Sex Hormone Receptors in Benign and Malignant Salivary Gland Tumors: Prognostic and Predictive Role
Abstract
:1. Introduction
2. Results
2.1. Characteristics of SGTs Patients
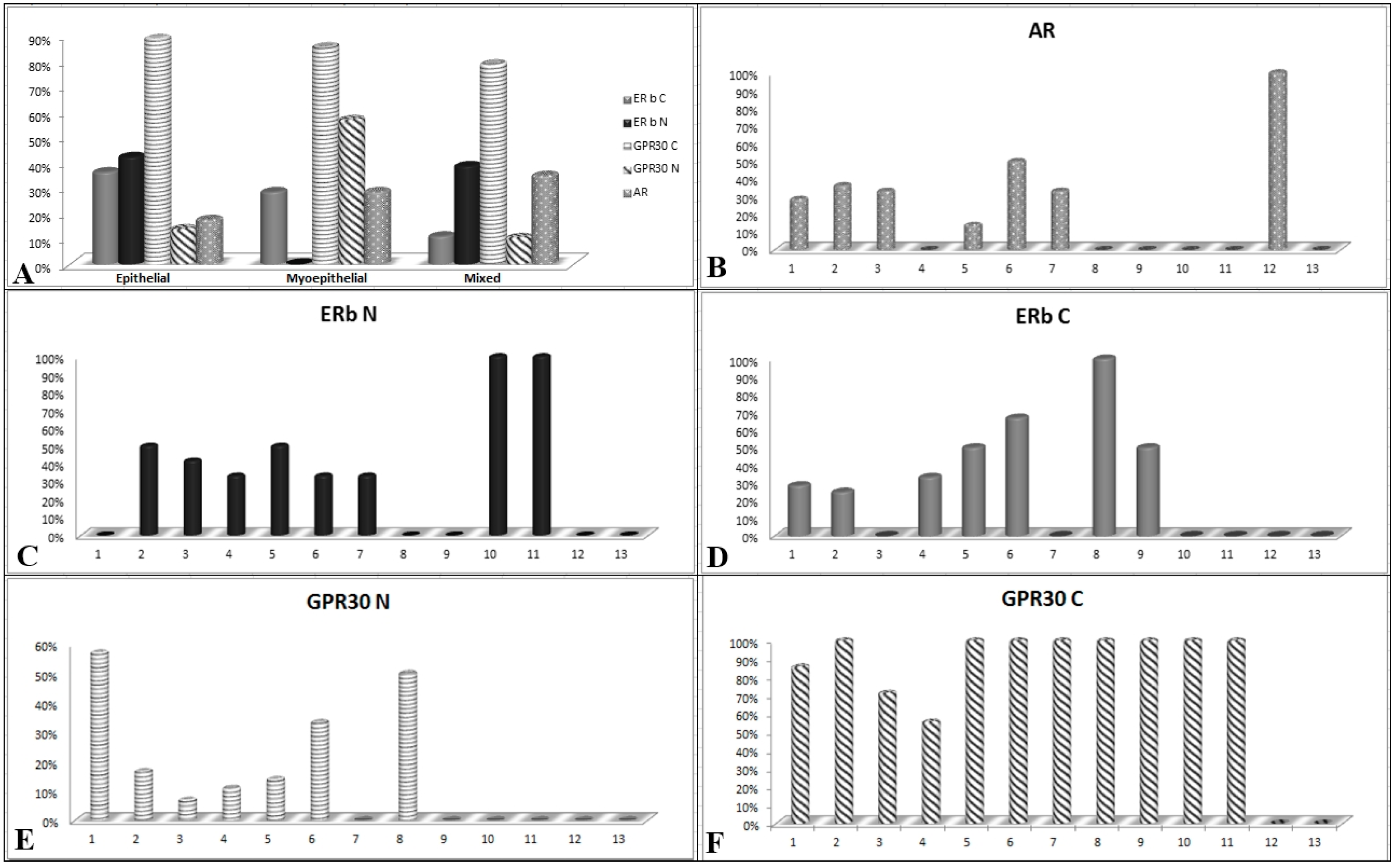
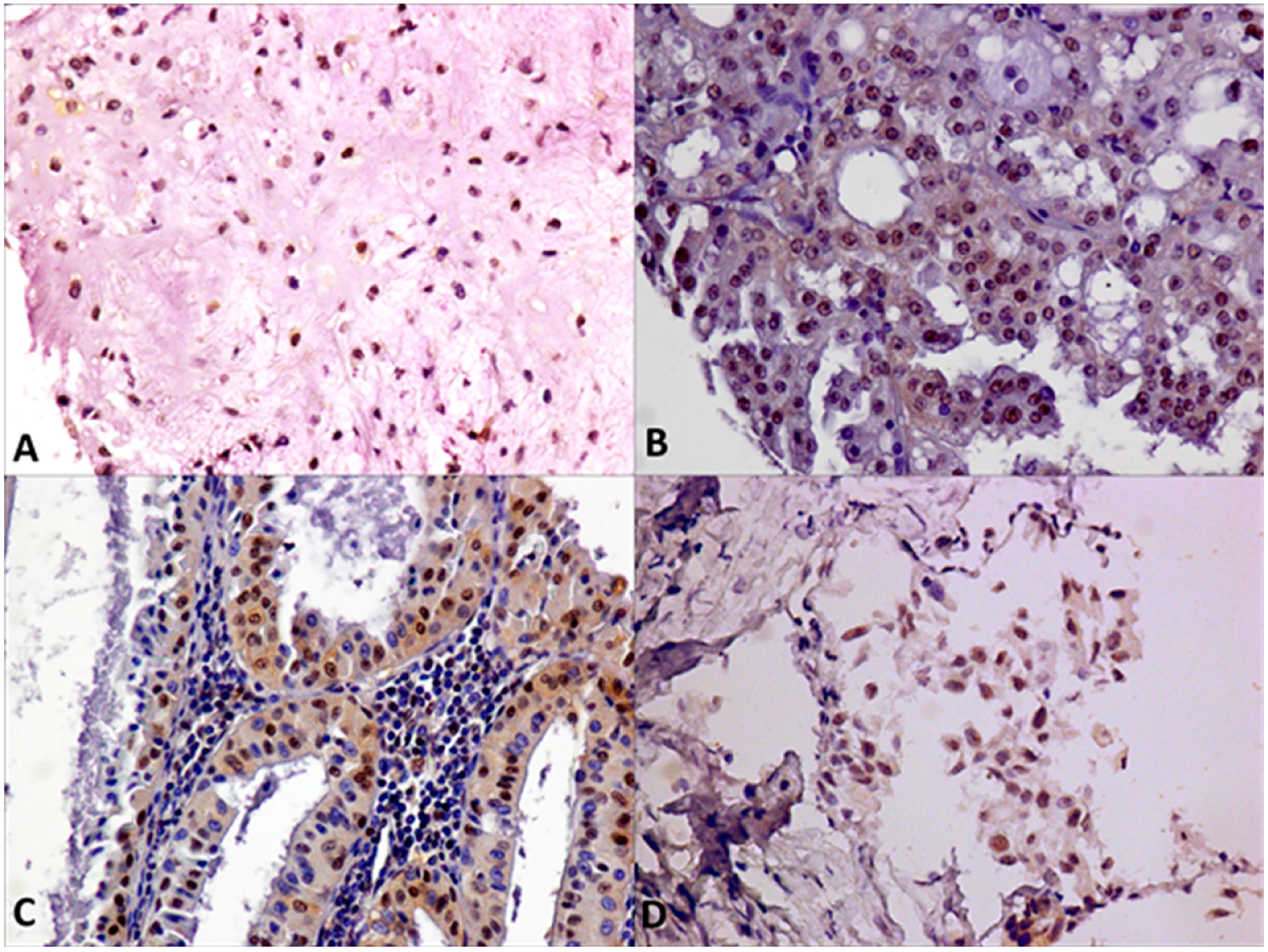
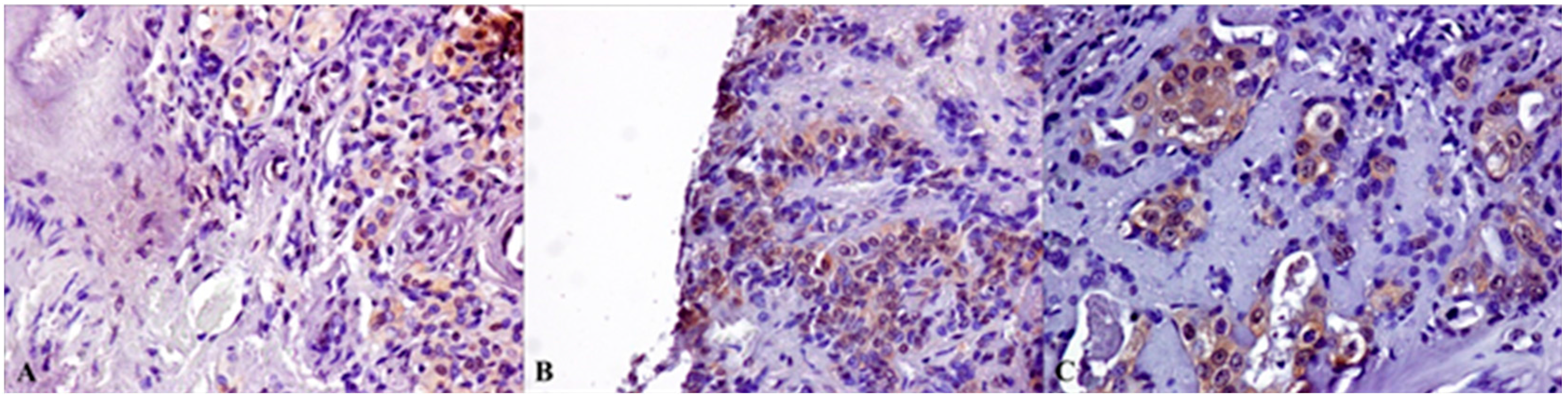
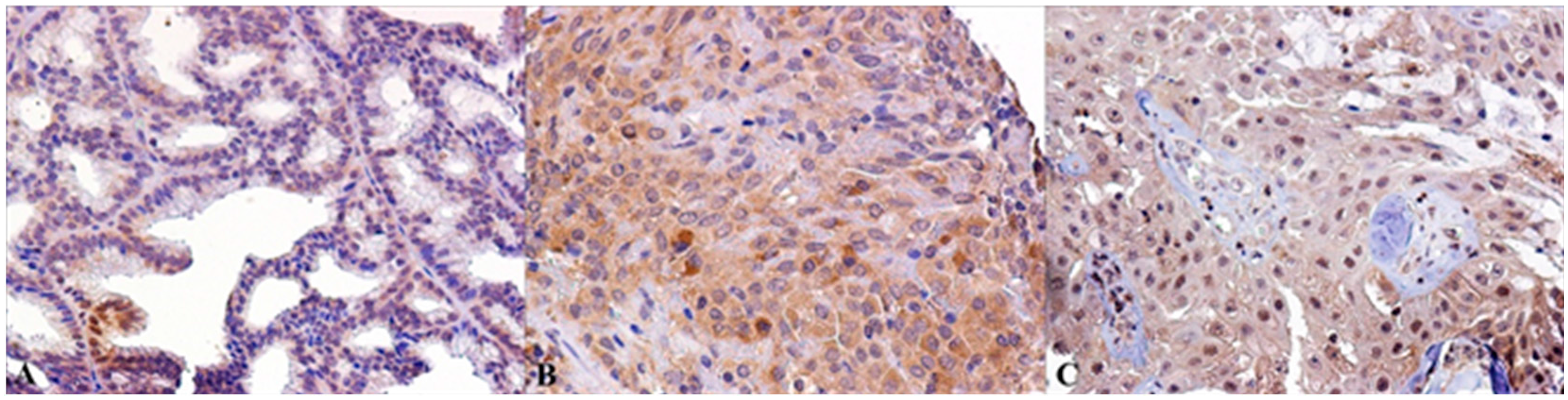
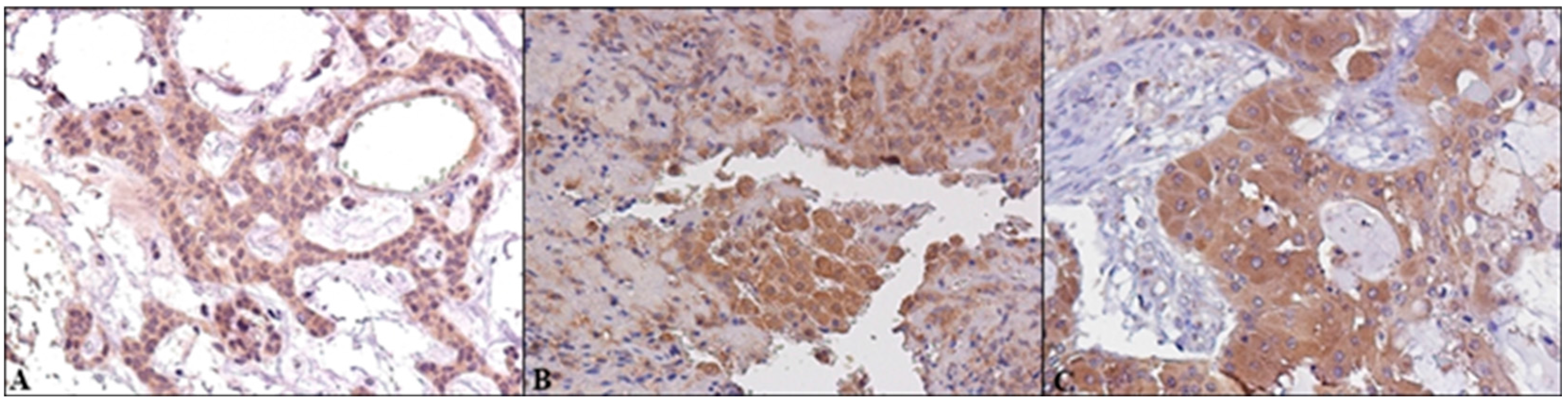
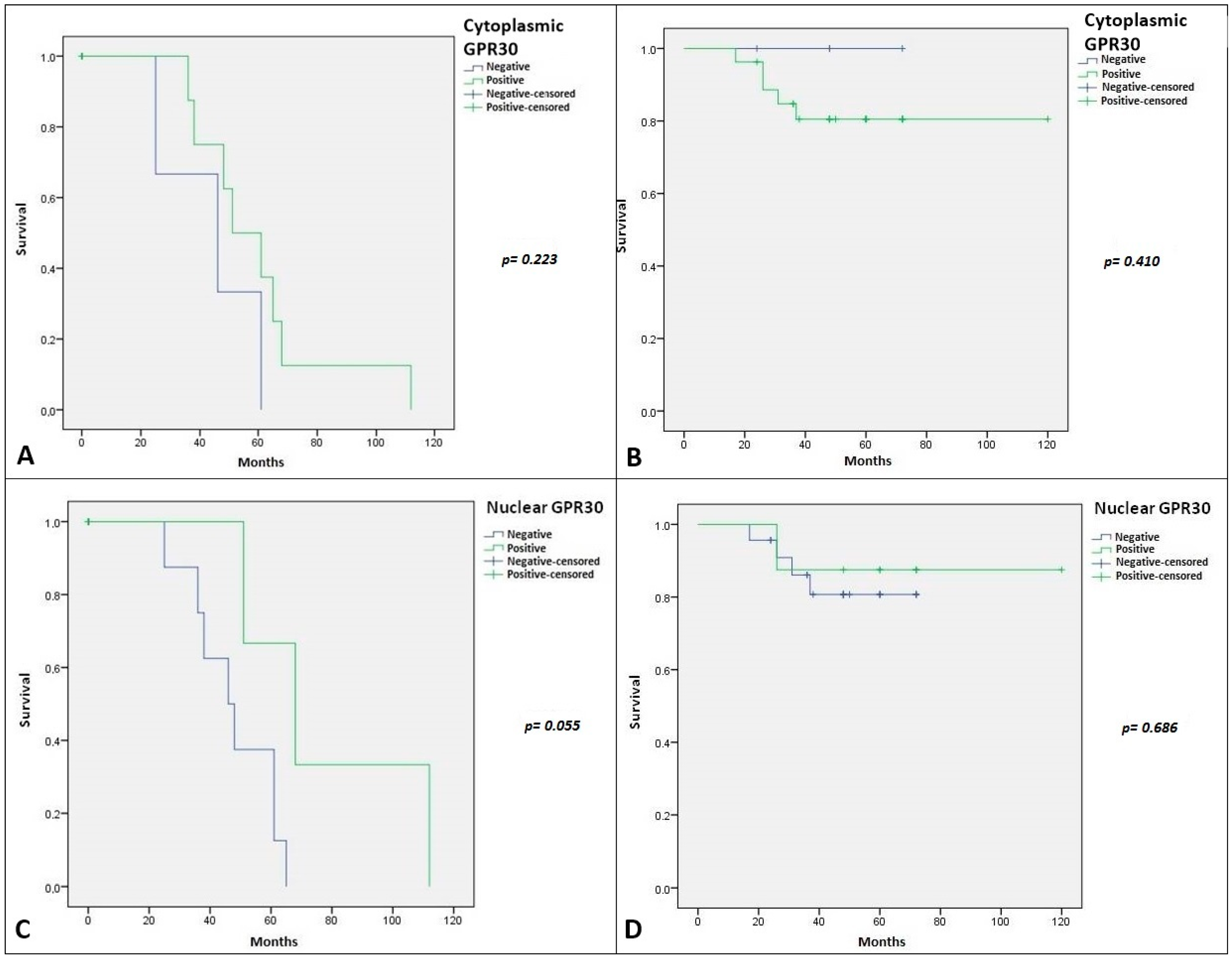
2.2. Immunohistochemical Expression of AR, ERβ and GPR30, and Relation with Clinical-Pathological Features and Survival in SGTs
3. Discussion
4. Material and Methods
4.1. Patients with Salivary Glands Tumors
4.2. TMA Building
4.3. Immunohistochemistry Analysis
4.4. Evaluation of Immunostaining
4.5. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheu, W.; Chan, J.K. Salivary gland tumours. In Diagnostic Histopathology of Tumours, 2nd ed.; Fletcher, C.D., Ed.; Churchill Livingstone: London, UK, 2000; p. 231. [Google Scholar]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. (Eds.) WHO Classification of Head and Neck Tumours, 4th ed.; IARC Press: Lyon, France, 2017; p. 160. [Google Scholar]
- Rochefort, H.; Chalbos, D. The role of sex steroid receptors on lipogenesis in breast and prostate carcinogenesis: A viewpoint. Horm. Cancer 2010, 1, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.A.; Miller, V.M.; Prakash, Y.S. Sex differences and sex steroids in lung health and disease. Endocr. Rev. 2012, 33, 1–47. [Google Scholar] [CrossRef] [PubMed]
- González-Arenas, A.; Agramonte-Hevia, J. Sex steroid hormone effects in normal and pathologic conditions in lung physiology. Mini Rev. Med. Chem. 2012, 12, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Cleve, A.; Fritzemeier, K.H.; Haendler, B.; Heinrich, N.; Möller, C.; Schwede, M.; Wintermantel, T. Pharmacology and clinical use of sex steroid hormone receptor modulators. Handb. Exp. Pharmacol. 2012, 214, 543–587. [Google Scholar]
