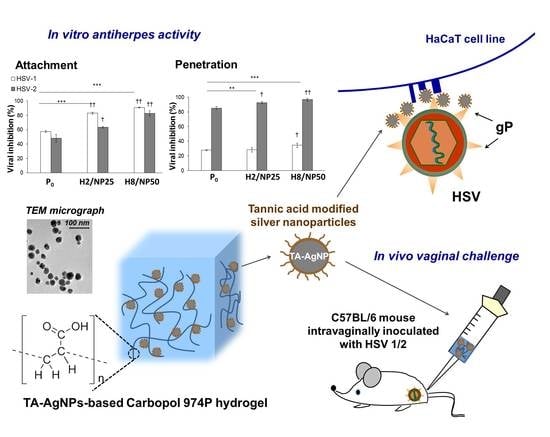
Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Results and Discussion
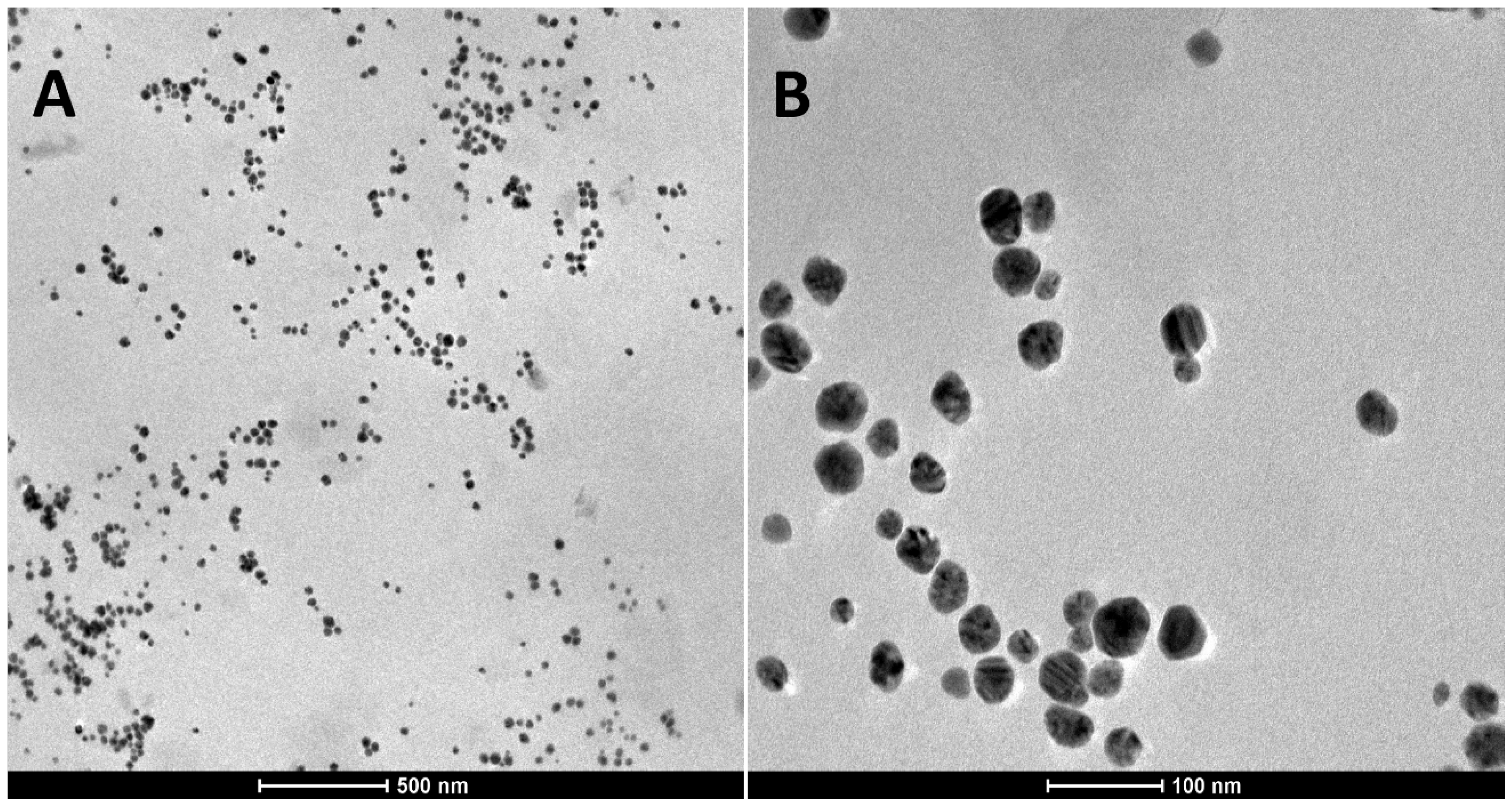
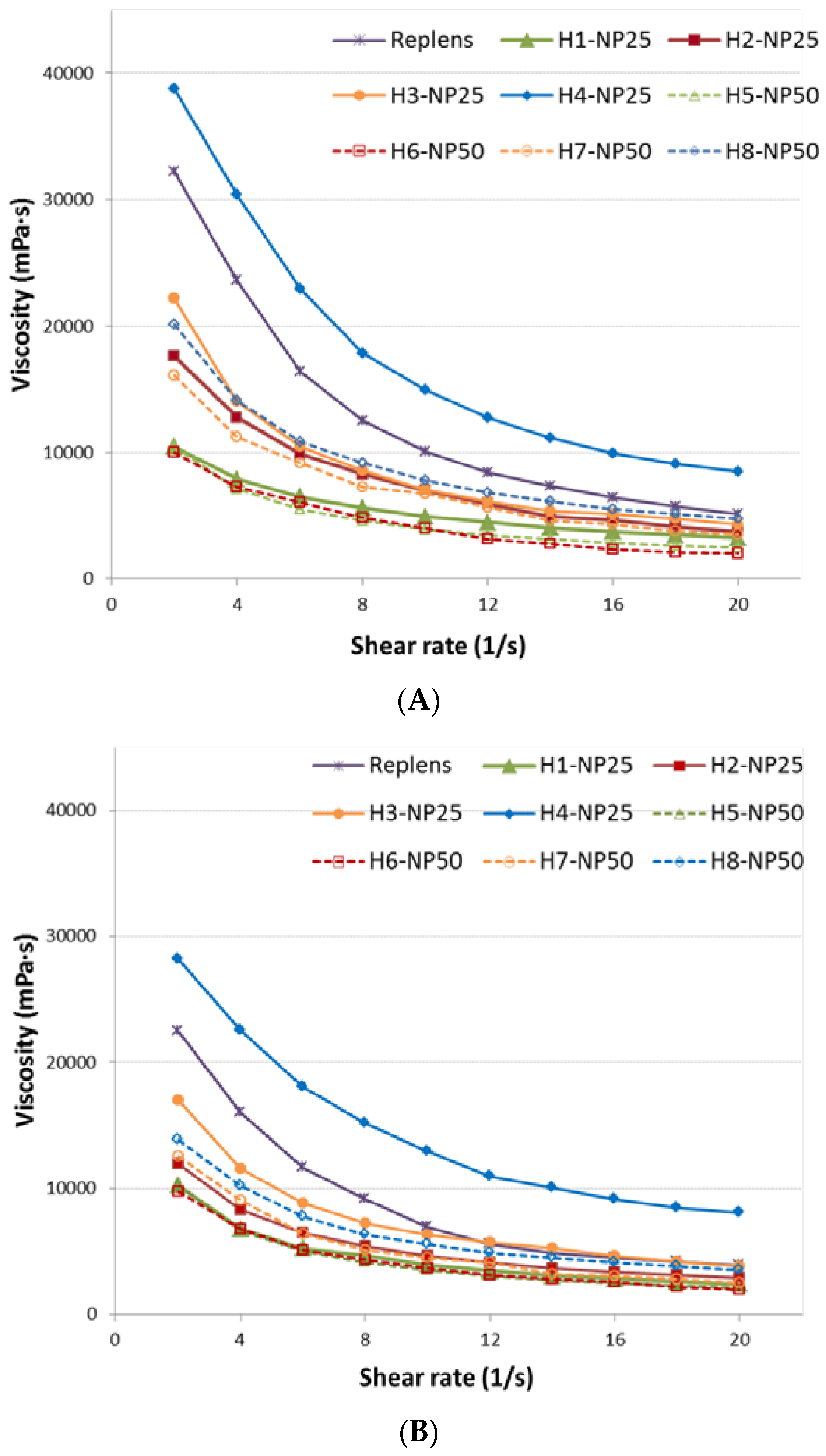
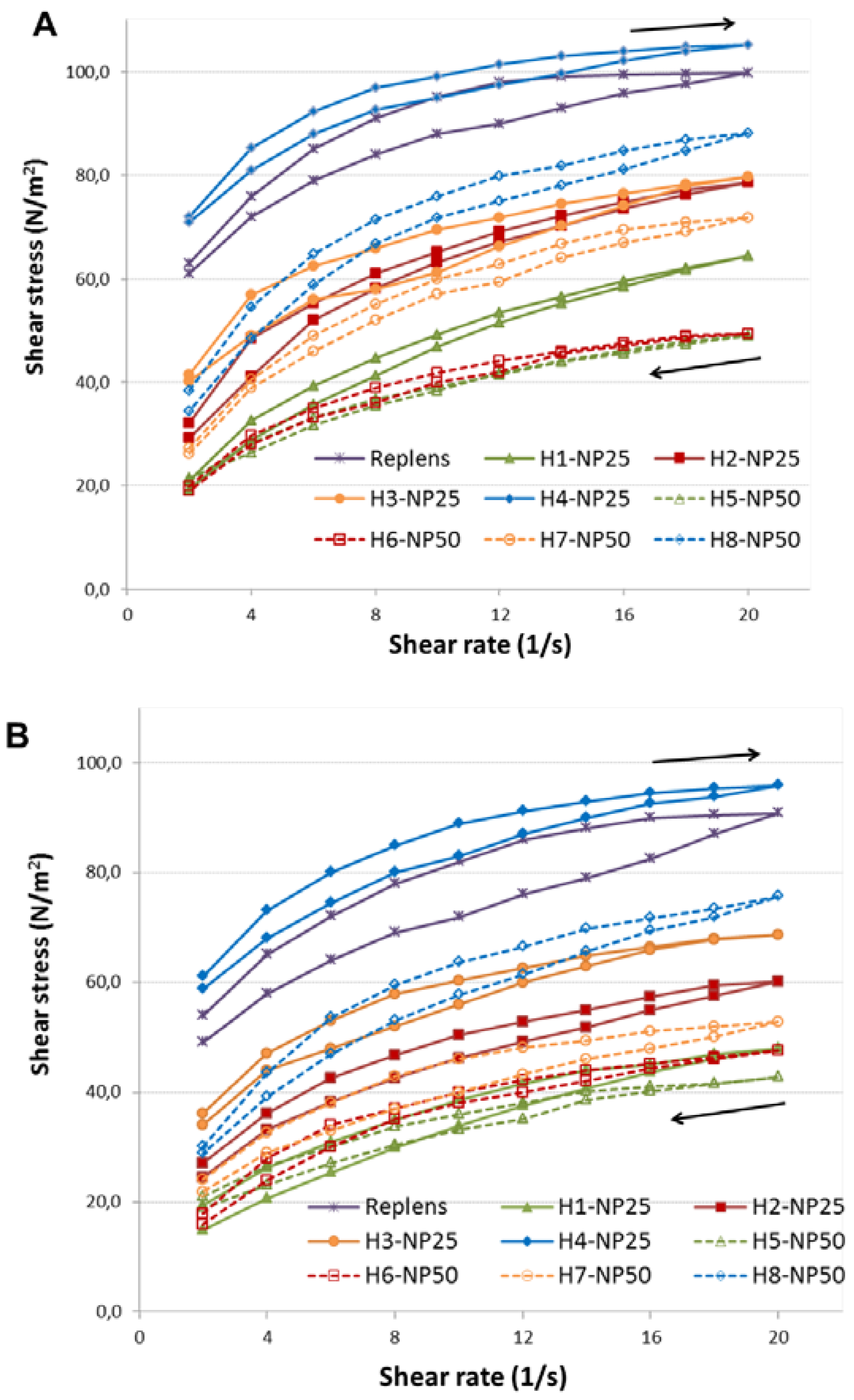
2.1. Characterization of TA-AgNPs-Based Hydrogels
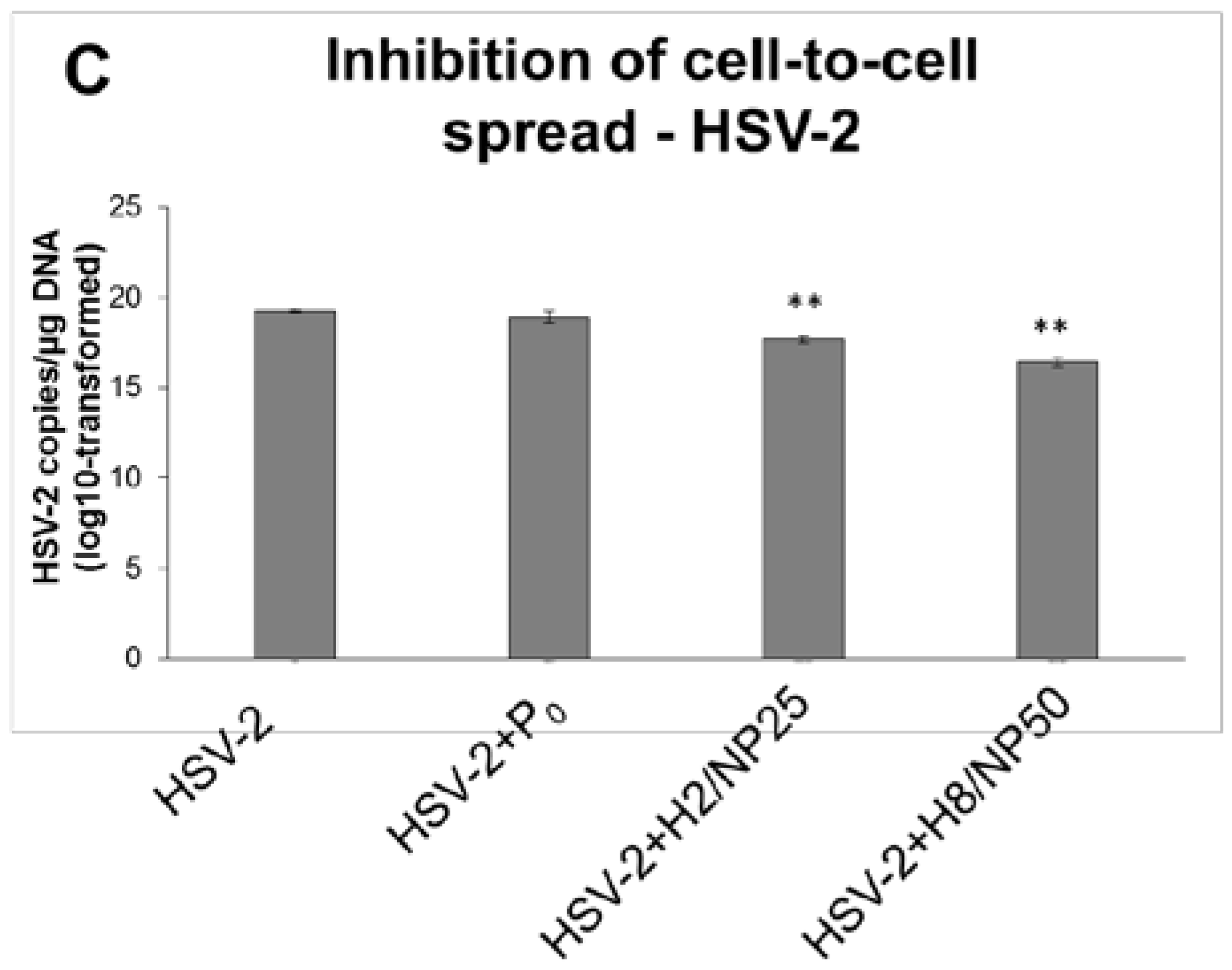
2.2. Assessment of In Vitro Antiviral Efficacy
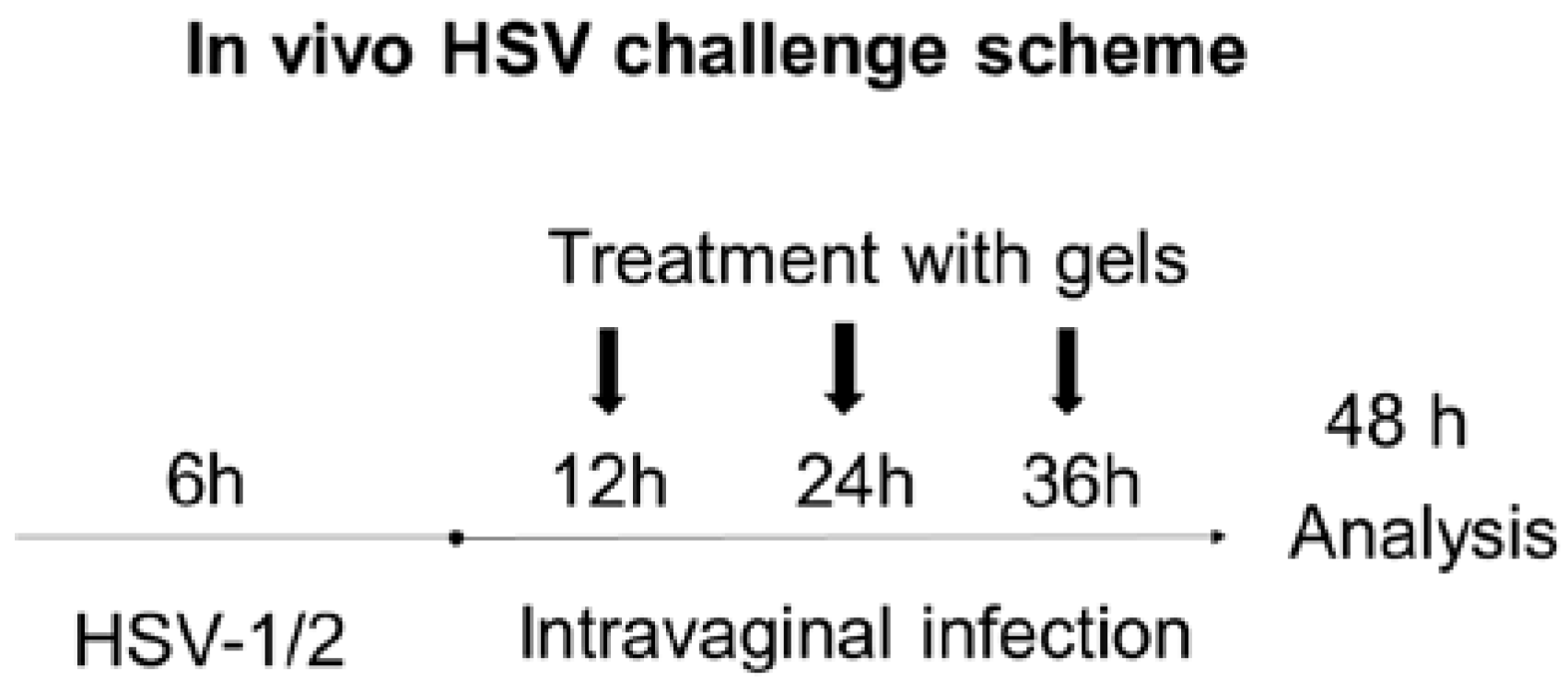
2.3. In Vivo HSV Vaginal Challenge
3. Materials and Methods
3.1. Materials
3.2. Nanoparticles
3.3. Cell Lines and Viruses
3.4. Preparation of TA-AgNPs-Based Hydrogels
3.5. Characerization of TA-AgNPs-Based Hydrogels
3.6. Ex Vivo Mucoadhesion Measurements
3.7. HSV Infection In Vitro
3.7.1. Virus Plaque Forming Assay
3.7.2. Virus Quantification by Real Time Polymerase Chain Reaction
3.7.3. HSV Inactivation Assay
3.7.4. HSV Attachment Assay
3.7.5. HSV Penetration Assay
3.7.6. HSV Cell-to-Cell Infection Assay
3.8. In Vivo HSV Challenge
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| b.w. | Body weight |
| Carb | Carbopol type 974P (Carbomer Homopolymer Type B) |
| D-MEM | Dulbecco’s modified eagle medium |
| FBS | Fetal bovine serum |
| FCS | Fetal calf serum |
| Fmax | Force of detachment |
| GMK-AH1 | Green monkey kidney cell line |
