Zaro, J.L.; Zhang, D.; Huang, N.; Simon, A.; Shen, W.-C. Characterization and Oral Delivery of Proinsulin-Transferrin Fusion Protein Expressed Using ExpressTec. Int. J. Mol. Sci. 2018, 19, 378. https://doi.org/10.3390/ijms19020378
Chen Y-S, Zaro JL, Zhang D, Huang N, Simon A, Shen W-C. Characterization and Oral Delivery of Proinsulin-Transferrin Fusion Protein Expressed Using ExpressTec. International Journal of Molecular Sciences. 2018; 19(2):378. https://doi.org/10.3390/ijms19020378
Chicago/Turabian StyleChen, Yu-Sheng, Jennica L. Zaro, Deshui Zhang, Ning Huang, Andrew Simon, and Wei-Chiang Shen. 2018. "Characterization and Oral Delivery of Proinsulin-Transferrin Fusion Protein Expressed Using ExpressTec" International Journal of Molecular Sciences 19, no. 2: 378. https://doi.org/10.3390/ijms19020378