- Higa, G.M.; Fell, R.G. Sex Hormone Receptor Repertoire in Breast Cancer. Int. J. Breast. Cancer 2013, 2013, 284036. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, P.; Di Donato, M.; Giraldi, T.; Auricchio, F. Targeting rapid action of sex steroid receptors in breast and prostate cancers. Front Biosci. 2011, 16, 2224–2232. [Google Scholar] [CrossRef]
- Kapadia, S.B.; Barnes, L. Expression of androgen receptor, gross cystic disease fluid protein, and CD44 in salivary duct carcinoma. Mod. Pathol. 1998, 11, 1033–1038. [Google Scholar] [PubMed]
- Fan, C.Y.; Melhem, M.F.; Hosal, A.S.; Grandis, J.R.; Barnes, E.L. Expression of androgen receptor, epidermal growth factor receptor, and transforming growth factor α in salivary duct carcinoma. Arch. Otolaryngol.-Head Neck Surg. 2001, 127, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Marques, Y.M.; Giudice, F.S.; Freitas, V.M.; Abreu e Lima Mdo, C.; Hunter, K.D.; Speight, P.M.; Machado de Sousa, S.C. Oestrogen receptor β in adenoid cystic carcinoma of salivary glands. Histopathology 2012, 60, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Dobbins, T.A.; Tseung, J.; Tran, N.; Lee, C.S.; O’Brien, C.J.; Clark, J.; Rose, B.R. Oestrogen receptor β expression in pleomorphic adenomas of the parotid gland. J. Clin. Pathol. 2009, 62, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, K.; Rayala, S.K.; Williams, M.D.; Kumar, R.; El-Naggar, A.K. Biological Role of Estrogen Receptor β in Salivary Gland Adenocarcinoma Cells. Clin. Cancer Res. 2006, 12, 5994–5999. [Google Scholar] [CrossRef] [PubMed]
- Albanito, L.; Madeo, A.; Lappano, R.; Vivacqua, A.; Rago, V.; Carpino, A.; Oprea, T.I.; Prossnitz, E.R.; Musti, A.M.; Andò, S.; Maggiolini, M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007, 67, 1859–6610. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Santolla, M.F.; Pupo, M.; Sinicropi, M.S.; Caruso, A.; Rosano, C.; Maggiolini, M. MIBE acts as antagonist ligand of both estrogen receptor α and GPER in breast cancer cells. Breast Cancer Res. 2012, 14, R12. [Google Scholar] [CrossRef] [PubMed]