| GRAS | Generally regarded as safe |
| HaCaT | Immortal human keratinocyte cell line |
| HSV | Herpes simplex virus |
| h p.i. | Hours post infection |
| α-MEM | Minimum essential medium with alpha modification |
| MOI | Multiplicity of infection |
| P0 | Nanoparticle-free hydrogel (placebo) |
| ppm | Parts per million |
| PBS | Phosphate buffer saline |
| PFU | Plaque forming units |
| SD | Standard deviation |
| RT-qPCR | Real time quantitative polymerase chain reaction |
| TA-AgNPs | Tannic acid modified silver nanoparticles |
| TEA | Triethylamine |
| TEM | Transmission electron microscopy |
| w/w | Weight/weight |
| Wad | Work of adhesion |
References
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Magaret, A.S.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE 2015, 10, e114989. [Google Scholar] [CrossRef] [PubMed]
- Pires de Mello, C.P.; Bloom, D.C.; Paixão, I.C. Herpes simplex virus type-1: Replication, latency, reactivation and its antiviral targets. Antivir. Ther. 2016, 21, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.G.; Porter, S.R. Herpes Simplex Virus Type 1 infection: Overview on relevant clinico-pathological features. J. Oral Pathol. Med. 2008, 37, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Warren, T.; Wald, A. Genital herpes. Lancet 2007, 370, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, A.M.; Rosenthal, S.L.; Stanberry, L.R. Current thinking on genital herpes. Curr. Opin. Infect. Dis. 2014, 27, 75–83. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. NIH Researchers Show How Anti-HIV Drug Acts to Block Herpes Virus. NIH News. [Updated 20 October 2011]. Available online: https://www.nih.gov (accessed on 7 December 2017).
- Roizman, B.; Knipe, D.M. Herpes simplex viruses and their replication. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 1, pp. 2399–2459. [Google Scholar]
- Schiffer, J.T.; Gottlieb, S.L. Biologic interactions between HSV-2 and HIV-1 and possible implications for HSV vaccine development. Vaccine 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.C.; Feng, H.; Lin, Y.C.; Guo, X.R. New strategies against drug resistance to herpes simplex virus. Int. J. Oral Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Donalisio, M.; Laine, C.; Cagno, V.; Civra, A.; Bianchini, E.P.; Zeghbib, N.; Bouchemal, K. Auto-associative heparin nanoassemblies: A biomimetic platform against the heparan sulfate-dependent viruses HSV-1, HSV-2, HPV-16 and RSV. Eur. J. Pharm. Biopharm. 2014, 88, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Agrahari, V.; Ezoulin, M.J.; Zhang, C.; Purohit, S.S.; Molteni, A.; Dim, D.; Oyler, N.A.; Youan, B.B.C. Tenofovir containing thiolated chitosan core/shell nanofibers: In vitro and in vivo evaluations. Mol. Pharm. 2016, 13, 4129–4140. [Google Scholar] [CrossRef] [PubMed]
- Ceña-Diez, R.; García-Broncano, P.; de la Mata, F.J.; Gómez, R.; Muñoz-Fernández, M.Á. Efficacy of HIV antiviral polyanionic carbosilane dendrimer G2-S16 in the presence of semen. Int. J. Nanomed. 2016, 11, 2443–2450. [Google Scholar] [CrossRef]
- Stella, B.; Arpicco, S.; Rocco, F.; Burgalassi, S.; Nicosia, N.; Tampucci, S.; Chetoni, P.; Cattel, L. Nonpolymeric nanoassemblies for ocular administration of acyclovir: Pharmacokinetic evaluation in rabbits. Eur. J. Pharm. Biopharm. 2012, 80, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celichowski, G.; Grobelny, J.; et al. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE 2014, 9, e104113. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.W. Drug delivery: Vaginal Route. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; Volume 2, pp. 1339–1361. [Google Scholar]
- The United States Food and Drug Administration. Inactive Ingredient Search for Approved Drug Products. [Updated 5 July 2017]. Available online: https://www.accessdata.fda.gov (accessed on 25 November 2017).