- Mau, M.; Mielenz, M.; Südekum, K.H.; Obukhov, A.G. Expression of GPR30 and GPR43 in oral tissues: Deriving new hypotheses on the role of diet in animal physiology and the development of oral cancers. J. Anim. Physiol. Anim. Nutr. 2011, 95, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Perrone, F.; Losa, M.; Mela, M.; Casieri, P.; Orsenigo, M.; Cortelazzi, B.; Negri, T.; Tamborini, E.; Quattrone, P.; et al. Treatment relevant target immunophenotyping of 139 salivaryglandcarcinomas (SGCs). Oral Oncol. 2009, 45, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric 2005, 1, 3–63. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S.M.; Faquin, W.C.; Dayal, Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Frequent expression of androgen receptor in a subset of malignant salivary gland tumors. Am. J. Clin. Pathol. 2003, 119, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.; Rao, U.; Contis, L.; Krause, J.; Schwartz, A.; Scalamogna, P. Salivary duct carcinoma. Part II. Immunohistochemical evaluation of 13 cases for estrogen and progesterone receptors, cathepsin D, and C-ERBB-2 protein. Oral. Surg. Oral. Med. Oral. Pathol. 1994, 78, 74–80. [Google Scholar] [CrossRef]
- Shick, P.C.; Riordan, G.P.; Foss, R.D. Estrogen and progesterone receptors in salivary gland adenoid cystic carcinoma. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 1995, 80, 440–444. [Google Scholar] [CrossRef]
- Dori, S.; Trougouboff, P.; David, R.; Buchner, A. Immunohistochemical evaluation of estrogen and progesterone receptors in adenoid cystic carcinoma of salivary gland origin. Oral Oncol. 2000, 36, 450–453. [Google Scholar] [CrossRef]
- Jeannon, J.P.; Soames, J.V.; Bell, H.; Wilson, J.A. Immunohistochemical detection of oestrogen and progesterone receptors in salivary tumours. Clin. Otolaryngol. Allied Sci. 1999, 24, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.R.; da Cruz Perez, D.E.; de Almeida, O.P.; Kowalski, L.P. Estrogen receptor expression in salivary gland mucoepidermoid carcinoma and adenoid cystic carcinoma. Pathol. Oncol. Res. 2004, 10, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.D.; Roberts, D.; Blumenschein, G.R., Jr.; Temam, S.; Kies, M.S.; Rosenthal, D.I.; Weber, R.S.; El-Naggar, A.K. Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: Biologic significance and potential role in therapeutic stratification of patients. Am. J. Surg. Pathol. 2007, 31, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Sygut, D.; Bień, S.; Ziółkowska, M.; Sporny, S. Immunohistochemical expression of androgen receptor in salivary gland cancers. Pol. J. Pathol. 2008, 59, 205–210. [Google Scholar] [PubMed]
- Moriki, T.; Ueta, S.; Takahashi, T.; Mitani, M.; Ichien, M. Salivary duct carcinoma: Cytologic characteristics and application of androgen receptor immunostaining for diagnosis. Cancer 2001, 93, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Kishimoto, T.; Nagai, Y.; Yamada, M.; Iida, Y.; Okamoto, Y.; Ishida, Y.; Nakatani, Y.; Ichinose, M. Expressions of androgen receptor and its co-regulators in carcinoma ex pleomorphic adenoma of salivary gland. Pathology 2009, 41, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, H.C.; Verbist, B.M.; Schoffelen, R.; Mattijssen, V.; Slootweg, P.J.; van der Graaf Winette, T.A.; Carla, M.L.; van Herpen, C.M. Androgen receptor-positive salivary duct carcinoma: A disease entity with promising new treatment options. J. Clin. Oncol. 2011, 29, e473–e476. [Google Scholar] [CrossRef] [PubMed]
- Välimaa, H.; Savolainen, S.; Soukka, T.; Silvoniemi, P.; Mäkelä, S.; Kujari, H.; Gustafsson, J.A.; Laine, M. Estrogen receptor-β is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J. Endocrinol. 2004, 180, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Collini, P.; Imbimbo, M.; Barisella, M.; Testi, A.; Licitra, L.F.; Löning, T.; Tiemann, K.; Quattrone, P.; Bimbatti, E.; et al. Immunohistochemical and molecular profile of salivary gland cancer in children. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Omoto, Y.; Iwase, H. Clinical significance of estrogen receptor β in breast and prostate cancer from biological aspects. Cancer Sci. 2015, 106, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Hopp, T.A.; Weiss, H.L.; Parra, I.S.; Cui, Y.; Osborne, C.K.; Fuqua, S.A. Low levels of estrogen receptor β protein predict resistance to tamoxifen therapy in breast cancer. Clin. Cancer Res. 2004, 10, 7490–7499. [Google Scholar] [CrossRef] [PubMed]
- Iwase, H.; Zhang, Z.; Omoto, Y.; Sugiura, H.; Yamashita, H.; Toyama, T.; Iwata, H.; Kobayashi, S. Clinical significance of the expression of estrogen receptors alpha and β for endocrine therapy of breast cancer. Cancer Chemother. Pharmacol. 2003, 52, S34–S38. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.C.; Peng, B.; Lewis, A.; Davie, J.R.; Leygue, E.; Kemp, A.; Ung, K.; Vendetti, M.; Shiu, S. Inducible upregulation of oestrogen receptor-β1 affects oestrogen and tamoxifen responsiveness in MCF7 human breast cancer cells. J. Mol. Endocrinol. 2005, 34, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gustafsson, J.Å. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011, 1, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; Green, A.R.; Karthik, S.; Alizadeh, Y.; Hughes, T.A.; Harkins, L.; Ellis, I.O.; Robertson, J.F.; Paish, E.C.; Saunders, P.T.; Groome, N.P. Nuclear and cytoplasmic expression of ERβ1, ERβ2, and ERβ5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008, 14, 5228–5235. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, I.; Zannoni, G.F.; Prisco, M.G.; Fagotti, A.; Tortorella, L.; Vizzielli, G.; Mencaglia, L.; Scambia, G.; Gallo, D. Cytoplasmic expression of estrogen receptor β (ERβ) predicts poor clinical outcome in advanced serous ovarian cancer. Gynecol Oncol. 2011, 122, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, G.F.; Prisco, M.G.; Vellone, V.G.; De Stefano, I.; Vizzielli, G.; Tortorella, L.; Fagotti, A.; Scambia, G.; Gallo, D. Cytoplasmic expression of oestrogen receptor β (ERβ) as a prognostic factor in vulvar squamous cell carcinoma in elderly women. Histopathology. 2011, 59, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Arterburn, J.B.; Sklar, L.A. GPR30: AG protein-coupled receptor for estrogen. Mol. Cell. Endocrinol. 2007, 265, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, H.M.; Cheng, S.B.; Christensen, E.M.; Filardo, E.J. The G-protein coupled estrogen receptor, GPER: The inside and inside-out story. Mol. Cell. Endocrinol. 2015, 3, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Graeber, C.T.; Quinn, J.A.; Filardo, E.J. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids. 2011, 76, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, E.P.; Noske, A.; Meisel, A.; Varga, Z.; Fink, D.; Imesch, P. The G Protein-Coupled Estrogen Receptor (GPER) Is Expressed in Two Different Subcellular Localizations Reflecting Distinct Tumor Properties in Breast Cancer. PLoS ONE 2014, 9, e83296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjöström, M.; Hartman, L.; Grabau, D.; Fornander, T.; Malmström, P.; Nordenskjöld, B.; Sgroi, D.C.; Skoog, L.; Stål, O.; Leeb-Lundberg, L.M.; et al. Lack of G protein-coupled estrogen receptor (GPER) in the plasma membrane is associated with excellent long-term prognosis in breast cancer. Breast Cancer Res. Treat. 2014, 145, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Bologa, C.G.; Revankar, C.M.; Young, S.M.; Edwards, B.S.; Arterburn, J.B.; Kiselyov, A.S.; Parker, M.A.; Tkachenko, S.E.; Savchuck, N.P.; Sklar, L.A.; et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2006, 2, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.K.; Burai, R.; Ramesh, C.; Petrie, W.K.; Alcon, S.N.; Nayak, T.K.; Bologa, C.G.; Leitao, A.; Brailoiu, E.; Deliu, E.; et al. In vivo effects of a GPR30 antagonist. Nat. Chem. Biol. 2009, 5, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.K.; Field, A.S.; Burai, R.; Ramesh, C.; Petrie, W.K.; Bologa, C.G.; Oprea, T.I.; Yamaguchi, Y.; Hayashi, S.; Sklar, L.A.; et al. Identification of a GPER/GPR30 Antagonist with Improved Estrogen Receptor Counter selectivity. J. Steroid Biochem. Mol. Biol. 2011, 127, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Petrie, W.K.; Dennis, M.K.; Hu, C.; Dai, D.; Arterburn, J.B.; Smith, H.O.; Hathaway, H.J.