- Orlowski, P.; Soliwoda, K.; Tomaszewska, E.; Bien, K.; Fruba, A.; Gniadek, M.; Labedz, O.; Nowak, Z.; Celichowski, G.; Grobelny, J.; et al. Toxicity of tannic acid-modified silver nanoparticles in keratinocytes: Potential for immunomodulatory applications. Toxicol. In Vitro 2016, 35, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, P.; Krzyzowska, M.; Zdanowski, R.; Winnicka, A.; Nowakowska, J.; Stankiewicz, W.; Tomaszewska, E.; Celichowski, G.; Grobelny, J. Assessment of in vitro cellular responses of monocytes and keratinocytes to tannic acid modified silver nanoparticles. Toxicol. In Vitro 2013, 27, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.; Yoshii, A.C.; Rocha e Silva, H.; Stringhetti Ferreira Cury, B.; Chorilli, M. Polyacrylic acid polymers hydrogels intended to topical drug delivery: Preparation and characterization. Pharm. Dev. Technol. 2015, 20, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, A.; Molteni, B.; Milani, M. Successful treatment of bacterial vaginosis with a policarbophil-carbopol acidic vaginal gel: Results from a randomised double-blind, placebo-controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 120, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Baloğlu, E.; Ozyazici, M.; Yaprak Hizarcioğlu, S.; Senyiğit, T.; Ozyurt, D.; Pekçetin, C. Bioadhesive controlled release systems of ornidazole for vaginal delivery. Pharm. Dev. Technol. 2006, 11, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, A.; Simmons, A.P.; Ugaonkar, S.R.; Watson, K.M.; Dezzutti, C.S.; Rohan, L.C.; Buckheit, R.W.; Kiser, P.F. Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob. Agents Chemother. 2011, 55, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Carbopol® 974P NF Polymer—Lubrizol. Available online: https://www.lubrizol.com/Life-Sciences/Products (accessed on 26 November 2017).
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Cevher, E.; Sensoy, D.; Taha, M.A.; Araman, A. Effect of thiolated polymers to textural and mucoadhesive properties of vaginal gel formulations prepared with polycarbophil and chitosan. AAPS PharmSciTech 2008, 9, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Engesland, A.; Kermany, B.P.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Erbeck, D.; Uckun, F.M. A study of the potential of the pig as a model for the vaginal irritancy of benzalkonium chloride in comparison to the nonirritant microbicide PHI-443 and the spermicide vanadocene dithiocarbamate. Toxicol. Pathol. 2005, 33, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, A.; Deinhardt, S.; Zell, R.; Wutzler, P. Phenotypic and genotypic characterization of acyclovir-resistant clinical isolates of herpes simplex virus. Antivir. Res. 2010, 86, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Bacon, T.H.; Levin, M.J.; Leary, J.J.; Sarisky, R.T.; Sutton, D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 2003, 16, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Bayer, L.L.; Jensen, J.T. Acidform: A review of the evidence. Contraception 2014, 90, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Stefanidou, M.; Mesquita, P.M.; Fakioglu, E.; Segarra, T.; Rohan, L.; Halford, W.; Palmer, K.E.; Herold, B.C. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 2013, 87, 6257–6269. [Google Scholar] [CrossRef] [PubMed]
- Ceña-Diez, R.; Vacas-Córdoba, E.; García-Broncano, P.; de la Mata, F.J.; Gómez, R.; Maly, M.; Muñoz-Fernández, M.Á. Prevention of vaginal and rectal herpes simplex virus type 2 transmission in mice: Mechanism of antiviral action. Int. J. Nanomed. 2016, 11, 2147–2162. [Google Scholar] [CrossRef]
- Campadelli-Fiume, G.; Menotti, L. Entry of alphaherpesviruses into the cell. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007; Chapter 7. [Google Scholar]
- Gerber, S.I.; Belval, B.J.; Herold, B.C. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology 1995, 214, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Naderi, M.; Chouljenko, V.N.; Walker, J.D.; Kousoulas, K.G. Amino acid differences in glycoproteins B (gB), C (gC), H (gH) and L (gL) are associated with enhanced herpes simplex virus type-1 (McKrae) entry via the paired immunoglobulin-like type-2 receptor α. Virol. J. 2012, 9, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Luck, G.; Liao, H.; Murray, N.J.; Grimmer, H.R.; Warminski, E.E.; Williamson, M.P.; Lilley, T.H.; Haslam, E. Polyphenols, astringency and proline-rich proteins. Phytochemistry 1994, 37, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Gianni, T.; Amasio, M.; Campadelli-Fiume, G. Herpes simplex virus gD forms distinct complexes with fusion executors gB and gH/gL in part through the C-terminal profusion domain. J. Biol. Chem. 2009, 284, 17370–17382. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Mantz, M.J.; Schlievert, P.M.; Davis, C.C. Porcine vagina ex vivo as a model for studying permeability and pathogenesis in mucosa. J. Pharm. Sci. 2008, 97, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Bagdonaite, I.; Nordén, R.; Joshi, H.J.; Dabelsteen, S.; Nyström, K.; Vakhrushev, S.Y.; Olofsson, S.; Wandall, H.H. A strategy for O-glycoproteomics of enveloped viruses—The O-glycoproteome of herpes simplex virus type 1. PLoS Pathog. 2015, 11, e1004784–e1004806. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Sosnowska, K.; Miltyk, W.; Rusak, M.; Basa, A.; Winnicka, K. The effect of β-glycerophosphate cross-linking on chitosan cytotoxicity and properties of hydrogels for vaginal application. Polymers 2015, 7, 2223–2244. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K.; Amelian, A.; Cwalina, U. Vaginal chitosan tablets with clotrimazole-design and evaluation of mucoadhesive properties using porcine vaginal mucosa, mucin and gelatine. Chem. Pharm. Bull. 2014, 62, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Navarro, D.; Paz, P.; Pereira, L. Domains of herpes simplex virus I glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology 1992, 186, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Namvar, L.; Olofsson, S.; Bergstrom, T.; Lindh, M. Detection and typing of herpes simplex virus (HSV) in mucocutaneous samples by TaqMan PCR targeting a gB segment homologous for HSV types 1 and 2. J. Clin. Microbiol. 2005, 43, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Krzyzowska, M.; Shestakov, A.; Eriksson, E.; Chiodi, F. Role of Fas/FasL in regulation of inflammation in vaginal tissue during HSV-2 infection. Cell Death Dis. 2011, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
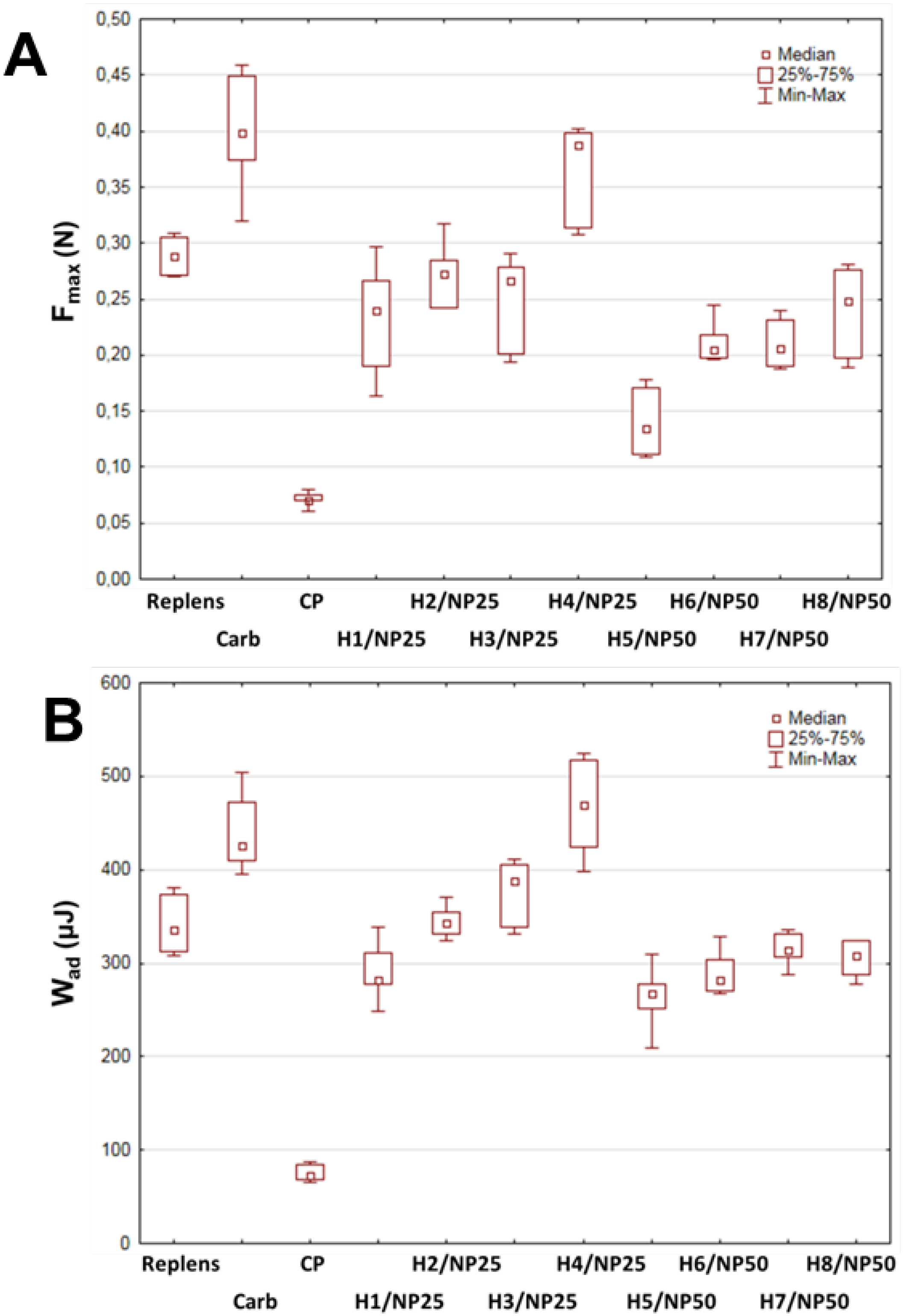
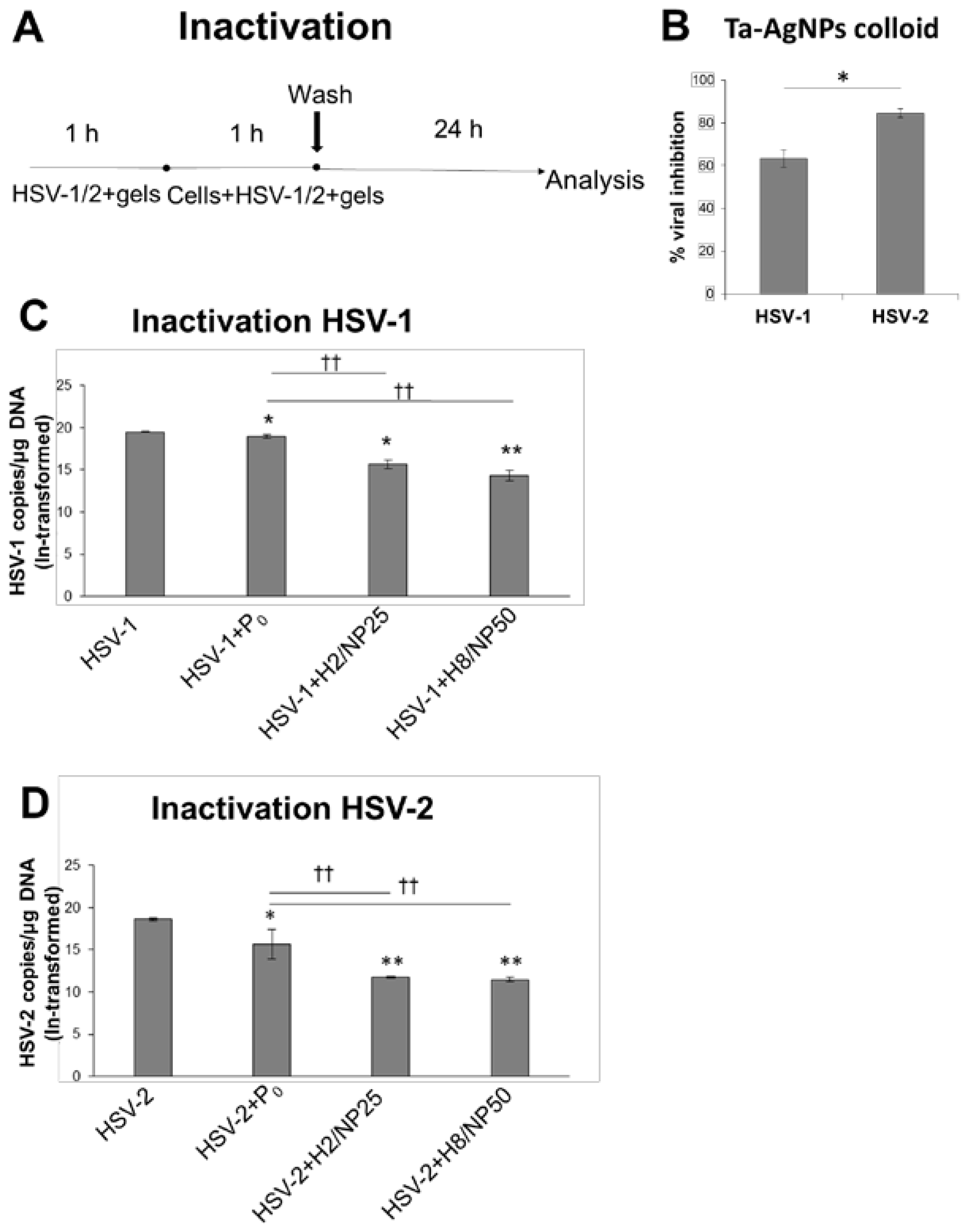
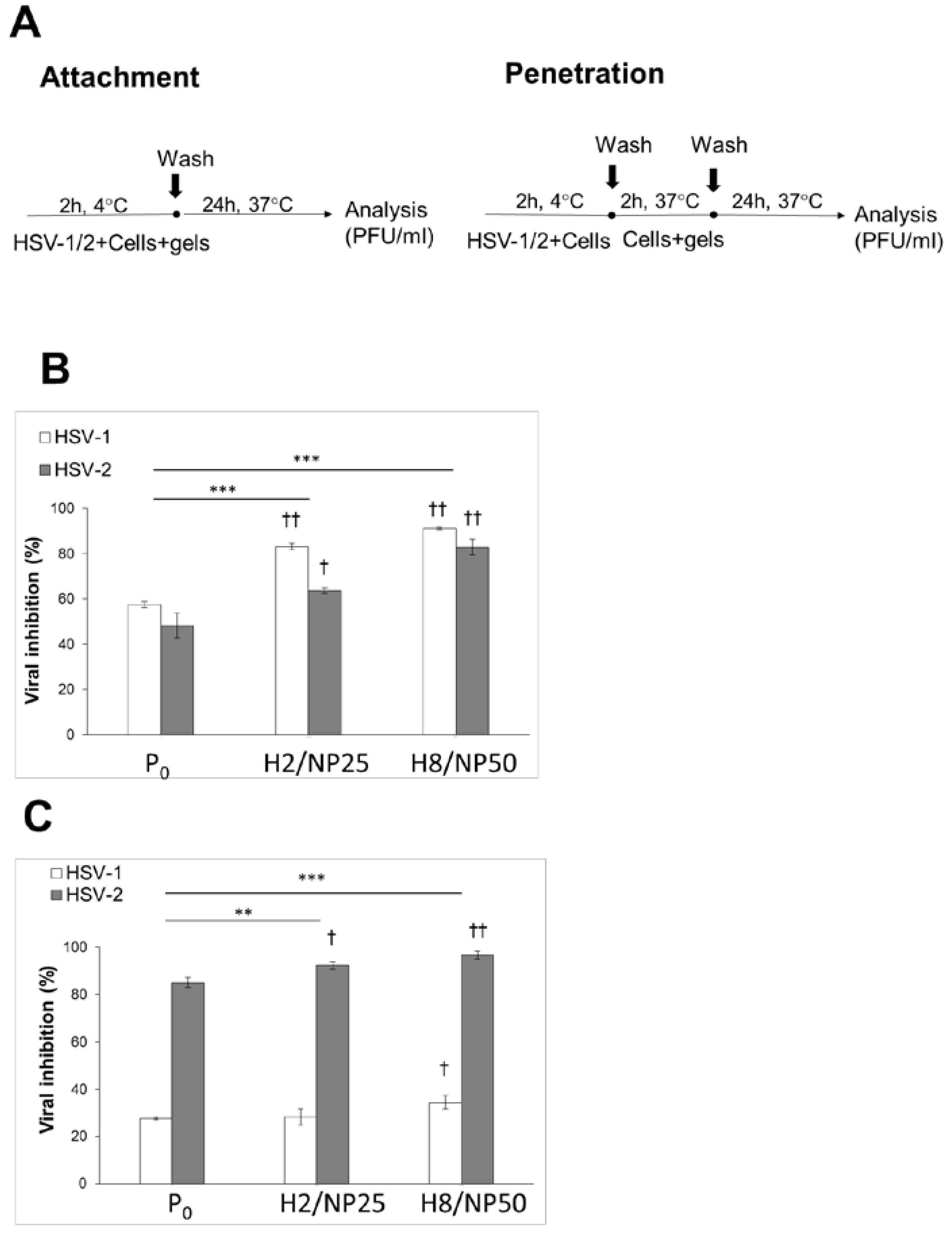
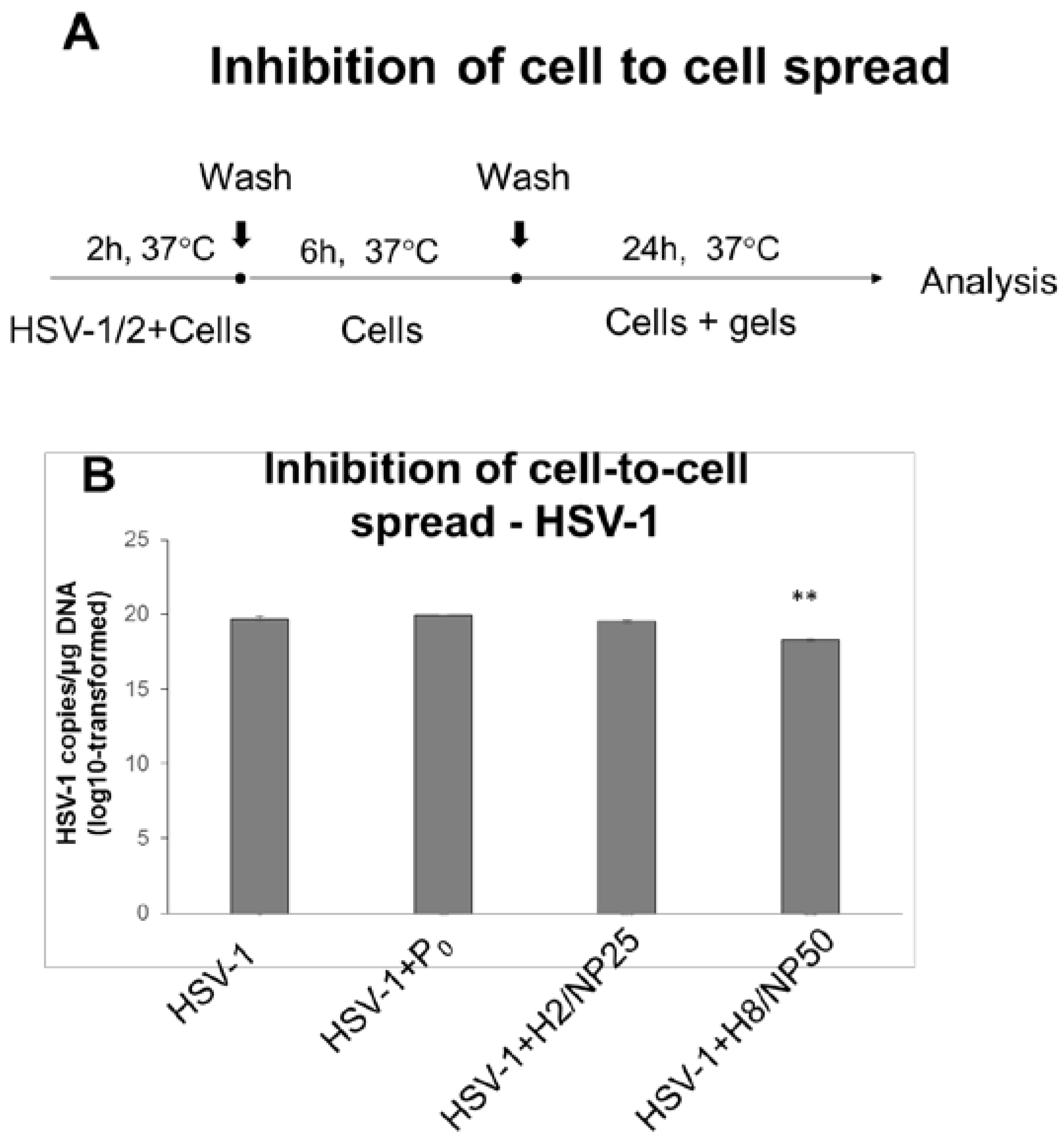
| Component (g) | Formulation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | P0 | With TA-AgNPs 25 ppm a | With TA-AgNPs 50 ppm b | |||||||