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor-Selective Ligands Modulate Endometrial Tumor Growth. Obstet. Gynecol. Int. 2013, 2013, 472720. [Google Scholar] [CrossRef] [PubMed]


| Patient Features | Number of Patients | 69 |
|---|---|---|
| Median Age (Range) | 60 (17–87) Years | |
| Sex | Male | 41 (59.4%) |
| Female | 28 (40.6%) | |
| Lesion | Benign | 36 (52.2%) |
| Malign | 33 (47.8%) | |
| Site | Parotid | 59 (85.5%) |
| SG | 10 (14.5%) | |
| Grading | G1 | 14 (42.4%) |
| G2/G3 | 19 (57.6%) | |
| Benign (without grading) | 36 | |
| Ki67 Score | ≤5% | 42 (60.9%) |
| >5% | 20 (29%) | |
| NA | 7 (10.1%) | |
| Cell Type Differentiation | Epithelial | 38 (55.1%) |
| Myoepithelial | 7 (10.1%) | |
| Mixed | 24 (34.8%) | |
| Histotype | MEC | 13 (18.8%) |
| ACC | 9 (13%) | |
| CA ex PA | 3 (4.3%) | |
| Adenocarcinoma | 2 (2.9%) | |
| AdCC | 1 (1.4%) | |
| SDC | 1 (1.4%) | |
| Oncocytic CA | 1 (1.4%) | |
| Mixed tumor | 3 (4.3%) | |
| PA | 18 (26.1%) | |
| Warthin’s tumors | 9 (13%) | |
| Myoepithelioma | 7 (10.1%) | |
| Oncocytoma | 1 (1.4%) | |
| Basal cell adenoma | 1 (1.4%) |
| Patient Features | Nuclear AR | Cytoplasmic ERβ | Nuclear ERβ | Cytoplasmic GPR30 | Nuclear GPR30 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | p-Value | R Pearson | Negative | Positive | p-Value | R Pearson | Negative | Positive | p-Value | R Pearson | Negative | Positive | p Value | R Pearson | Negative | Positive | p-Value | R Pearson | ||
| Age | ≤60 | 24 | 10 | 0.326 | −0.126 | 25 | 6 | 0.133 | 0.197 | 18 | 13 | 0.331 | −0.128 | 3 | 29 | 0.235 | −0.151 | 24 | 8 | 0.122 | −0.196 |
| >60 | 22 | 5 | 17 | 10 | 19 | 8 | 6 | 24 | 27 | 3 | |||||||||||
| Sex | Male | 27 | 8 | 0.715 | 0.047 | 25 | 10 | 0.836 | −0.027 | 22 | 13 | 0.855 | −0.024 | 3 | 32 | 0.130 | −0.192 | 30 | 5 | 0.417 | 0.103 |