| H1/NP25 | H2/NP25 | H3/NP25 | H4/NP25 | H5/NP50 | H6/NP50 | H7/NP50 | H8/NP50 | |||
| TA-AgNPs colloid | - | 25.0 | 25.0 | 25.0 | 25.0 | 50.0 | 50.0 | 50.0 | 50.0 | |
| Carbopol 974P | 0.5 | 0.60 | 0.40 | 0.50 | 0.75 | 1.0 | 0.40 | 0.50 | 0.75 | 1.0 |
| TEA | 1.0 | 0.9 | 0.6 | 1.0 | 1.12 | 1.5 | 0.6 | 1.0 | 1.12 | 1.5 |
| Parameter | ||||||||||
| pH (n = 3) | 7.3 ± 0.3 | 7.1 ± 0.1 | 6.6 ± 0.1 | 6.6 ± 0.2 | 7.4 ± 0.2 | 7.5 ± 0.1 | 6.6 ± 0.2 | 7.0 ± 0.3 | 6.9 ± 0.1 | 7.0 ± 0.1 |
| Viscosity at 25 ± 1 °C (mPa·s) (n = 3) c | n.d. | 7520 ± 131 | 4921 ± 115 | 7005 ± 102 | 7000 ± 124 | 14970 ± 198 | 3910 ± 98 | 3940 ± 101 | 6610 ± 108 | 7670 ± 134 |
| Viscosity at 37 ± 1 °C (mPa·s) (n = 3) c | n.d. | 5080 ± 152 | 3950 ± 78 | 4480 ± 89 | 6290 ± 106 | 12915 ± 198 | 3490 ± 102 | 3670 ± 84 | 4500 ± 85 | 5530 ± 112 |
| Hardness (g) (n = 5) | 248.1 ± 21.0 | 206.4 ± 29.0 | 178.4 ± 20.1 | 207.3 ± 4.6 | 267.1 ± 18.1 | 542.6 ± 29.9 | 155.6 ± 9.1 | 167.2 ± 11.9 | 191.4 ± 7.9 | 218.3 ± 10.1 |
| Adhesiveness (g·s) (n = 5) | 3498 ± 109 | 3210 ± 125 | 2820 ± 186 | 3041 ± 79 | 3210 ± 250 | 3894 ± 198 | 1633 ± 156 | 1710 ± 148 | 1900 ± 109 | 2851 ± 185 |
| Consistency (g) (n = 5) | 441.8 ± 9.8 | 352.8 ± 39.6 | 341.4 ± 19.2 | 391.9 ± 15.3 | 471.6 ± 28.0 | 801.7 ± 27.8 | 251.2 ± 19.1 | 302.5 ± 14.0 | 348.2 ± 20.1 | 378.4 ± 22.7 |
| Virus | Control a | P0 b | H2/NP25 c | H8/NP50 d |
|---|---|---|---|---|
| HSV-1 | 15.61 ± 0.17 | 15.33 ± 0.24 | 14.42 ± 0.08 | 14.98 ± 0.15 |
| HSV-2 | 16.58 ± 0.06 | 16.71 ± 0.06 | 12.65 ± 1.15 * | 14.21 ± 0.65 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobelny, J.; Basa, A.; Krzyżowska, M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 387. https://doi.org/10.3390/ijms19020387
Szymańska E, Orłowski P, Winnicka K, Tomaszewska E, Bąska P, Celichowski G, Grobelny J, Basa A, Krzyżowska M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 2018; 19(2):387. https://doi.org/10.3390/ijms19020387
Chicago/Turabian StyleSzymańska, Emilia, Piotr Orłowski, Katarzyna Winnicka, Emilia Tomaszewska, Piotr Bąska, Grzegorz Celichowski, Jarosław Grobelny, Anna Basa, and Małgorzata Krzyżowska. 2018. "Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies" International Journal of Molecular Sciences 19, no. 2: 387. https://doi.org/10.3390/ijms19020387