| Female | 19 | 7 | 17 | 6 | 15 | 8 | 6 | 21 | 21 | 6 | |||||||||||
| Site | Parotid | 41 | 13 | 0.795 | 0.033 | 35 | 14 | 0.695 | −0.051 | 32 | 17 | 0.576 | 0.073 | 8 | 45 | 0.754 | 0.040 | 42 | 11 | 0.132 | −0.191 |
| SG | 5 | 2 | 7 | 2 | 5 | 4 | 1 | 8 | 9 | 0 | |||||||||||
| Lesion | Benign | 22 | 9 | 0.413 | −0.105 | 21 | 6 | 0.394 | 0.112 | 18 | 9 | 0.671 | 0.056 | 4 | 27 | 0.718 | −0.046 | 24 | 7 | 0.319 | −0.127 |
| Malignant | 24 | 6 | 21 | 10 | 19 | 12 | 5 | 26 | 27 | 4 | |||||||||||
| Grade | G1 | 10 | 2 | 0.709 | 0.068 | 12 | 2 | 0.052 | 0.349 | 9 | 5 | 0.756 | 0.056 | 4 | 10 | 0.087 | 0.307 | 13 | 1 | 0.385 | 0.156 |
| G2–G3 | 14 | 4 | 9 | 8 | 10 | 7 | 1 | 16 | 14 | 3 | |||||||||||
| Ki67 | ≤5% | 29 | 9 | 0.904 | −0.016 | 26 | 9 | 0.392 | 0.116 | 23 | 12 | 0.570 | 0.077 | 8 | 31 | 0.150 | 0.191 | 32 | 7 | 0.704 | 0.050 |
| >5% | 14 | 4 | 12 | 7 | 11 | 8 | 1 | 17 | 14 | 4 | |||||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, G.; Collina, F.; Sabatino, R.; Cerrone, M.; Longo, F.; Ionna, F.; Losito, N.S.; De Cecio, R.; Cantile, M.; Pannone, G.; et al. Sex Hormone Receptors in Benign and Malignant Salivary Gland Tumors: Prognostic and Predictive Role. Int. J. Mol. Sci. 2018, 19, 399. https://doi.org/10.3390/ijms19020399
Aquino G, Collina F, Sabatino R, Cerrone M, Longo F, Ionna F, Losito NS, De Cecio R, Cantile M, Pannone G, et al. Sex Hormone Receptors in Benign and Malignant Salivary Gland Tumors: Prognostic and Predictive Role. International Journal of Molecular Sciences. 2018; 19(2):399. https://doi.org/10.3390/ijms19020399
Chicago/Turabian StyleAquino, Gabriella, Francesca Collina, Rocco Sabatino, Margherita Cerrone, Francesco Longo, Franco Ionna, Nunzia Simona Losito, Rossella De Cecio, Monica Cantile, Giuseppe Pannone, and et al. 2018. "Sex Hormone Receptors in Benign and Malignant Salivary Gland Tumors: Prognostic and Predictive Role" International Journal of Molecular Sciences 19, no. 2: 399. https://doi.org/10.3390/ijms19020399






